
- •Preface
- •Contents
- •1 Extracellular and Intracellular Signaling – a New Approach to Diseases and Treatments
- •1.1 Introduction
- •1.1.1 Linear Model of Drug Receptor Interactions
- •1.1.2 Matrix Model of Drug Receptor Interactions
- •1.2 Experimental Approaches to Disease Treatment
- •1.3 Adipokines and Disease Causation
- •1.4 Questions in Disease Treatment
- •1.5 Toxic Lifestyles and Disease Treatment
- •References
- •2.1 Introduction
- •2.2 Heterogeneity of Adipose Tissue Composition in Relation to Adipokine and Cytokine Secretion
- •2.3 Feedback between FA and the Adipocyte
- •2.6 Metabolic Programming of Autocrine Signaling in Adipose Tissue
- •2.8 Cell Heterogeneity in the Pancreatic Islet
- •2.16 Concluding Remarks
- •Acknowledgements
- •References
- •3 One Receptor for Multiple Pathways: Focus on Leptin Signaling
- •3.1 Leptin
- •3.2 Leptin Receptors
- •3.3 Leptin Receptor Signaling
- •3.3.4 AMPK
- •3.3.5 SOCS3
- •3.4 Leptin Receptor Interactions
- •3.4.1 Apolipoprotein D
- •3.4.2 Sorting Nexin Molecules
- •3.4.3 Diacylglycerol Kinase Zeta
- •3.4.4 Apolipoprotein J
- •References
- •4.1 Introduction
- •4.2 Leptin: A Brief Introduction
- •4.3 Expression of Leptin Receptors in Cardiovascular Tissues
- •4.6 Post Receptor Leptin Signaling
- •4.6.2 Mitogen Activated Protein Kinase Stimulation
- •4.7 Adiponectin
- •4.7.1 Adiponectin and Cardiovascular Disease
- •4.7.2 Adiponectin and Experimental Cardiac Hypertrophy
- •4.8 Resistin
- •4.8.1 Cardiac Actions of Resistin
- •4.8.1.1 Experimental Studies on the Cardiac Actions of Resistin
- •4.9 Apelin
- •4.9.1 Apelin and Heart Disease
- •4.10 Visfatin
- •4.11 Other Novel Adipokines
- •4.12 Summary, Conclusions and Future Directions
- •Acknowledgements
- •References
- •5 Regulation of Muscle Proteostasis via Extramuscular Signals
- •5.1 Basic Protein Synthesis
- •5.2.1 Hormones
- •5.2.1.1 Mechanisms of Action: Glucocorticoids
- •5.2.1.2 Mechanisms of Action: TH (T3)
- •5.2.1.3 Mechanisms of Action: Testosterone
- •5.2.1.4 Mechanisms of Action: Epinephrine
- •5.2.2 Local Factors (Autocrine/Paracrine)
- •5.2.2.1 Mechanisms of Action: Insulin/IGF Spliceoforms
- •5.2.2.2 Mechanisms of Action: Fibroblast Growth Factor (FGF)
- •5.2.2.3 Mechanisms of Action: Myostatin
- •5.2.2.4 Mechanisms of Action: Cytokines
- •5.2.2.5 Mechanisms of Action: Neurotrophins
- •5.2.2.7 Mechanisms of Action: Extracellular Matrix
- •5.2.2.8 Mechanisms of Action: Amino Acids (AA)
- •5.3 Regulation of Muscle Proteostasis in Humans
- •5.3.1 Nutrients as Regulators of Muscle Proteostasis in Man
- •5.3.2 Muscular Activity (i.e. Exercise) as a Regulator of Muscle Proteostasis
- •5.4 Conditions Associated with Alterations in Muscle Proteostasis in Humans
- •5.4.2 Disuse Atrophy
- •5.4.3 Sepsis
- •5.4.4 Burns
- •5.4.5 Cancer Cachexia
- •References
- •6 Contact Normalization: Mechanisms and Pathways to Biomarkers and Chemotherapeutic Targets
- •6.1 Introduction
- •6.2 Contact Normalization
- •6.3 Cadherins
- •6.4 Gap Junctions
- •6.5 Contact Normalization and Tumor Suppressors
- •6.6 Contact Normalization and Tumor Promoters
- •6.7 Conclusions
- •References
- •7.1 Introduction
- •7.2 Background on Migraine Headache
- •7.3 Migraine and Neuropathic Pain
- •7.4 Role of Astrocytes in Pain
