
книги студ / Color Atlas of Pathophysiology (S Silbernagl et al, Thieme 2000)
.pdf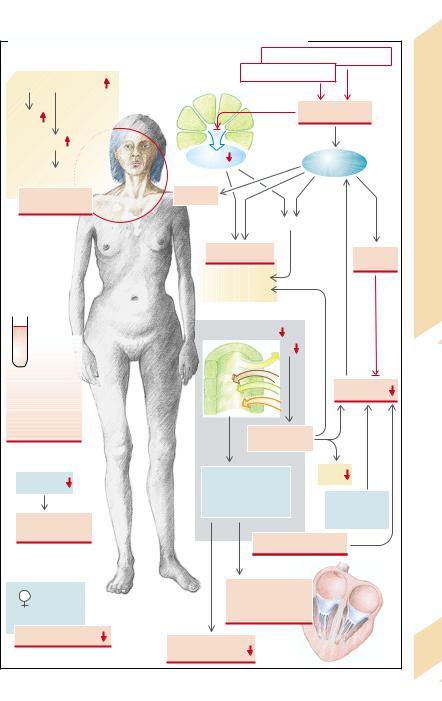
A. Effects and Symptoms of Adrenocortical Hormone Deficiency |
|
|
||||||
|
|
|
|
|
|
Decreased gluconeogenesis |
|
|
Proopiomelanocortin |
|
|
|
Increased glycolysis |
|
|||
|
|
|
|
1 |
|
|
||
|
|
|
|
|
|
|
|
|
ACTH |
3 |
|
|
|
|
|
Hypoglycemia |
|
|
|
|
|
|
|
|
||
MSH |
|
|
|
|
|
|
|
|
|
|
Insulin |
|
|
|
Epinephrine |
|
|
|
|
|
|
|
|
|
|
|
Melanotropic effect |
|
|
|
|
|
|
|
Disease |
Brown discolor- |
|
Sweating |
|
|
|
|
|
|
ation of the skin |
|
|
|
|
2 |
|
|
|
|
|
|
|
|
|
Lipolysis |
|
Addison’s |
|
|
|
Protein |
|
Tachy- |
|||
|
|
|
breakdown |
|
||||
|
|
|
|
|
|
|
cardia |
|
|
|
Muscle weakness |
|
|
9.8 |
|||
|
|
|
Weight loss |
|
|
|||
|
|
|
|
|
Plate |
|||
|
|
|
|
|
|
|
|
|
|
|
Renal Na+ retention |
|
|
||||
4 |
|
|
|
|
|
Na+ |
|
|
Anemia, |
|
|
H |
+ |
|
|
Blood |
|
neutropenia, |
|
|
|
|
|
|
||
|
|
K+ |
|
|
pressure |
|
||
eosinophilia, |
|
|
Mg |
2+ |
|
|
|
|
thrombopenia, |
|
|
|
|
|
|
||
|
|
|
|
|
|
|
|
|
lymphocytosis |
|
|
|
|
Hypotonic |
|
|
|
|
|
5 |
|
|
|
|
||
|
|
|
|
dehydration |
|
|
||
Hydrochloric |
|
Acidosis, |
|
GFR |
|
|||
acid |
|
hyperkalemia, |
|
|
|
|||
|
|
|
Reduced |
|
||||
6 |
|
hypermagnesemia |
|
|||||
|
|
|
|
|
|
|
catecholamine |
|
Gastrointestinal |
|
|
|
|
|
|
sensitivity |
|
|
|
|
|
|
|
|
|
|
infections |
|
|
|
|
|
|
|
|
|
|
|
|
|
Electrolyte disturbances |
|
||
7 |
|
|
|
Abnormal action |
|
|
||
|
|
|
potential generation |
|
||||
|
|
|
|
|
||||
Androgen deficiency |
|
|
|
and conduction |
|
|
||
|
|
|
in the heart |
|
|
|
||
Pubic hair |
Neuromuscular |
8 |
|
|
||||
|
|
|
|
271 |
||||
|
|
excitability |
|
|
|
|
||
Silbernagl/Lang, Color Atlas of Pathophysiology © 2000 Thieme
All rights reserved. Usage subject to terms and conditions of license.

Causes and Effects of Androgen Excess and Deficiency
9 Hormones
Follitropin (FSH) and lutropin (LH) are released in the anterior pituitary, stimulated by pulsatile release of gonadoliberin (gonadotropin-re- leasing hormone, GnRH) (→ A1). The pulsatile secretion of these gonadotropins is inhibited by prolactin (→p. 260). LH controls the release of testosterone from the Leydig cells in the testes. Testosterone, by means of a negative feedback, inhibits the release of GnRH and LH (→A2). The formation of inhibin, which inhibits the release of FSH, and of androgen-binding protein (ABP) is promoted by FSH in the testicular Sertoli cells (→ A3).
