
- •Preface
- •Contents
- •1 Extracellular and Intracellular Signaling – a New Approach to Diseases and Treatments
- •1.1 Introduction
- •1.1.1 Linear Model of Drug Receptor Interactions
- •1.1.2 Matrix Model of Drug Receptor Interactions
- •1.2 Experimental Approaches to Disease Treatment
- •1.3 Adipokines and Disease Causation
- •1.4 Questions in Disease Treatment
- •1.5 Toxic Lifestyles and Disease Treatment
- •References
- •2.1 Introduction
- •2.2 Heterogeneity of Adipose Tissue Composition in Relation to Adipokine and Cytokine Secretion
- •2.3 Feedback between FA and the Adipocyte
- •2.6 Metabolic Programming of Autocrine Signaling in Adipose Tissue
- •2.8 Cell Heterogeneity in the Pancreatic Islet
- •2.16 Concluding Remarks
- •Acknowledgements
- •References
- •3 One Receptor for Multiple Pathways: Focus on Leptin Signaling
- •3.1 Leptin
- •3.2 Leptin Receptors
- •3.3 Leptin Receptor Signaling
- •3.3.4 AMPK
- •3.3.5 SOCS3
- •3.4 Leptin Receptor Interactions
- •3.4.1 Apolipoprotein D
- •3.4.2 Sorting Nexin Molecules
- •3.4.3 Diacylglycerol Kinase Zeta
- •3.4.4 Apolipoprotein J
- •References
- •4.1 Introduction
- •4.2 Leptin: A Brief Introduction
- •4.3 Expression of Leptin Receptors in Cardiovascular Tissues
- •4.6 Post Receptor Leptin Signaling
- •4.6.2 Mitogen Activated Protein Kinase Stimulation
- •4.7 Adiponectin
- •4.7.1 Adiponectin and Cardiovascular Disease
- •4.7.2 Adiponectin and Experimental Cardiac Hypertrophy
- •4.8 Resistin
- •4.8.1 Cardiac Actions of Resistin
- •4.8.1.1 Experimental Studies on the Cardiac Actions of Resistin
- •4.9 Apelin
- •4.9.1 Apelin and Heart Disease
- •4.10 Visfatin
- •4.11 Other Novel Adipokines
- •4.12 Summary, Conclusions and Future Directions
- •Acknowledgements
- •References
- •5 Regulation of Muscle Proteostasis via Extramuscular Signals
- •5.1 Basic Protein Synthesis
- •5.2.1 Hormones
- •5.2.1.1 Mechanisms of Action: Glucocorticoids
- •5.2.1.2 Mechanisms of Action: TH (T3)
- •5.2.1.3 Mechanisms of Action: Testosterone
- •5.2.1.4 Mechanisms of Action: Epinephrine
- •5.2.2 Local Factors (Autocrine/Paracrine)
- •5.2.2.1 Mechanisms of Action: Insulin/IGF Spliceoforms
- •5.2.2.2 Mechanisms of Action: Fibroblast Growth Factor (FGF)
- •5.2.2.3 Mechanisms of Action: Myostatin
- •5.2.2.4 Mechanisms of Action: Cytokines
- •5.2.2.5 Mechanisms of Action: Neurotrophins
- •5.2.2.7 Mechanisms of Action: Extracellular Matrix
- •5.2.2.8 Mechanisms of Action: Amino Acids (AA)
- •5.3 Regulation of Muscle Proteostasis in Humans
- •5.3.1 Nutrients as Regulators of Muscle Proteostasis in Man
- •5.3.2 Muscular Activity (i.e. Exercise) as a Regulator of Muscle Proteostasis
- •5.4 Conditions Associated with Alterations in Muscle Proteostasis in Humans
- •5.4.2 Disuse Atrophy
- •5.4.3 Sepsis
- •5.4.4 Burns
- •5.4.5 Cancer Cachexia
- •References
- •6 Contact Normalization: Mechanisms and Pathways to Biomarkers and Chemotherapeutic Targets
- •6.1 Introduction
- •6.2 Contact Normalization
- •6.3 Cadherins
- •6.4 Gap Junctions
- •6.5 Contact Normalization and Tumor Suppressors
- •6.6 Contact Normalization and Tumor Promoters
- •6.7 Conclusions
- •References
- •7.1 Introduction
- •7.2 Background on Migraine Headache
- •7.3 Migraine and Neuropathic Pain
- •7.4 Role of Astrocytes in Pain
- •7.5 Adipokines and Related Extracellular Signalling
- •7.6 The Future of Signaling Research to Migraine
- •Acknowledgements
- •References
- •8.1 Alzheimer’s Disease
- •8.1.2 Target for AD Therapy
- •8.2 AD and Metabolic Dysfunction
- •8.2.1 Impaired Glucose Metabolism
- •8.2.2 Lipid Disorders
- •8.2.3 Obesity
- •8.3 Adipokines
- •8.3.1 Leptin
- •8.3.2 Adiponectin
- •8.3.3 Resistin
- •8.3.4 Visfatin
- •8.3.5 Plasminogen Activator Inhibitor
- •8.3.6 Interleukin-6
- •8.4 Conclusions
- •References
- •9.1 Introduction
- •9.1.1 Structure and Function of Astrocytes
- •9.1.1.1 Morphology
- •9.1.1.2 Astrocyte Functions
- •9.1.2 Responses of Astrocytes to Injury
- •9.1.2.1 Reactive Astrocytosis
- •9.1.2.2 Cell Swelling
- •9.1.2.3 Alzheimer Type II Astrocytosis
