
Understanding the Human Machine - A Primer for Bioengineering - Max E. Valentinuzzi
.pdf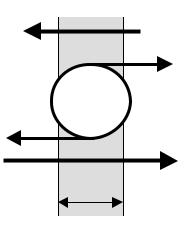
58 |
Understanding the Human Machine |
Algebraic sum of all charges = zero |
(2.48) |
The first postulate of the Ionic Theory of Excitation states that, within the cell membrane, there is an active ionic pump that extrudes sodium from the cell and carries potassium into it (Figure 2.18). For the time being, let us assume that the ionic exchange is 1:1, or, for each sodium out, one potassium is brought in. It is active because the pump consumes energy supplied by the cell itself. Its slowing down or inactivation tends to level concentrations off and, in the end, kills the cell. The amount of evidence collected in favor of the Na+ – K+ pump over the years in countless laboratories all over the world is enormous and overwhelming, indeed; however, perhaps there has not been yet a final conclusive demonstration, so offering one relatively weak side to those questioning the theory. Notice the semantic difference between evidence and demonstration. In fact, other ionic pumps have been described having become a widely
Sodium back |
|
|
diffusion |
|
|
ICF |
|
Na |
|
|
|
[K+] |
Active |
|
ionic |
ECF |
|
[A-] |
Pump |
[Na+] |
K |
|
|
|
|
|
PK >> PNa |
Em |
Potassium back |
|
diffusion |
|
|
|
+ |
Figure 2.18. MEMBRANE RESTING POTENTIAL. The two vertical lines represent, respectively, the internal (left) and external (right) sides of the cell membrane, hence delimiting its thickness. The active ionic pump maintains the ionic concentration gradients (potassium is higher in the ICF than in the ECF and sodium is just the opposite). Besides, in the resting condition, the permeability of the membrane to potassium is much higher than its permeability to sodium, thus, potassium back-diffuses easier to the ECF than sodium does it to the ICF. Since both carry positive electric charges, the net result is an accumulation of positive charge on the ECF side, breaking neutrality and so giving rise to the resting membrane potential, Em, positive outside and negative inside.
Chapter 2. Source: Physiological Systems and Levels |
59 |
used concept in physiology.
The second postulate of the Ionic Theory says that, in the resting condition, the permeability, PK, of the membrane to potassium ion is higher than its permeability, PNa, to sodium. This is a demonstrated fact with a numerical ratio between the two of about 50 to 75. Due to the concentration gradients, there is a passive (with no energy expenditure) back diffusion of both ions; one tends to return sodium to he ICF and the other pushes potassium to the ECF. However, because of the large permeability difference, for each Na+ getting back inside the cell, there are 50 to 75 K+ leaving it, so accumulating positive charges on the ECF side, depleting the ICF side of them and, hence, creating a charge imbalance on both sides with the outer face positive to the inner. Thus, here we have the membrane resting potential, Em (Figure 2.18), which obviously breaks neutrality.
The membrane is impermeable to the large proteic anions and they stay within the cell, but chloride can pass through it passively following the electric field sustained by the resting potential and reaching finally an equilibrium state. Due to osmotic forces that depend on the concentrations of the osmotically active particles (largely the small ions), water also moves across the membrane so determining cell volume.
Exercise: Suppose the permeability ratio potassium to sodium is one half of the stated value, would the resulting membrane potential increase, stay the same or decrease? Suppose both permeabilities were equal, what resting potential would result? Suppose the pumping rate drops to one half of its normal value, would it affect the membrane potential? Suppose a toxin stops the pump, what would Em be? Does the pump directly contribute to the resting membrane potential? For the latter question consider a 1:1 pump ratio. Note: do not confuse membrane ionic permeability ratio with ionic pumping ratio. This exercise is important for the full understanding of changes in membrane potential due to permeability changes.
Exercise: Suppose the pump ratio changed to 2Na+/3K+ and both permeabilities were equal, would the pump contribute to the membrane potential? If the answer were affirmative, which side would be positive to the other? Suppose now the ratio changed to 3Na+/2K+ keeping always both permeabilities equal, which side would be positive?
In real life, very rarely if ever, the pump is electrically neutral (1:1 ratio). The ionic exchange ratio varies from tissue to tissue and, even within the same tissue, histologically different fibers may show also different ratios. A typical example is found in the heart. In fact, the determination of the sodium-potassium pump exchange ratios is still a subject of research.