Testosterone or dihydrotestosterone (5-α- DHT) which is formed from testosterone in the Sertoli cells and in some organs, promotes the growth of the penis, seminiferous tubules, and scrotum (→ A4). Testosterone and FSH are both necessary for the formation and maturation of spermatozoa. In addition, testosterone stimulates the secretory activity of the prostate
(reduced viscosity of the ejaculate) and the seminal vesicle (admixture of fructose and prostaglandins), as well as the secretory activity of the sebaceous and sweat glands in the axillae and the genital region. Testosterone increases skin thickness, scrotal pigmentation, and erythropoiesis. It also influences height and stature by promoting muscle and bone growth (protein anabolism), longitudinal growth, and bone mineralization as well as fusion of the epiphyseal plates. Testosterone stimulates laryngeal growth (deepening of the voice), hair growth in the pubic and axillary regions, on the chest and in the face (beard); its presence is essential for hair loss in the male. The hormone stimulates libido and aggressive behavior. Lastly, it stimulates the renal retention of electrolytes, reduces the concentration of high density lipoprotein (HDL) in blood, and influences fat distribution.
receptors. Other causes are inhibition of pulsatile gonadotropin release by prolactin as well as damage to the hypophysis (trauma, infarct, autoimmune disease, tumor, hyperplasia) or to the testes (genetic defect, severe systemic disease). Lastly, androgen effects can be impaired by enzyme defects in hormone synthesis, for example, genetic reductase deficiency (→p. 264) or by a defect of the testosterone receptors.
Effects of deficient testosterone action in the male fetus are absent sexual differentiation (→ p. 278); in juveniles they are failure of the voice to break and absence of adult body hair, delayed bone growth, but also ultimately excess longitudinal growth of the limbs due to delayed epiphyseal fusion. Other effects (in juveniles and adults) are infertility, decreased libido and aggressiveness, reduced muscle and bone mass, and slightly decreased hematocrit. If there is no androgen effect at all, there will not even be any feminine pubic and axillary hair.
Possible causes of androgen excess are enzyme defects in steroid hormone synthesis (→p. 264), a testosterone-producing tumor, or iatrogenic androgen supply (→ A2, A3).
Effects of testosterone excess are male sex differentiation and hair growth, even in the female, an increase in erythropoiesis, muscle and bone mass as well as of libido and aggressiveness. Amenorrhea (!) and impaired fertility (" and !) are caused by inhibition of GnRH and gonadotropin release.
The generative function of the testes can, however, also be impaired without appreciable abnormality of the sex hormones, as in undescended testis (cryptorchidism), genetic defects, or damage to the testes (e.g., inflammation, radiation, abnormal blood perfusion due to varices).
Decreased release of androgens can be due to a lack of GnRH. Even nonpulsatile GnRH secretion stimulates androgen formation inadequately. Both can occur with damage to the hypothalamus (tumor, radiation, abnormal perfusion, genetic defect) as well as psychological or physical stress. Persistently high concentra-
272tions of GnRH (and its analogs) decrease gonadotropin release by down-regulation of the
Silbernagl/Lang, Color Atlas of Pathophysiology © 2000 Thieme
All rights reserved. Usage subject to terms and conditions of license.
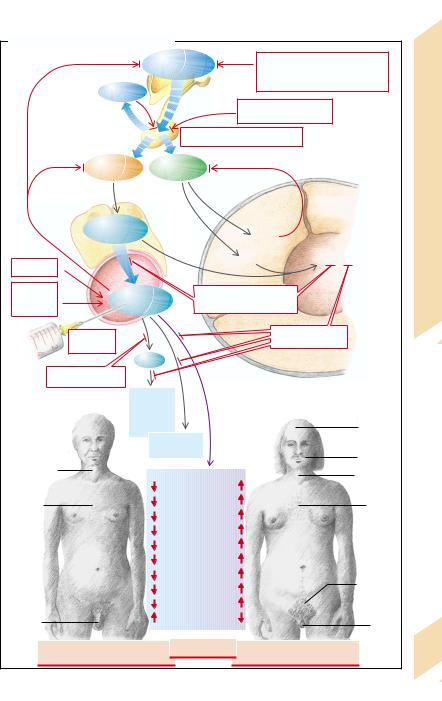
A. Androgen Excess and Deficiency |
|
|
|
|
|
||
|
|
Gonadoliberin |
|
Cachexia, |
|
|
|
|
|
(GnRH) |
|
damage to hypothalamus, |
|
||
|
Prolactin |
|
|
genetic defects |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Persistently increased |
|
Deficiency |
|
|
1 |
|
|
GnRH analogues |
|
||
|
|
|
|
|
|
||
|
|
Damage to pituitary |
|
|
|||
|
|
|
|
|
|
|
|
|
Lutropin |
Follitropin |
|
|
|
|
and |
|
(LH) |
(FSH) |
|
|
|
|
|
|
Leydig |
|
|
|
Sertoli cells |
|
Excess |
|
|
|
|
of the |
|
||
|
cells |
|
|
|
|
||
|
|
|
|
seminiferous |
|
Androgen |
|
|
|
|
|
|
|
||
|
Testosterone |
|
|
|
tubules |
|
|
|
3 |
|
Inhibin |
|
|
||
|
|
|
|
|
|||
Tumors |
|
|
|
ABP |
Spermiogenesis |
||
|
|
|
|
|
|
||
Blut |
|
|
|
|
|
9.9 |
|
|
|
|
|
|
|
||
Adreno- |
2 |
|
|
|
|
|
|
|
|
Testicular damage, |
Lumen |
|
Plate |
||
genital |
Testosterone |
|
|||||
genetic damage |
|
|
|||||
syndrome |
|
|
|
|
|||
|
Iatrogenic |
|
|
Receptor defect |
|
|
|
|
supply |
|
|
|
|
|
|
|
DHT |
|
|
|
|
|
|
Reductase deficiency |
|
|
|
|
|
|
|
|
Penis, |
|
|
|
|
|
|
|
scrotum, |
|
|
|
|
|
|
|