- •7.5 Adipokines and Related Extracellular Signalling
- •7.6 The Future of Signaling Research to Migraine
- •Acknowledgements
- •References
- •8.1 Alzheimer’s Disease
- •8.1.2 Target for AD Therapy
- •8.2 AD and Metabolic Dysfunction
- •8.2.1 Impaired Glucose Metabolism
- •8.2.2 Lipid Disorders
- •8.2.3 Obesity
- •8.3 Adipokines
- •8.3.1 Leptin
- •8.3.2 Adiponectin
- •8.3.3 Resistin
- •8.3.4 Visfatin
- •8.3.5 Plasminogen Activator Inhibitor
- •8.3.6 Interleukin-6
- •8.4 Conclusions
- •References
- •9.1 Introduction
- •9.1.1 Structure and Function of Astrocytes
- •9.1.1.1 Morphology
- •9.1.1.2 Astrocyte Functions
- •9.1.2 Responses of Astrocytes to Injury
- •9.1.2.1 Reactive Astrocytosis
- •9.1.2.2 Cell Swelling
- •9.1.2.3 Alzheimer Type II Astrocytosis
- •9.2 Intracellular Signaling System in Reactive Astrocytes
- •9.2.1 Oxidative/Nitrosative Stress (ONS)
- •9.2.2 Protein Kinase C (PKC)
- •9.2.5 Signal Transducer and Activator of Transcription 3 (STAT3)
- •9.3 Signaling Systems in Astrocyte Swelling
- •9.3.1 Oxidative/Nitrosative Stress (ONS)
- •9.3.2 Cytokines
- •9.3.3 Protein Kinase C (PKC)
- •9.3.5 Protein Kinase G (PKG)
- •9.3.7 Signal Transducer and Activator of Transcription 3 (STAT3)
- •9.3.10 Ion Channels/Transporters/Exchangers
- •9.4 Conclusions and Perspectives
- •Acknowledgements
- •References
- •10.1 Adipokines, Toxic Lipids and the Aging Brain
- •10.1.1 Toxic Lifestyles, Adipokines and Toxic Lipids
- •10.1.2 Ceramide Toxicity in the Brain
- •10.3 Oxygen Radicals, Hydrogen Peroxide and Cell Death
- •10.4 Gene Transcription and DNA Damage
- •10.5 Conclusions
- •References
- •11.1 Introduction
- •11.2 Cellular Signaling
- •11.2.1 Types of Signaling
- •11.2.2 Membrane Proteins in Signaling
- •11.3 G Protein-Coupled Receptors
- •11.3.1 Structure of GPCRs
- •11.3.1.1 Structure Determination
- •11.3.1.2 Structural Diversity of Current GPCR Structures
- •11.3.1.3 Prediction of GPCR Structure and Ligand Binding
- •11.3.2 GPCR Activation: Conformation Driven Functional Selectivity
- •11.3.2.2 Ligand or Mutation Stabilized Ensemble of GPCR Conformations
- •11.3.2.4 GPCR Dimers and Interaction with Other Proteins
- •11.3.3 Functional Control of GPCRs by Ligands
- •11.3.3.1 Biased Agonism
- •11.3.3.2 Allosteric Ligands and Signal Modulation
- •11.3.4 Challenges in GPCR Targeted Drug Design
- •11.4 Summary and Looking Ahead
- •Acknowledgements
- •References
- •12.1 Introduction
- •12.5.1 Anthocyanins
- •12.5.2 Gallates
- •12.5.3 Quercetin
- •12.5.5 Piperine
- •12.5.6 Gingerol
- •12.5.7 Curcumin
- •12.5.8 Guggulsterone
- •12.6.1 Phytanic Acid
- •12.6.2 Dehydroabietic Acid
- •12.6.3 Geraniol
- •12.7 Agonists of LXR that Reciprocally Inhibit NF-jB
- •12.7.1 Stigmasterol
- •12.7.3 Ergosterol
- •12.8 Conclusion
- •References
- •13.1 Introduction
- •13.2 Selective Dopaminergic Neuronal Death
- •13.3 Signaling Pathways Involved in Selective Dopaminergic Neuronal Death
- •13.3.1 Initiators and Signaling Molecules
- •13.3.1.1 Response to Oxidative and Nitrosative Stress
- •13.3.1.2 Response to Altered Proteostasis
- •13.3.1.3 Response to Glutamate
- •13.3.1.4 Other Initiators
- •13.3.2 Signal Transducers, Intracellular Messengers and Upstream Elements
- •13.3.2.2 Small GTPases