60 |
Understanding the Human Machine |
Therefore, one portion (usually less than one third) of Em is contributed by the pump. Ionic pumps, which sustain potentials, are called electrogenic. In other words, the resting membrane potential recognizes two sources, one (the largest), comes from the large difference in permeabilities via back diffusion, in turn dependent on concentration gradients sustained by the sodium-potassium pump, and the other (much smaller), directly produced by the pump due to a not neutral pumping sodium-potassium ratio, different from 1:1.
− Action potential
It was said above that an adequate stimulus applied to the cell triggers a response characterized by a change in the electrical stable state to another level, E2, remaining at the second one for a very specific length of time, td; thus, it can be called metastable. Let us describe the phenomenon with more detail: The external side of the membrane is electrically positive. If some of these charges are removed, i.e., if the membrane is depolarized,
|
ENa |
|
|
membrane |
PNa+/ PK+ =20 |
PK+/ PNa+ =50 to 75 |
|
Overshoot |
|
||
cell |
0 |
|
|
across |
time |
||
|
|||
Critical level |
|
||
Voltage |
Threshold |
||
Em |
|||
|
|||
EK |
|
||
|
|
Influx
+ Na
Outflux
+ K
Purmp restores
andinside
+ K
outside
+ Na
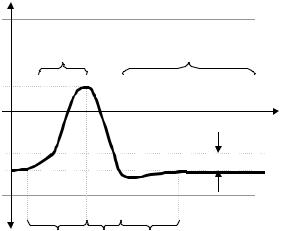
Figure 2.19. ACTION POTENTIAL AND ITS BOUNDARIES. The action potential is always bounded by the potassium potential, near the membrane potential, and the sodium potential above, which is a limiting unreachable value. See text.

Chapter 2. Source: Physiological Systems and Levels |
61 |
0
mV
–100
|
|
|
Figure 2.20. ACTUAL ACTION PO- |
|
|
|
TENTIAL. Recorded with microelectrode |
|
|
|
from a cat dorsal root nerve fiber. Ob- |
|
|
|
serve the stimulus artifact just before the |
|
|
|
depolarization upstroke, where the zero |
|
|
|
time is placed. The overall duration of the |
|
|
|
event is shorter than 1 ms. The positive |
|
|
|
overshoot appears clearly depicted. The |
|
|
|
resting potential lies around –90 mV. |
|
|
|
Reproduced from Ruch and Patton, |
|
|
|
Physiology and Biophysics, Copyrigth |
0 |
0.5 |
1.0 |
1966, Chapter 2 by Walter Wodbury, |
|
mS |
|
figure 2C, page 30, with permission from |
Elsevier.
the resting potential shifts towards zero (Figure 2.19). If depolarization continues, the decrease in Em (less negative inside) eventually reaches a level called the critical or the firing or the threshold potential. At the latter level, the membrane takes command of its own depolarization which rapidly proceeds until reaching a maximum, E2, to, thereafter and somewhat slower, returning to the initial resting state (Figure 2.19). Beyond the critical or firing level, the applied stimulus loses control completely. Figure 2.20 is an actual nerve action potential photographed on the oscilloscope screen after sweep synchronization with the stimulus (seen as a small spike preceding the fast depolarization upstroke). By comparison of both Figure 2.19 and Figure 2.20, the student can easily identify potential levels and durations. Notice the conceptual difference, many times mixed up in the daily jargon, between critical (or firing) threshold potential and threshold stimulus: the first one is just the negative potential measured from the zero line (in Figure 2.20, it could be in the order of – 70 mV), above the resting potential Em (about –90 mV), while the latter is defined as the threshold potential minus the membrane resting potential, or
Sth = Eth − Em |
(2.49) |
which, using the numerical values stated above to illustrate, would yield (–70) – (–90) = 20 mV. This equation clearly says that the applied stimulating voltage must overcome the difference separating the resting level from the threshold potential and, since it is applied via an internal microelectrode, it has to be a positive pulse.