seminiferous |
|
|
|
Receding |
|
|
|
tubules |
|
|
|
|
||
|
|
Prostate, |
|
|
|
forehead |
|
|
|
seminal |
|
|
|
Growth of |
|
|
|
vesicles |
|
|
|
|
|
Female voice |
|
|
|
|
|
beard |
|
|
Libido and |
|
|
Male voice |
|
||
|
|
|
|
|
|||
|
|
|
|
|
|
||
Absence of |
|
aggressive behavior |
|
|
|
|
|
|
Bone growth |
|
|
Hair on |
|
||
male hair |
|
|
|
|
|||
|
|
Electrolyte retention |
|
|
chest |
|
|
|
|
|
|
|
|
||
|
|
Epiphyseal fusion |
|
|
|
|
|
Feminine |
|
Protein synthesis |
|
Amenorrhea |
|
||
|
|
|
|
|
|||
|
Muscle growth |
|
|
|
|
||
fat |
|
|
|
|
|
||
|
|
|
|
|
|
|
|
distribution |
|
Erythropoiesis |
|
|
Rhomboid |
|
|
|
|
Skin thickness |
|
|
|
||
|
|
|
|
pubic hair |
|
||
Hypotrophic |
|
Sebaceous glands |
|
|
|
|
|
|
HDL |
|
|
|
|
|
|
genitalia |
|
|
|
|
Hyper- |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
trophic |
|
Feminization of the male |
Infertility |
Masculinization of the female |
clitoris |
273 |
|||
|
|||||||
due to testosterone deficiency |
|
due to testosterone excess |
|
||||
|
|
|
|||||
Silbernagl/Lang, Color Atlas of Pathophysiology © 2000 Thieme
All rights reserved. Usage subject to terms and conditions of license.

9 Hormones
274
Release of Female Sex Hormones
The gonadotropic hormones FSH and LH are released from the anterior lobe of the pituitary gland in a pulsative manner (every 60 to 90 min for 1 min) after pulsatile stimulation by GnRH from the hypothalamus at the same frequency (→A2; see also p. 272). FSH and LH are essential for the maturing of the follicles and for the temporal coordination of the production of female sex hormones. In the female organism FSH promotes the maturation of the follicles and estrogen production in the granulosa cells of the follicles (→A2). The estrogens (estrone, estradiol, estriol) at first stimulate the further release of gonadotropins (positive feedback) until the maturation of a follicle leads to ovulation and corpus luteum formation. Progestogens (progesterone and analogs), formed by the corpus luteum under the influence of LH, and the estrogens (after ovulation) inhibit further release of gonadotropins (→A3). The concentration of gonadotropins falls again, as does, after some delay, that of the estrogens and progestogens (→A4). As a rule this cycle takes 28 days, although the interval between menstruation and ovulation varies greatly. The granulosa cells also form inhibin and activin, while the theka cells form the androgens androstenedione and testosterone. Activin promotes gonadotropin release, while inhibin suppresses it (see p. 272 for the effect of testosterone). Prolactin produced in the anterior pituitary inhibits the pulsatile release of gonadotropins. It also decreases the ovary’s responsiveness to gonadotropins.
An excess of female sex hormones is usually due to an exogenous supply (contraceptive pills). In addition, some tumors produce sex hormones.
A lack of estrogens and progestogens is frequently the result of a decreased GnRH release in severe psychological or physical stress (e.g., malnutrition, serious systemic disease, highperformance sport). GnRH release can also be reduced through the influence of the neurotransmitters norepinephrine, dopamine, serotonin, and endorphins (→A1).
However, it is not only reduced, but also persistently high concentrations of GnRH (or its analogs) that decrease the release of gonadotropins (down-regulation of the GnRH recep-
tors). Even if the hypothalamus is undamaged, gonadotropin release can be impaired by damage to the pituitary (hemorrhage, ischemia, inflammation, trauma), by displacement of go- nadotropin-producing cells by tumors, or by inhibition due to a raised concentration of sex hormones (ovulation inhibitors, anabolic substances with androgen action, tumors, adrenogenital syndrome; →p. 264).
If androgen production is raised, the release of FSH is inhibited and follicle maturation is thus interrupted. Polycystic ovaries are formed. Some of the androgens are transformed into estrogens which, via stimulation of LH release, promote further formation of ovarian androgens.
It is relatively common for a reduction in gonadotropin release to be due to raised prolactin secretion, for example, as a result of the absence of inhibition of pituitary secretion of prolactin or a prolactin-producing pituitary tumor (→p. 260). Gonadotropin release can be inhibited by dopaminergic drugs that cause a rise in prolactin secretion. Lastly, gonadotropin release can be inhibited by damage to the pituitary through head trauma, abnormal anlage or maturation, radiation, tumors, degenerative or inflammatory disease, or defective biosynthesis.