- •13.3.3 Intracellular Signaling Cascades
- •13.3.3.1 Mitogen Activated Protein Kinases (MAPK) Pathway
- •13.3.3.2 PI3K/Akt Pathway
- •13.3.3.4 Unfolded Protein Response (UPR)
- •13.3.4 Potentially Involved Intracellular Signaling Components
- •13.3.4.3 PINK1
- •13.3.5.2 Dopamine Metabolism
- •13.3.5.3 Cell Cycle
- •13.3.5.4 Autophagy
- •13.3.5.5 Apoptosis
- •13.4 Conclusions
- •References
- •Subject Index
136 |
Chapter 8 |
support the use of statins, fibrates and other lipid-lowering agents to diminish the risk of AD.59 In the brain, cholesterol concentrates in lipid rafts where it a ects the activity of several enzymes.60 In fact the activity of b- and g- secretases and of a-secretase are respectively positively and negatively modulated by high cholesterol concentrations.61–63 The opposite, i.e. a decrease of Ab formation, occurs when cholesterol levels are lowered.64 Hence high cholesterol seems to positively correlate to Ab synthesis in a direct way. However, evidence also exists to support a more indirect link between cholesterol homeostasis and Ab production. More precisely, inhibition of acyl CoA cholesterol transferase (ACAT), responsible for cholesterol esterification, reduces Ab production in cultured cells65 and amyloid pathology in Alzheimer transgenic mice.66 Moreover, the ATP-binding cassette (ABC) transporter A1, which in the CNS prevents cholesterol accumulation by facilitating its flux toward the periphery, is known to have a role in reducing Ab accumulation in the brain.67 Reduction or knockout of ABCA1 results in increased Ab plaque load,68–70 whereas ABCA1 over-expression increases amyloid accumulation in AD transgenic mice.71
Dyslipidemia accompanies hyperinsulinemia and insulin resistance. Under these conditions, in fact, free fatty acids levels rise, causing an elevation of very low density lipoproteins (VLDL), which results in postprandial hyperlipidemia. Increased VLDL has been associated with increased Ab deposition in the brain72 and with elevated risk of AD.73
8.2.3Obesity
One of the main causes of insulin resistance is obesity, with over 80% of obese people being insulin resistant.74 Combination of these two conditions is responsible for persistent free fatty acid (FFA) elevation. FFAs inhibit IDE,
the metalloprotease that participates not only in insulin signaling but also in Ab clearance.75 FFAs favor also aggregation of Ab and tau in vitro.76,77 A strong
correlation has been demonstrated between obesity and AD risk. More specifically, midlife obesity is considered a risk factor for dementia,57,78,79 whereas
later in life a low80 and high body mass index have been related to higher and lower AD risk, respectively.81 To explain this apparent paradox, weight loss often precedes dementia onset82 and may precede diagnosis by more than ten years.83 Interestingly, distribution of adiposity seems to play a role in the risk of dementia as already reported for cardiovascular diseases and diabetes.84 In particular, central distribution of fat, known as visceral adiposity, can be especially dangerous, even for patients who are not overweight, increasing the risk of dementia.85 This suggests that there might be something intrinsic to the condition of central adiposity that increases this risk.