62 |
Understanding the Human Machine |
Initial depolarizing trigger
Cycle
Hodgkin
More |
|
|
|
|
Less |
|||
depolarization |
|
|
|
|
|
depolarization |
||
|
|
|
|
Depolarization |
|
|
||
|
|
|
|
|
|
|||
|
|
|
|
|
|
|
|
|
Sodium |
|
Delay |
|
|
||||
Permeability |
|
|
||||||
Increases |
|
|
|
|
|
|||
|
|
|
|
|
Sodium |
Potassium |
||
|
|
|
|
|
||||
|
|
|
|
|
Permeability |
Permeability |
||
|
|
|
|
|
||||
Rapid |
|
Decreases |
Increases |
|||||
Sodium |
|
|
|
|
|
|||
Inflow |
|
|
|
|
|
|||
|
|
|
|
|
Less sodium |
Potassium |
||
|
|
|
|
|
||||
|
|
|
|
|
||||
|
|
|
|
|
goes in |
|||
|
|
|
|
|
Outflow |
|||
Increases
Repolarization
Cycle
Repolarization
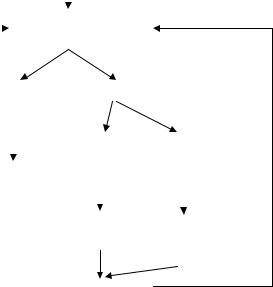
Figure 2.21. HODGKIN ACTIVATION CYCLE. Depolarization produces an increase of the membrane permeability to sodium leading to a positive feedback loop. With some delay, depolarization starts an increase of the permeability to potassium so initiating repolarization. See text.
Thinking exercise: An adequate stimulus was mentioned above, implying some conditions to be met by the stimulating rectangular pulse. Two conditions were already introduced. Search for them in the preceding text. Think of a third, which is easily fulfilled by any electronic stimulator. Hint: Do practical rectangular pulses change instantly from one level to the other?
Study subject: Find out what the strength-duration curve is. From it, the important concepts of utilization time, rheobase, and chronaxy are derived. This experimental relationship was introduced in 1909 by the French physiologist Louis Lapicque (1866 – 1952). Old beautiful theoretical and still valid in many respects descriptions were given by Nicholas Rashevsky in his classic book (Rashevsky, 1960). The student is encouraged to at least take a look at some of them, as for example, Blair’s Theory of Excitation. Notice that these theories were developed long before Hodgkin’s.
Chapter 2. Source: Physiological Systems and Levels |
63 |
So far, we merely described the Hodgkin's group contributions. A
action potential. Let us continue with third postulate of the Ionic Theory is
that depolarization produces an increase in the membrane permeability to sodium ions which, due to the outside-inside concentration gradient, leads to an increase in sodium influx with more positive charges toward the internal face and more depolarization to the membrane (recall that depolarization means either negative charges on the external side or positive on the inside). Beyond the critical level, however, this process is greatly and irreversibly accelerated so that the external stimulating front is no longer needed because more depolarization brings about higher permeability to sodium with a rapid positive ionic entrance and even more depolarization. A positive feedback loop, called Hodgkin activation cycle, is established. It is characterized by a fast inward sodium current (Figure 2.21). However, after a certain delay, the effect of depolarization on sodium permeability slows down and eventually there is a reversal while, simultaneously, the permeability of the membrane to potassium ions increases. Thus, less sodium gets into the cell and a quite rapid and steady potassium outflow takes place, both leading to depolarization slowing down and reversal to repolarization. The latter inactivation cycle is characterized by potassium extrusion.
Ionic membrane permeabilities to both ions are, thus, main actors in the resting state and during excitation. Changes are rapid and dramatic, from a stable potassium to sodium ratio of 50 to 75, depending on the tissue, to a full reversal of sodium to potassium in the order of 20, at the metastable state or overshoot. Permeabilities act, indeed, as true controllers. Associated with these changes, there are ionic currents, a very fast sodium one (depolarization) and a somewhat slower but also fast potassium efflux (repolarization). Ionic exchange has become a tremendously important physiological tool. Now the specialist talks in terms of ionic channels, because the mechanisms involved may be different even for the same ionic species, thus, they refer to, say, potassium channel 1 and potassium channel 2. Whole scientific conferences are being devoted to the subject with participation of electrophysiologists, cell physiologists, molecular biologists, geneticists and, why not, biomedical engineers. They discuss concepts such as channel gating, channel pore, channel associated proteins, assembly of channels, channel regulation, and ionic channel dysfunction associated with disease. The student is encouraged
64 |
Understanding the Human Machine |
to enter the American Physiological Society INTERNET Web Site to search for these and related subjects.