The formation of estrogens and/or progestogens can be impaired by ovarian insufficiency caused by an abnormal development (→ p. 278) or by damage (e.g., radiation, chemotherapeutic agents). Inadequate follicular maturation or transformation in the corpus luteum (corpus luteum insufficiency) can cause the deficiency. Lack of estrogen can also be due to an enzyme defect. In the resistant ovary syndrome the ovaries are refractory to the action of gonadotropins. This may be caused by defective receptors or inactivating antibodies. The result is a lack of estrogens despite an increased release of gonadotropins.
Silbernagl/Lang, Color Atlas of Pathophysiology © 2000 Thieme
All rights reserved. Usage subject to terms and conditions of license.
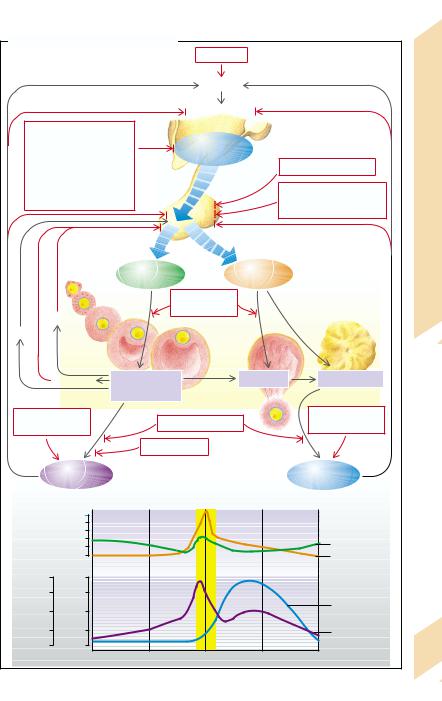
A. Release of Female Sex Hormones |
|
|
|
|
|
|||||
|
|
|
|
|
|
Stress |
|
|
|
|
|
|
|
|
|
|
Psyche |
|
|
|
|
|
|
|
|
|
|
Hypothalamus |
|
|
Release |
|
Inflammation, ischemia, |
|
|
|
|
|
|
||||
tumors, trauma, |
|
|
Gonadoliberin |
|
|
|||||
malnutrition, |
|
|
|
|
Hormones: |
|||||
|
|
(GnRH) |
|
|
|
|||||
drugs, |
|
|
|
|
Androgens, prolactin |
|||||
|
|
|
|
|
||||||
genetic defects, |
|
1 |
|
|
||||||
|
|
|
|
|
||||||
norepinephrine, dopamine, |
|
|
|
|
||||||
|
|
|
Bleeding, ischemia, |
|||||||
serotonin, endorphins, |
|
Hypophysis |
|
|||||||
radiation |
|
|
|
inflammation, trauma, |
||||||
|
|
|
Female Sex |
|||||||
|
|
|
|
|
|
|
|
hypophyseal insufficiency |
||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Follitropin |
|
Lutropin |
|
|
9.10 |
|
|
|
|
|
(FSH) |
|
(LH) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Resistant ovary |
|
|
|
Plate |
|
Inhibin |
|
|
|
syndrome |
|
3 |
|
||
|
|
|
|
|
|
|
|
|||
Activin |
|
|
|
|
|
|
|
|
|
|
|
|
|
2 |
|
|
|
|
|
|
|
|
|
Androgens |
Follicle |
|
Ovulation |
Corpus luteum |
|
|||
|
|
maturation |
|
|
||||||
|
|
|
|
|
|
|
|
|
||
|
|
|
|
Granulosa cells |
|
|
|
|
|
|
Ovulation inhibitors, |
|
Ovarian insufficiency |
|
Ovulation inhibitors, |
|
|||||
|
tumors |
|
|
|
|
tumors |
|
|||
|
|
|
|
|
Enzyme defects |
|
|
|
|
|
|
|
Estrogens |
|
|
|
|
Progestogens |
|
||
4 |
|
|
50 |
|
|
|
|
|
|
|
Units/L |
|
|
|
|
|
|
|
|||
|
|
|
|
|
|
|
|
|||
|
|
|
|
|
|
|
|
|
FSH |
|
|
|
|
0 |
|
|
|
|
|
LH |
|
ng/mL |
ng/mL |
|
|
|
|
|
|
|
||
|
16 |
|
0.4 |
|
|
|
|
|
|
|
Progestogens |
12 |
|
0.3 |
|
|
|
|
|
|
|
8 |
Estrogens |
0.2 |
|
|
|
|
|
Progestogens |
|
|
|
|
|
|
|
|
|
||||
4 |
0.1 |
|
|
|
|
|
Estrogens |
|
||
0 |
0 |
|
|
|
|
|
|
275 |
||
|
|
|
|
|
|
|
|
|||
|
|
|
1 |
|
7 |
14 |
21 |
28 |
Days |
|
|
|
|
|
|
||||||
Silbernagl/Lang, Color Atlas of Pathophysiology © 2000 Thieme
All rights reserved. Usage subject to terms and conditions of license.