- •9.2 Intracellular Signaling System in Reactive Astrocytes
- •9.2.1 Oxidative/Nitrosative Stress (ONS)
- •9.2.2 Protein Kinase C (PKC)
- •9.2.5 Signal Transducer and Activator of Transcription 3 (STAT3)
- •9.3 Signaling Systems in Astrocyte Swelling
- •9.3.1 Oxidative/Nitrosative Stress (ONS)
- •9.3.2 Cytokines
- •9.3.3 Protein Kinase C (PKC)
- •9.3.5 Protein Kinase G (PKG)
- •9.3.7 Signal Transducer and Activator of Transcription 3 (STAT3)
- •9.3.10 Ion Channels/Transporters/Exchangers
- •9.4 Conclusions and Perspectives
- •Acknowledgements
- •References
- •10.1 Adipokines, Toxic Lipids and the Aging Brain
- •10.1.1 Toxic Lifestyles, Adipokines and Toxic Lipids
- •10.1.2 Ceramide Toxicity in the Brain
- •10.3 Oxygen Radicals, Hydrogen Peroxide and Cell Death
- •10.4 Gene Transcription and DNA Damage
- •10.5 Conclusions
- •References
- •11.1 Introduction
- •11.2 Cellular Signaling
- •11.2.1 Types of Signaling
- •11.2.2 Membrane Proteins in Signaling
- •11.3 G Protein-Coupled Receptors
- •11.3.1 Structure of GPCRs
- •11.3.1.1 Structure Determination
- •11.3.1.2 Structural Diversity of Current GPCR Structures
- •11.3.1.3 Prediction of GPCR Structure and Ligand Binding
- •11.3.2 GPCR Activation: Conformation Driven Functional Selectivity
- •11.3.2.2 Ligand or Mutation Stabilized Ensemble of GPCR Conformations
- •11.3.2.4 GPCR Dimers and Interaction with Other Proteins
- •11.3.3 Functional Control of GPCRs by Ligands
- •11.3.3.1 Biased Agonism
- •11.3.3.2 Allosteric Ligands and Signal Modulation
- •11.3.4 Challenges in GPCR Targeted Drug Design
- •11.4 Summary and Looking Ahead
- •Acknowledgements
- •References
- •12.1 Introduction
- •12.5.1 Anthocyanins
- •12.5.2 Gallates
- •12.5.3 Quercetin
- •12.5.5 Piperine
- •12.5.6 Gingerol
- •12.5.7 Curcumin
- •12.5.8 Guggulsterone
- •12.6.1 Phytanic Acid
- •12.6.2 Dehydroabietic Acid
- •12.6.3 Geraniol
- •12.7 Agonists of LXR that Reciprocally Inhibit NF-jB
- •12.7.1 Stigmasterol
- •12.7.3 Ergosterol
- •12.8 Conclusion
- •References
- •13.1 Introduction
- •13.2 Selective Dopaminergic Neuronal Death
- •13.3 Signaling Pathways Involved in Selective Dopaminergic Neuronal Death
- •13.3.1 Initiators and Signaling Molecules
- •13.3.1.1 Response to Oxidative and Nitrosative Stress
- •13.3.1.2 Response to Altered Proteostasis
- •13.3.1.3 Response to Glutamate
- •13.3.1.4 Other Initiators
- •13.3.2 Signal Transducers, Intracellular Messengers and Upstream Elements
- •13.3.2.2 Small GTPases
- •13.3.3 Intracellular Signaling Cascades
- •13.3.3.1 Mitogen Activated Protein Kinases (MAPK) Pathway
- •13.3.3.2 PI3K/Akt Pathway
- •13.3.3.4 Unfolded Protein Response (UPR)
- •13.3.4 Potentially Involved Intracellular Signaling Components
- •13.3.4.3 PINK1
- •13.3.5.2 Dopamine Metabolism
- •13.3.5.3 Cell Cycle
- •13.3.5.4 Autophagy
- •13.3.5.5 Apoptosis
- •13.4 Conclusions
- •References
- •Subject Index

CHAPTER 4
Cell Signaling Mechanisms
Underlying the Cardiac Actions
of Adipokines
MORRIS KARMAZYN* AND VENKATESH
RAJAPUROHITAM
Department of Physiology and Pharmacology, University of Western Ontario, Schulich School of Medicine and Dentistry, London, Ontario, Canada N6A 5C1
4.1 Introduction
The identification of adipokines as potent bioactive compounds has made a major impact on the area of endocrinology and physiology as it is now generally recognized that adipocytes represent endocrine organs secreting potent biologically active molecules producing a wide array of responses on di erent target tissues.1 The present review centers primarily on adipokines that have been shown to modify cardiac function and that appear to play potentially important roles in pathology. Attention is particularly given to leptin and adiponectin since these compounds have been extensively studied, at least relative to other adipokines, in terms of their cardiac e ects. Some of the latter such as resistin, apelin and visfatin are also discussed in this review, although their cardiac e ects have been studied to a substantially lesser degree than either leptin or adiponectin.