Study subject: There is a famous figure, after Hodgkin and collaborators, reprinted over and over in papers and textbooks, which clearly shows an action potential and both ionic membrane permeabilities, the two latter depicted as electrical conductances. In the figure, the delay is very well seen. Find this figure; inspect units and times taken for each phase (depolarization and repolarization). If you cannot find it, go to the library and check in Hodgkin and Huxley (1952) original paper (p 530, its figure 17). It would be a good exercise at least to glance it over.
A previous experimental finding associated the action potential with a temporal membrane impedance change. It was a superb biomedical engineering accomplishment carried out by Cole and his collaborator, H.J. Curtis, in 1939 in the USA, with the cell membrane forming one arm of an impedance bridge and rather rudimentary but well designed vacuum tube electronics. We urge the student to search also for this frequently reprinted oscilloscope photograph. Hodgkin et al. broke up the impedance change in its two sodium and potassium ionic components.
− Nernst equation
Figure 2.19 displays two horizontal lines marking two levels: one, positive and above the overshoot, called the sodium equilibrium potential, ENa, and another, negative and below the resting level, called the potassium equilibrium potential, EK. They represent theoretical potentials delimiting precisely the band within which the action potential occurs. It never can go beyond such band, neither above nor below it.
We need the concept of Nernst–Gibbs–Donnan passive equilibrium potential. Hermann Walther Nernst (1864–1941) was a Prussian born physicist who developed methods for measuring dielectric constants. He was awarded the Nobel Prize (Chemistry, 1920) for his theoretical work on the Third Law of Thermodynamics. Probably, he is best known to electrochemists for his elucidation of the now called Nernst equation. A simple, illustrative, and perhaps not too rigorous demonstration is given below, encompassing both, electrolytic and solid semiconductor solutions.
Let us assume two compartments, 1 and 2, separated by a semipermeable interface, holding respectively concentrations C1 and C2 of electrically charged carriers, which can be either ions in electrolytic solutions or electrons/holes in doped semiconductor materials (such as silicium or
germanium or the like). If C1 > C2, a diffusion current, iDIF, appears, proportional to the concentration gradient,

Chapter 2. Source: Physiological Systems and Levels |
65 |
iDIF = q ×D(∂C / ∂x) |
(2.50) |
where q stands for the electric charge of the carrier, D represents the diffusion constant, and (∂C/∂x) is the concentration gradient in the x- direction. In fact, the gradient should be written in space, but to simplify the mathematics we will stick to the one-variable case. It does not affect the concept.
The diffusion current across the semipermeable interface (a membrane in electrolytic solutions or the pn-junction in semiconductors) creates an electric field because of accumulation and depletion of charges on the sides, thus producing an opposing current called a drift current, iDRI, or
iDRI = q ×µ×D(∂U / ∂x) |
(2.51) |
where q is the carrier charge in coulombs, as above, µ represents the mobility of the carriers, C stands for the concentration of carriers, and (∂U/∂x) is the linear electrical potential gradient created by the diffusion current. The same comment as above is valid, i.e., a three dimensional gradient should be considered. Carrier mobility is defined as the velocity per unit of electric field, obviously measured in [m/s]/[V/m]. Ions have a much lower mobility than holes and electrons. Observe that the charge transfer across the interface breaks neutrality on each side.
Both currents (diffusion and drift) soon reach an equilibrium when they become equal, fully opposed, and thus stopping any further exchange, iDIF = iDRI, or after equating eqs. (2.50 and 2.51),
D(∂C ∂x)= µC(∂U ∂x) |
(2.52) |
which, after separation of variables and integration between C1 and C2, on one side of the equation, and between U1 and U2, on the other, yields,
∆U =U2 −U1 = (D / µ)ln(C2 / C1) |
(2.53) |
Eq. (2.53) describes the equilibrium difference of potential, passively developed, when two compartments containing different concentrations of electrically charged carriers are separated by a semipermeable interface. This is already an expression of Nernst equation for electrolytic solutions or the junction potential well-known in semiconductor pn- junctions. Such potential difference, however, is unable to sustain a current and, thus, cannot act as a generator.
The student is encouraged to make a little drawing showing both compartments, their respective concentrations, marking the above-mentioned currents and the resulting voltage

66 |
Understanding the Human Machine |
difference. Review carefully the rationale so far developed trying to understand its essentials.