Effects of Female Sex Hormones
Estrogens
|
Estrogens promote the development of the fe- |
|
male sex characteristics, i.e., the transforma- |
|
tion of the Müller ducts into Fallopian tubes, |
|
uterus and vagina, as well as the secondary |
|
sexual characteristics (e.g., development of |
|
the mammary glands and female fat distribu- |
|
tion). They require the cooperation of andro- |
|
gens in order to stimulate axillary and pubic |
|
hair growth. Estrogens also influence the psy- |
|
chological development of women. In sexually |
|
mature women estrogens and progestogens |
|
have partly opposite actions. |
|
Estrogens promote the proliferation of the |
Hormones |
uterine mucosa. In the cervix and vagina they |
the vaginal flora to lactic acid. The resulting |
|
|
reduce the viscosity of the cervical mucus and |
|
accelerate the exfoliation of the vaginal epi- |
|
thelium, whose glycogen is broken down by |
9 |
fall in pH stops pathogens from penetrating. |
|
Estrogens stimulate the formation of ducts in |
|
the mammary glands. They promote protein |
|
anabolism and increase the formation of HDL |
|
and VLDL. Conversely, they reduce the concen- |
|
tration of low density lipoproteins (LDL), and |
|
thus lower the risk of atherosclerosis. On the |
|
other hand, estrogens increase the coagulabil- |
|
ity of blood. Additionally, they raise electrolyte |
|
retention in the kidneys as well as the mineral- |
|
ization of the bones via hydroxylation of vita- |
|
min D3 and the inhibition of parathyroid hor- |
|
mone (PTH). In children they promote bone |
|
growth and maturation and accelerate epiphy- |
|
seal fusion. |
|
Progesterone |
|
In the uterus progesterone promotes the mat- |
|
uration and secretory activity of the uterine |
|
mucosa and decreases the contractility of the |
|
uterine muscle. When estrogen concentration |
|
falls at the end of the menstrual cycle, the mu- |
|
cosa is shed (menstruation). In the cervix and |
|
vagina progestogens raise the viscosity of cer- |
|
vical mucosa, narrow the cervical orifice, and |
|
inhibit fallopian motility. Furthermore, they |
276 |
inhibit the proliferation and exfoliation of vag- |
inal epithelium. They also promote the forma- |
|
|
tion of alveoli in the mammary glands. Proges- |
togens (progesterone and its analogs) raise the body’s metabolism and temperature, trigger hyperventilation, and reduce sensitivity to insulin in the periphery. Additionally, they have moderate glucocorticoid and antimineralocorticoid (natriuretic) actions. They lower the production of cholesterol and the plasma concentration of HDL and LDL.
Effects of Excess and Deficiency
In excess of female sex hormones (→A2) gonadotropin release is inhibited, there is no maturation of the follicles, no regular shedding of the uterine mucosa, and the woman will be infertile. An excess of estrogens can cause thrombosis due to a raised clotting tendency. In children high estrogen concentrations lead to premature sexual maturation and accelerate growth. However, premature epiphyseal fusion may eventually result in short stature. Increased progestogen action causes natriuresis, a rise in body temperature and hyperventilation, and via insulin resistance it can promote the development of diabetes mellitus.
A deficiency of female sex hormones
(→A3), like their excess, means that a normal menstrual cycle is not possible. In estrogen deficiency the phase of uterine proliferation is absent and the progestogens are not able to bring about maturation; in progestogen deficiency the uterine mucosa does not mature. In both these cases the woman is infertile and there is no menstrual bleeding (amenorrhea). The lack of estrogens also expresses itself in reduced manifestation of the external sex characteristics, in a tendency toward vaginal infections, in osteoporosis, and in an increased risk of atherosclerosis. In children there will be a delayed epiphyseal fusion that, despite slowed growth, may ultimately lead to tall stature.
The reproductive functions of a woman can also be abnormal independently of the sex hormones, for example, due to malformations or disease of the ovaries, fallopian tubes, or uterus.
Silbernagl/Lang, Color Atlas of Pathophysiology © 2000 Thieme
All rights reserved. Usage subject to terms and conditions of license.