RSC Drug Discovery Series No. 10 Extracellular and Intracellular Signaling
Edited by James D. Adams, Jr. and Keith K. Parker r Royal Society of Chemistry 2011
Published by the Royal Society of Chemistry, www.rsc.org
57
58 |
Chapter 4 |
4.2 Leptin: A Brief Introduction
As noted above, among the adipokines primary attention has been directed towards leptin, a 16-kDa protein secreted primarily by adipocytes but also produced by many tissues including the heart.2–4 The production of cardiomyocyte-derived leptin is increased by both endothelin-1 and angiotensin II suggesting a paracrine or autocrine role of leptin in the regulation of cardiac functions, particularly under pathological conditions.3 Indeed, leptin mediates the prohypertrophic e ect of both endothelin-1 and angiotensin II in cultured neonatal rat ventricular myocytes.3 The primary cardiac response to leptin in terms of physiological function appears to be a negative inotropic response, which has been shown primarily in cardiomyocytes and which is mediated by endogenously produced nitric oxide.4
Circulating total leptin levels are generally positively related with body mass index and the degree of adiposity with plasma levels ranging from 5 to 15 ng/ml in non-obese individuals and greater than 100 ng/ml in very obese subjects.5 Interestingly, circulating leptin exists primarily in the free form in obesity whereas in lean individuals leptin circulates primarily bound to plasma proteins.5 This di erence may be of biological importance since it suggests that, in obesity, substantially greater amounts of leptin are available to exert biological e ects. The e ects of leptin occur through leptin binding to its receptors, termed OBR, LEPR or LR, although the OBR designation will be used in this review for consistency. OBRs are expressed as splice variants classified as short (OBRa, c, d and f), secreted (OBRe) and long (OBRb) forms with OBRb generally considered as the primary functional isoform linked to full cell signaling processes.6 These receptors are expressed abundantly in many di erent cells including cardiomyocytes and intact myocardium.2,3 The intracellular domain of OBRb belongs to the Janus kinase signal transduction and translation system (Jak2/STAT3). As will be discussed below, it has been reported that leptin leads to the activation of various kinases in cardiomyocytes including RhoA/ROCK, ERK1/2, p38 MAPK, phosphoinositide 3-kinase (PI 3-kinase), Akt and protein kinase C.
4.3Expression of Leptin Receptors in Cardiovascular Tissues
The first demonstration of the presence of OBR gene expression in cardiac tissue was reported in 1996 upon the discovery of the gene encoding the db/db mutation.7 Further characterization of OBR isoforms indicated that cardiac tissue expressed OBRa, OBRb and OBRe.8,9 Recent work from the authors’ laboratory suggest that OBR gene expression in the heart di ers in terms of regional distribution and is also a ected by gender.2 Semi-quantitative real-time polymerase chain reaction revealed that in both males and females all three isoforms investigated were expressed in both atria, left and right ventricular walls as the interventricular septum, although the greatest gene abundance was found in the atria. In terms of
Cell Signaling Mechanisms Underlying the Cardiac Actions of Adipokines |
59 |
gender di erences, OBR expression was generally higher in tissues from female rats, especially in the right atria.2
The functions of each of the OBR isoforms in the heart, the relevance of regional distribution expression patterns or the influence of gender are currently unclear, although some potential functions for leptin signaling in the heart will be discussed later in this review. The identification of OBRe in cardiac tissue was of particular interest since this soluble receptor represents the primary binding protein for leptin in plasma and may thus dictate leptin availability to tissues. It is possible that the presence of OBRe in cardiac tissues
is a consequence of proteolytic cleavage of the extracellular domains of one of the other isoforms.10,11 Although the function of OBRe in the heart is currently
unknown, it is interesting to speculate that its local tissue production serves to ‘‘fine tune’’ leptin concentrations in that specific tissue, which would be in keeping with its role as a clearance receptor, although evidence for this hypothesis needs to be obtained with further studies.