Now, Nernst equation will be rewritten in two other mathematical forms using well-known relations in physics. One of Einstein’s equations relates several physical constants and parameters,
D µ = kT q |
(2.54) |
where k = Boltzmann constant, T = absolute temperature, and q = carrier charge. Besides, Boltzmann constant is also given by R/N, with R = constant of gases, and N = Avogadro's number. The student should search for the numerical values and units associated with these classical parameters. After replacement of the above in eq. (2.53), it obtains,
∆U = (RT / Nq)ln(C2 / C1)= (RT / FZ )ln(C2 / C1) |
(2.55) |
where Nq = FZ represents the electric charge of one mole of substance, with F being Faraday's constant and Z standing for the chemical valence of the element species. Eq. (2.55) is the form usually found in electrochemistry books. When the ion species is monovalent, Z = 1, further simplifying the right hand expression. However, semiconductor physicists prefer to write the equation as
∆U = kT / q ×ln(C2 / C1) |
(2.56a) |
||
easily rewritten as |
|
||
C |
2 |
= C e[q∆U / kT ] |
(2.56b) |
|
1 |
|
|
The latter equation obviously is nothing but the well-known relationship between hole and electron concentrations in a pn-slab. An interesting consequence of eq. (2.55) is that the product of the concentrations on one side equals the product on the other side, leading to the so-called Donnan equilibrium (Donnan, 1925-6).
When Nernst eq. (2.55) is applied to potassium and sodium concentrations, respectively, found in the ECF and ICF compartments separated by the semipermeable and excitable biological membrane, the following typical values can be calculated.
EK + = RT / F ×ln[Ko ]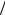 [Ki ]= 61.5log10 31 = −93mV
[Ki ]= 61.5log10 31 = −93mV
ENa+ = RT / F ×ln [Nao ]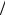 [Nai ]= 61.5log10 (1/ 24)=84 mV
[Nai ]= 61.5log10 (1/ 24)=84 mV
The latter are, respectively, the potassium and sodium equilibrium Nernst–Gibbs–Donnan potentials, if the system were a passive one dominated either by the first or by the second ion. Hence, they are only
Chapter 2. Source: Physiological Systems and Levels |
67 |
theoretical potentials impossible to coexist. Obviously, they are different and of different sign (Figure 2.19). In the resting state, the membrane potential, Em, however, is rather close to the potassium potential, EK, say, –90 mV versus –92 mV, or thereabouts, depending on the specific tissue. Thus, it is said that the resting membrane potential is dominated by potassium. On the other hand, the sodium potential is way up (about +84 mV). The positive overshoot (in the order of +20 mV) tends to the sodium potential but it falls rather short of reaching it. As stated at the beginning of this section, both theoretical equilibrium potentials mark the lower and upper limits of the action potential operating band.
The student should check the numerical values of the equilibrium potentials applying concentrations quoted in the textbooks for nerve and muscle tissues. The temperature is, by and large, body temperature, but other physiological values are valid as well (as the case may be in amphibians or in vitro preparations).
Thinking exercise. Many experiments have been performed and reported modifying sodium and potassium concentrations and measuring the possible influence on the resting potential and on the overall amplitude of the action potential. With the preceding information, the student should be able to answer the following questions:
If the concentration of potassium ions were increased in the ECF, (a) would Em change? (b) if the answer is yes, in what direction? (c) Is the amplitude of the action potential affected?
If the concentration of potassium were decreased in the ECF, (a) would the resting membrane potential change? (b) Would the stimulus threshold be different? (c) If the answer is yes, would a larger or smaller stimulus be needed?
If the concentration of sodium were decreased in the ECF, (a) would the resting potential be affected? (b) Would the action potential overshoot change? (c) If the answer is yes, would it be smaller or larger?
Draw an equivalent electric circuit modeling the membrane in the resting and in the maximum activity conditions. Permeabilities to sodium and potassium are represented by variable resistors and the equilibrium potentials by ... (complete the text). This circuit can be constructed and tested.
When the stimulus amplitude does not reach the firing level the action potential (is)(is not) triggered and the response, if any, is called subliminal. Select one of the answers between parentheses.
Demonstrate the Donnan equilibrium product in a two compartment NaCl system separated by a semipermeable membrane. Hint: equate the electrochemical potentials.
From the above, it is clearly seen that Nernst equation cannot describe neither the resting nor the maximum activity membrane potential. A modified empirical version was proposed by Hodgkin’s group, called the