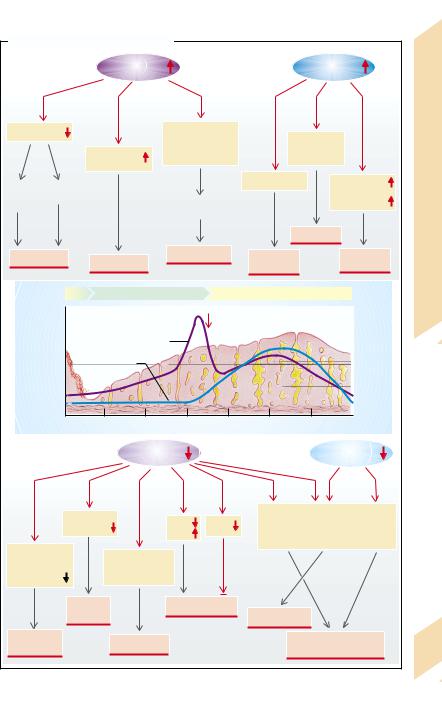
A. Effects of Female Sex Hormones |
|
|
|
|
|
|
|
||||
2 |
|
|
Estrogens |
|
|
|
|
Progestogens |
|
||
Excess |
|
|
|
|
|
|
|
||||
|
|
|
|
|
|
|
|
|
|
|
|
Gonadotropins |
|
|
In children: |
|
|
|
|
|
|
Effects |
|
|
|
accelerated |
|
|
Antimineralo- |
|
|||||
|
|
|
|
|
|
|
|||||
|
|
Clotting |
sexual maturation |
|
corticoid |
|
Hormones: |
||||
|
|
and growth |
|
|
action |
|
|
||||
|
|
tendency |
|
|
|
|
|||||
|
|
|
|
|
|
|
|
|
|||
No |
No |
|
|
|
|
|
Insulin resistance |
|
Basal |
||
|
|
|
|
|
|
|
metabolism, |
||||
follicular |
menstruation |
|
Premature |
|
|
|
|
|
body |
||
maturation |
|
|
|
|
|
|
|
temperature |
Sex |
||
|
|
|
|
epiphyseal fusion |
|
|
|
|
|||
|
|
|
|
|
|
|
|
Natriuresis |
|
Female |
|
Infertility |
Thrombosis |
Retarded growth |
Diabetes |
|
Hyper- |
||||||
|
|
|
|
|
|||||||
|
|
|
|
|
mellitus |
|
ventilation |
9.11 |
|||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Proliferative phase |
|
|
Secretory phase |
|
|
Plate |
||
|
|
|
|
Ovulation |
|
|
|
|
|||
1 |
|
|
Estrogens |
|
|
|
|
|
|
|
|
Normal |
|
|
|
|
|
|
|
|
|
||
|
|
|
|
|
|
|
|
|
|
||
|
|
Progesterone |
|
|
|
|
|
|
|
|
|
|
1 |
4 |
8 |
12 |
|
16 |
20 |
24 |
28 Days |
|
|
3 |
|
|
Estrogens |
|
|
|
|
Progestogens |
|
||
Deficiency |
|
|
|
|
|
|
|||||
|
|
|
|
|
|
|
|
|
|
||
|
|
25-OH-D3 |
|
|
|
|
|
No build-up of |
|
|
|
|
|
|
HDL, |
VLDL |
mammary |
uterus |
mammary |
|
|||
|
|
formation |
|
|
|||||||
|
|
|
LDL |
gland |
|
mucosa |
gland |
|
|||
|
|
|
|
|
|
tubes |
|
|
alveoli |
|
|
|
|
|
|
|
|
|
|
|
|
||
Proliferation and |
In children: |
|
|
|
|
|
|
|
|
||
desquamation |
|
|
|
|
|
|
|
|
|
||
|
delayed |
|
|
|
|
|
|
|
|
||
of vaginal |
|
|
|
|
|
|
|
|
|
|
|
|
|
epiphyseal fusion |
|
|
|
|
|
|
|
||
epithelium |
|
|
|
|
|
|
|
|
|||
|
|
|
|
|
|
|
|
|
|
||
|
|
Osteo- |
|
Atherosclerosis |
|
|
|
|
|
||
|
|
porosis |
|
|
|
|
Amenorrhea |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||
Vaginal |
|
|
Tall stature |
|
|
|
|
No distinct sexual |
|
||
infections |
|
|
|
|
|
characteristics |
|
277 |
|||
|
|
|
|
|
|
|
|||||
Silbernagl/Lang, Color Atlas of Pathophysiology © 2000 Thieme
All rights reserved. Usage subject to terms and conditions of license.

9 Hormones
278
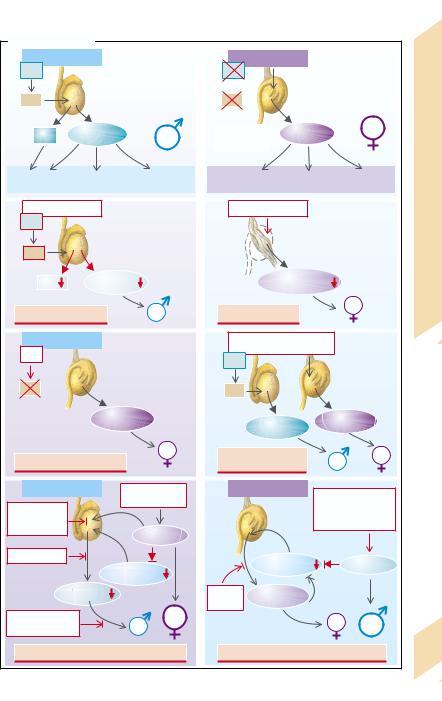
Intersexuality
The development of the gonadal anlagen to ovaries and testes is fixed by the presence or absence of the testis-determining factor (TDF), which is encoded on the sex determining region of the Y chromosome (SRY) and is responsible for testicular development (→A1). Ovaries develop if TDF is absent (→A2). The gonads determine the formation of female and male sexual hormones. Testosterone is formed in the Leydig cells of the testes, while antiMüller hormones are formed in the Sertoli cells (Müller inhibition factor [MIF]; →A1). However, not only androgens but also progestogens (some of them precursors of testosterone formation) and estradiol (predominantly by peripheral transformation of testosterone) are formed in the male. Progestogens and estrogens and, to a lesser extent, also androgens (mainly androstendion) are produced in the ovaries (→A2).
The development of the Wolffian ducts to internal male genitals (epididymis and vas deferens) is stimulated by the androgens, while the development of the Müller ducts to form the internal female genitals (fallopian tubes, uterus, vagina) is suppressed by the anti-Müller hormone from the Sertoli cells. The external sexual characteristics are determined, first and foremost, by the concentration of androgens (→p. 272), whereby the development of the female genitals and some of the sexual characteristics is promoted by estrogens.