In addition to cardiac tissue, leptin receptors have also been identified in both cerebral and coronary vessels.12,13 With respect to the latter it was proposed that OBR-mediated leptin-induced vasodilatation occurs through a nitric- oxide-dependent process and which was abolished by hyperleptinemia. This finding emphasizes the potential dual role of leptin on vascular tissue, a direct NO-dependent vasodilatation and vasoconstriction occurring secondarily to central stimulation of the sympathetic nervous system.
4.4 E ect of Leptin on Cardiomyocyte Function
Under in vivo conditions, the cardiovascular actions of leptin can be predicted based on the central sympathetic stimulatory e ect of the polypeptide resulting in sympathetic nervous system-dependent e ects such as elevations in blood pressure and positive inotropic and chronotropic e ects. However, leptin can exert direct e ects on both the heart and blood vessels through OBR-dependent cell signaling mechanisms. In isolated ventricular myocytes leptin produces a negative inotropic e ect via a NO-dependent pathway as the e ect was abrogated by NO synthase inhibition with L-NAME and associated with increased
NO synthase activity.4 The negative inotropism is also associated with both JAK-STAT and MAP kinase p38 activation.8,14 Leptin has also been shown to
stimulate fatty acid oxidation in working perfused rat hearts in the absence of any e ect on glucose oxidation while lowering cardiac triglyceride content.15
4.5 Cardiomyocyte Hypertrophic E ects of Leptin
Evidence for leptin as a hypertrophic and pro-growth factor stems primarily from studies examining the direct e ect of the polypeptide on myocyte preparations. For example, our laboratory reported that leptin produces marked hypertrophy in cultured neonatal rat ventricular myocytes as manifested by increased cells size, elevated protein synthesis and upregulation of a number of
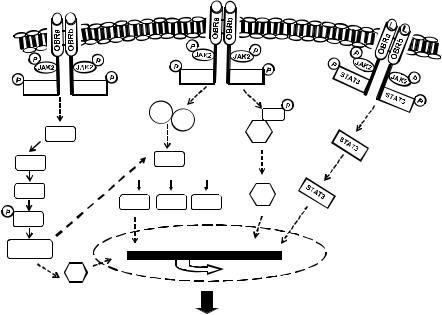
60 |
Chapter 4 |
genetic hypertrophic markers.16 As summarized in Figure 4.1, the hypertrophic e ect of leptin is likely associated with numerous cell signaling processes, some of which act in concert to modify transcriptional regulation resulting in the hypertrophic phenotype. For example, initial studies revealed that leptininduced hypertrophy was associated with MAPK activation including both the p44/42 and p38 pathways whereas the hypertrophy was prevented only by p38 inhibition.16 Xu and coworkers demonstrated that leptin-induced endothelin-1 release from neonatal rat ventricular myocytes results in activation of the endothelin-1 ETA receptor, which then stimulates production of reactive oxygen species, the latter inducing cardiomyocyte hypertrophy.17 This study suggests that leptin does not induce hypertrophy directly per se but rather as a consequence of upregulation of other pro-hypertrophic factors. Accordingly, both ETA receptor blockade and catalase were e ective in abrogating the hypertrophic response.17 In view of the fact that endothelin-1 and other hypertrophic factors such as angiotensin II are upregulated in obesity,18 this study describes an important potential synergistic relationship between various neurohumoral factors in the overall hypertrophic process. This relationship between leptin is further highlighted by evidence from our laboratory that leptin mediates the hypertrophic e ects of endothelin-1 and angiotensin II in cultured myocytes.3 In that study, the
|
|
|
|
|
L |
L |
L |
L |
|
|
|
|
|
|
|
|
|
STAT3 |
STAT3 |
|
STAT3 |
STAT3 |
|
|
|
|
|
|
|
SHP2 |
|
|
IkB |
|
|
|
|
Grb2 |
|
||
RhoA |
|
|
|
|
NF-kB |
|
|
|
|
|
|
||
ROCK |
|
RAS |
|
|
|
|
|
|
|
|
|
|
|
LIMK |
|
|
|
|
|
NF-kB |
|
|
|
|
|
||
|
|
|
|
|
||
|
|
|
|
|
|
|
|
p38 |
JNK |
ERK |
|
||
Cofilin
 G/F ratio
G/F ratio
 Transcription
Transcription
SRF
Hypertrophy
Figure 4.1 Summary of the multiplicity of cell signaling mechanisms underlying the hypertrophic e ects of leptin in the cardiomyocyte. See text for detailed discussion.