8.3 Adipokines
Emerging evidence is accumulating on the relevance of adipokines, biologically active substances released by adipose tissue, that make this tissue actively
Adipokines and Alzheimer’s Disease |
137 |
participate in metabolic functions. Adipokines can be synthesized by fat as well as non-fat cells of the adipose tissue but they can also be synthesized at di erent sites and exert independent actions. Many of them exert proinflammatory e ects, including leptin, tumor necrosis factor-a (TNFa), interleukin-6 (IL-6) angiotensinogen, resistin, plasminogen activator inhibitor (PAI), whereas a few others exert mainly anti-inflammatory activity. Among the latter are adiponectin and transforming growth factor b (TGFb). Most of the adipokines identified to date have high circulating plasma levels in obese subjects. Some of them reflect the mild inflammatory condition that characterizes obesity in its initial phases, others have more inflammatory features and their origin is debated.86 Additional adipokines may be endowed with anti-inflammatory e ect and be at higher circulating levels in obesity because of a compensatory response to inflammatory mediators. IL-10 as well as IL-6 may be considered in this context.87,88 As a whole, adipokines are considered fundamental regulators of carbohydrate and lipid metabolism. This gains importance in terms of deposition and mobilization of fatty acids and regulation of obesity-related comorbidities.89 However, the e ect of adipokines is not restricted to the periphery since some of them can cross the BBB or modulate the function of endothelial cells.90
8.3.1Leptin
The more well known and characterized adipokine, leptin, which has allowed the identification of this large family of proteins all related to lipid metabolism, was initially recognized because of its anorexigenic and appetite suppressive e ects. Both e ects are exerted within the brain. Since then, interest in the e ects of adipokines in the brain has increased and knowledge of this specific issue has broadened. Leptin is a 16-kDa peptide that exerts its biological actions by binding to Lep-Rb (ObR, a–f), all members of the IL-6 receptor family of the class I cytokine receptor superfamily, and divided into long, short and soluble forms.91 Six di erent isoforms of the ObR have been described. The long form ObRb seems to mediate most of the actions of leptin. Short forms of the receptor such as ObRa and ObRc are abundantly expressed in cerebral vessels of the BBB suggesting a role for these short forms of receptor in the transport of leptin from the periphery into the brain. Interestingly, leptin binds also megalin/LRP2, a multi-ligand receptor
expressed in choroid plexus epithelial cells that is known to participate also in Ab clearance from the brain to the blood.92,93 Transport across the BBB
seems to be crucial although leptin is also known to be produced in very low amounts within the brain. ObRb is devoid of autophosphorylation and signals mainly through JAK2 responsible for phosphorylation of STAT, above all STAT3, that dimerizes and translocates to the nucleus to control the expression of a series of genes. Alternatively JAK2 activates the SH2/SH3 domain-containing adaptor protein growth factor receptor-bound protein 2 (GRB2), responsible for downstream involvement of the mitogen-activated

138 |
Chapter 8 |
kinase (MAPK) pathway. The latter is the only signaling cascade activated by the short forms of ObR.94
Leptin via its central actions does not control exclusively food intake, but recognizes receptors also in the hippocampus where it controls cognitive processes. It in fact facilitates learning and memory events, increases long-term potentiation (LTP)95 and enhances synaptic density.96 Accordingly, impairment of LTP is reported in db/db mice, which carry a leptin receptor mutation and do not express functional leptin receptors,97 whereas leptin treatment increases hippocampal concentrations of synaptic proteins including synapsin 2A and synaptophysin.98 Leptin is also endowed with neuroprotective e ects as shown by decreased neuronal death following either serum or neurotrophic factor deprivation.99 In addition, leptin has been proven to counteract the loss of dopaminergic neurons observed in experimental models of Parkinson’s disease, to be neuroprotective against ischemic stroke and to exert anticonvulsant properties in most seizure models.100