The sex of an individual can be defined on the basis of the chromosomal set (XX or XY, respectively), of the gonads (ovary or testis), of the internal organs or of external appeareance.
Intersexuality occurs when the various sex characteristics have not developed unequivocally or are more or less pronounced.
An abnormal chromsome set occurs, for example, in Klinefelter’s syndrome (XXY), in which the testes are formed in such a way that spermatogenesis is possible, but androgen production is impaired (→A3). The androgen deficiency then leads to an inadequately male appearance. Only mild clinical symptoms are present in the XYY syndrome. A similar condition prevails in the XX male syndrome, which is probably due to translocation of an SRY-con-
taining Y chromosome fragment onto an X chromsome. In Turner’s syndrome (XO) connective tissue strands are formed in place of normal ovaries and the external features are more likely to be female (→A4). The condition is characterized by a number of additional malformations (e.g., of the heart and kidneys; dwarfism, webbed neck).
In certain mutations of the SRY gene no functional TDF is formed, despite the presence of a male chromosome set (XY), and ovaries develop (→A5).
In true hermaphroditism both testes and ovaries are simultaneously formed (→A6). An XY/XO mosaic can be a cause. Translocation of some parts of the Y chromosome, including of the SRY gene, onto an X chromosome (as in the XX male, see above) can lead to the formation of bisexual gonads and the appearance of intersexual sex characteristcs.
In pseudohermaphroditism the gonads correspond to the chromosomal sex, but the sex organs and secondary sex characteristics diverge or are not unequivocal. In male pseudohermaphroditism intersexual or female sex characteristics are present (→A7). A gonadotropin deficiency may be a cause, for example when gonadotropin release is suppressed due to an increased formation of female sexual hormones by a tumor. Other causes can be defects in the gonadotropin receptor, aplasia of the Leydig cells, enzyme defects of testosterone synthesis (→p. 264), defective testes, absent conversion of testosterone into dihydrotestosterone (reductase deficiency), or defective androgen receptors (→p. 272). In rare cases the formation of the female genitals may not be suppressed owing to a defect in the release or action of the anti-Müller hormone. Female pseudohermaphroditism (→
A8) can be the result of iatrogenic administration or increased formation of androgens, for example in an androgen-producing tumor, or can be due to an enzymatic defect in adrenocortical hormone synthesis, or a defect of aromatase, which transforms androstendion or rather testosterone into estrogens (→p. 264).
Silbernagl/Lang, Color Atlas of Pathophysiology © 2000 Thieme
All rights reserved. Usage subject to terms and conditions of license.
A. Intersexuality |
|
|
|
|
|
|
|
|
|
||
XY chromosomes |
|
|
XX chromosomes |
|
|
|
|
||||
SRY |
|
|
|
|
SRY |
|
|
|
|
|
|
TDF |
|
Testicular development |
TDF |
|
Ovarian development |
|
|||||
|
|
|
|
|
|
|
|||||
|
|
|
|
|
|
|
|
|
|
||
MIF |
|
Testosterone |
|
No MIF and |
Estrogens |
|
|
|
|||
|
|
|
|
|
testosterone |
|
|
|
|
|
|
1 |
|
|
|
Normal |
2 |
|
|
|
|
Normal |
|
Genitals |
Sexual characteristics |
Psyche |
Genitals |
Sexual characteristics |
Psyche |
|
|||||
XXY chromosomes |
|
X0 chromosomes |
|
|
|
Intersexuality |
|||||
SRY |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Hypotrophic testis |
|
|
Gonadal dysgenesis |
|||||
TDF |
|
|
|
|
|
|
|
|
|||
MIF |
Testosterone |
|
|
Sexual hormones |
|
|
9.12 |
||||
3 |
|
|
|
|
4 |
|
|
|
|
|
Plate |
Klinefelter’s syndrome |
|
Turner’s syndrome |
|
|
|
||||||
|
|
|
|
|
|||||||
XY chromosomes |
|
|
XX chromosomes + SRY |
|
|
|
|||||
SRY |
|
|
|
|
|
or XY/X0 mosaic |
|
|
|
|
|
|
|
|
|
SRY |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Ovaries |
|
|
|
|
|
Hybrid |
|
|
TDF |
|
|
|
|
TDF |
|
|
|
gonads |
|
|
No TDF |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Estrogens |
|
|
Testosterone |
Estrogens |
|
|||
5 |
|
|
|
|
6 |
|
|
|
|
||
|
|
|
|
|
|
|
|
|
|
||
Mutation of SRY gene |
|
True |
|
|
|
|
|
|
|||
|
hermaphroditism |
|
|
|
|
||||||
XY chromosomes |
Tumors, |
|
XX chromosomes |
Enzyme defects of |
|
||||||
|
|
|
exogenous supply |
|
|
|
|
||||
Abnormal |
|
|
|
|
|
steroid hormone |
|
||||
|
|
|
|
|
|
|
|
||||
|
|
|
|
|
|
|
synthesis, tumors, |
|
|||
testicular |
|
|
|
|
|
|
|
|
|||
|
|
|
|
|
|
|
exogenous supply |
|
|||
differentiation |
|
|
|
|
|
|
|||||
Estrogens |
|
|
|
|
|
|
|
||||
|
|
|
|
|
|
|
|
|
|
||
Enzyme defects |
|
|
|
Gonadotropins |
|
Testosterone |
|
||||
|
|
|
Gonadotropins |
|
|
|
|||||
|
|
|
|
|
|
|
|
|
|
||
7 |
|
Testosterone |
|
Enzyme |
Estrogens |
|
|
|
|
||
|
|
|
|
|
defects |
|
|
|
|
|
|
Reductase |
|
|
|
|
|
|
|
|
|
|
|
deficiency |
|
|
|
|
8 |
|
|
|
|
|
|
Receptor defects |
|
|
|
|
|
|
|
|
|||
|
|
|
|
|
|
|
|
|
|||
Male pseudohermaphroditism |
Female pseudohermaphroditism |
279 |
|||||||||
Silbernagl/Lang, Color Atlas of Pathophysiology © 2000 Thieme
All rights reserved. Usage subject to terms and conditions of license.