Among various adipokines, leptin is the one that has been more convincingly related to AD. In fact, observational studies have demonstrated an inverse relationship between blood leptin levels on one side and human cognitive impairment and AD development on the other side.101 Conversely, high peripheral leptin concentrations correlate to a low incidence of dementia and AD.102 Leptin can a ect AD pathogenesis by acting at multiple sites (Figure 8.1). Evidence exists in fact to suggest that it a ects not only Ab but also NFT formation. In particular, due to its lypolitic e ect, leptin modifies lipid raft composition and a ects indirectly BACE activity, thus decreasing Ab formation.103 It increases also Ab clearance by facilitating ApoE-dependent Ab uptake through LRP1.103 These in vitro data find support in animal studies
reporting the ability of leptin to reduce Ab load in a transgenic model of AD103,104 and to improve cognitive performance in AD mice.105 The e ects of
leptin are not limited to modulation of Ab synthesis and degradation as leptin decreases also tau phosphorylation in neurons,106 through modulation
Aβ
leptin 

ObRb
MAPK JAK2/STAT3
 BACE =
BACE =  Ab synthesis
Ab synthesis
 LRP1 =
LRP1 =  Ab clearance
Ab clearance
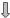 AMPK =
AMPK =  ptau
ptau
Figure 8.1 Mechanisms involving leptin in the pathogenesis of AD.
Adipokines and Alzheimer’s Disease |
139 |
of AMP-activated protein kinase (AMPK)107 and ensuing inactivation of GSK-3b,108 which is known to be involved with tau hyperphosphorylation and neurofibrillary tangle formation. Of note, AMPK seems to mediate not only the e ect of leptin on tau phosphorylation, but also the reducing action of leptin on Ab production. Moreover, the e ects of leptin, through AMPK activation, involve two independent downstream signaling pathways, with peroxisome proliferator-activated receptor-g (PPAR-g) a ecting Ab formation through BACE and GSK3b modifying tau phosphorylation.107 All these experimental data have identified leptin as a novel potential therapeutic tool in AD104 and have also indicated leptin-induced intracellular signals as a potential target in the optic of novel treatments of AD.
8.3.2Adiponectin
Adiponectin is a 224 amino acid protein whose main function is decreasing glucose synthesis in the liver and enhancement of fatty acid oxidation, thus exerting in anti-atherogenic, anti-diabetic and insulin-sensitizing properties.109 Accordingly, decreased serum concentrations of adiponectin correlate with obesity, type 2 diabetes and insulin resistance. Adiponectin binds two di erent receptors, ADIPOR1 and ADIPOR2. Stimulation of both receptors leads to subsequent activation of AMPK, PPAR-a and PPAR-g. An action of adiponectin at the CNS has not been clearly demonstrated as it does not cross the BBB. However, an upregulation of adiponectin receptors has been observed in brain endothelium during fasting.110 Thus adiponectin seems to exert only indirect e ects in the CNS, such as reduced release of IL-6 observed in brain endothelial cells following treatment with adiponectin.111 To date, the ability of adiponectin to modify IL-6 (or other proinflammatory cytokines) in the brain represents the only link between this adipokine and AD. However, the possibility that adiponectin plays an indirect role by reducing insulin resistance, diabetes, obesity, all conditions considered main risk factors for AD, has also to be taken into account.
8.3.3Resistin
Resistin is a member of the resistin-like molecule (RELM) family of cysteinerich proteins, which appears to increase in obesity, type 2 diabetes and insulin resistance, although some controversies regarding its role in humans still exist.112 It is clear that resistin is upregulated in inflammatory processes and a reciprocal control exists between resistin, TNFa and IL-6. Thus, resistin has been suggested to be involved in several inflammatory conditions including inflammatory bowel disease, coronary artery disease and rheumatoid arthritis.112 Resistin is present in the brain and its expression increases under conditions of hypoxia/ischemia, traumatic brain injury or cerebral LPS injection.113 Although leptin and resistin synergize to control glucose homeostasis in diabetes, they a ect STAT3 and AMPK in an opposite way. Thus, resistin counteracts central leptin action and nullifies its signaling.114