9 Hormones
280
Causes of Hypothyroidism, Hyperthyroidism and Goitre
The hormones thyroxine (T4) and triiodothyronine (T3) are formed in the epithelial cells (thyrocytes) that surround the follicles of the thyroid gland. Their synthesis is achieved in several steps, each of which can be disrupted. Iodine is essential for the synthesis of the hormones and has to be supplied in food (→A1). Iodine is taken from the blood into the follicular epithelial cells by means of a transporter coupled to Na+ (→A2). At the apical membrane of the cells it passes into the follicular lumen by exocytosis and is oxidized there (→A3).
A tyrosine-rich protein (thyroglobulin, TG) is formed in the epithelial cells (→A4) and secreted into the follicular lumen, too. Here the tyrosine residues of the globulin are iodized to the residues of diiodotyrosine (DIT) or of monoiodotyrosine (MIT) (→A6). The thyroid hormones are stored as thyroglobulin colloid in the follicular lumen. When stimulated to do so by the thyroid-stimulating hormone (TSH; see below), globulin is again taken up into the follicular epithelial cells and thyroxine and to a lesser extent triiodothyronine, is split off from the globulin (→A7). One iodine is removed from T4 in the periphery by a deiodinating enzyme (deiodinase) and thus converted into the more active T3 (→A8).
Regulation. Formation and release of T3 and T4 as well as growth of the thyroid gland are stimulated by thyrotropin (TSH) from the anterior pituitary. Its release is, in turn, stimulated by thyroliberin (TRH) from the hypothalamus. Stress and estrogens increase TSH release, while glucocorticoids, somatostatin, and dopamine inhibit it.
The causes of a lowered release of thyroid hormone (hypothyroidism) are usually found in the thyroid itself. Abnormal synthesis of thyroid hormones can be brought about by any one of the following steps in their synthesis:
1.Decreased iodine intake in food;
2.Impaired iodine uptake in the thyroid cells (genetically defective carrier or inhibition of transport by perchlorate, nitrate, thiocyanate (rhodanate);
3.Peroxidase deficiency (genetic) or peroxidase inhibition by thiouracil or iodine ex-
cess (inhibition of H2O2 formation by excessive I–);
4.Abnormal breakdown of thyreoglobulin;
5.Defective iodine incorporation (peroxidase is involved in this, too);
6.Defective coupling of two iodinated tyrosine residues;
7.Inability to release thyroxine and triiodothyronine, from thyroglobulin (genetically determined or lithium);
8.Lack of sensitivity of the target organs due to receptor defects or inadequate conver-
sion into the more effective T3 decreases T3/ T4 effectiveness even if T3/T4 release is nor-
mal or even raised.
Furthermore, mutations of the TSH receptors can change the degree to which the thyroid can be stimulatd by TSH. However, genetic defects of receptors and enzymes of T3/T4 synthesis are rare.
Two very common causes of hypothyroidism are inflammatory damage to the thyroid gland or surgical removal of the gland (due to thyroid cancer). More rarely hypothyroidism is due to a deficiency of TSH (e.g., in pituitary insufficiency) or of TRH (e.g., in damage to the hypothalamus).
The most common cause of an increased release of thyroid hormone (hyperthyroidism) is long-acting thyroid stimulator (LATS) or thy- roid-stimulating immunoglobulin (TSI), an IgG that apparently “fits” into the TSH receptor (Graves’ disease). This results, among other effects, in stimulation of hormonal release and thyroid enlargement. TSH release is suppressed by a high T3/T4 level. Other causes of hyperthyroidism are orthotopic or ectopic thyroid hormone–producing tumors, inflammation of the thyroid (thyroiditis), increased release of TSH, or excessive supply of thyroid hormones.
Enlargement of the thyroid gland (goitre) is the result of uncontrolled growth (tumor), or of increased stimulation by TSH or TSI. In this situation release of thyroid hormones can either be reduced (e.g., in marked iodine deficiency and the above-mentioned enzyme defects) or increased (e.g., in Graves’ disease).
Silbernagl/Lang, Color Atlas of Pathophysiology © 2000 Thieme
All rights reserved. Usage subject to terms and conditions of license.
