
Книги по МРТ КТ на английском языке / Advanced Imaging of the Abdomen - Jovitas Skucas
.pdf
985
ABDOMINAL VASCULATURE
false aneurysms are saccular in outline and communicate with the aortic lumen. Some false lumens spiral around the aortic circumference; in these, dissection the inner lumen is invariably the true lumen (21).
Aortography was the traditional gold standard in detecting a dissection, identifying an intimal flap, and establishing an entry site and possible exit site. Postcontrast CT and lately MRI are evolving as noninvasive alternatives to aortography in suggesting the extent and length of a dissection (Fig. 17.5). A helical CT study
A
evaluating various protocols concluded that an optimal CT study consists of two separate but adjacent scans—3-mm collimation for the aortic arch and 5-mm collimation for remaining aorta (22); such a study achieved almost a 100% sensitivity and specificity for detecting a dissection and identifying entry and exit sites.
Some acute dissecting aneurysms have an eccentric hyperdense aortic wall on precontrast CT. A hyperdense wall is also found in some intramural hematomas and nondissecting
B C
Figure 17.5. Dissecting abdominal aortic aneurysm. Transverse (A), coronal (B), and 3D (C) CT reconstructions each provide slightly different information about the shape and scope of this aneurysm. (Courtesy of Patrick Fultz, M.D., University of Rochester.)

986
aneurysms, but with the latter the hyperdensity is circumferential rather than eccentric.
Contrast-enhanced CT in a minority of patients with a dissecting aneurysm reveals linear hypodense structures in the false lumen; these fibroelastic bands, described as aortic cobwebs, extend from the intimal flap and represent portions of aortic wall incompletely sheared during dissection. These cobwebs identify the false lumen and distinguish it from the true lumen.
Ultrasonography identifies most true and false lumens, separated by an intimal flap. Some false-positive Doppler imaging results are probably due to a heavily calcified aorta acting as a strong acoustic reflector, resulting in mirror image artifacts. At times a crescent-shaped hypoechoic zone between a thrombus and aortic wall suggests dissection, but Doppler US does not detect any flow in this region, a condition called pseudodissection.
Intravascular US also differentiates the true from the false lumen. It identifies an acute angle between the dissecting flap and false lumen outer wall, and differentiates the three-layered true lumen wall from single-layered false lumen wall. Intravascular US is also useful to differentiate causes of branch vessel compromise— whether a dissection intersects a branch vessel origin or whether a vessel origin was spared but a dissection flap simply compresses the true lumen and covers its origin.
Magnetic resonance detects an intimal flap and identifies both true and false lumens. Flowing blood results in a signal void in both lumens with a SE technique, a finding modified by slow flow or a thrombus. As an aneurysm diameter increases, the false-to-true lumen cross-sectional area ratio also increases, but peak average velocity in the true lumen decreases, findings obtained from MR phasecontrast images. T1-weighted SGE images reveal slow flow in either a true or false lumen as high signal intensity, although, in general, postcontrast 2D or 3D techniques better identify both lumens and an intimal flap, and differentiate slow flow from a thrombus. Contrast-enhanced MR establishes flow patterns in a dissection, including flow in major vessels, and is useful if iodine-based contrast agents are contraindicated. Yet exceptions do occur; in an occasional patient aortic wall thickening is the only sign of dissection on T1-weighted MRI.
ADVANCED IMAGING OF THE ABDOMEN
The diagnosis is difficult with a thrombosed false lumen; the appearance is similar to that of an eccentric mural thrombus. An intimal flap is not detected with a thrombosed false lumen. Inward displacement of intimal calcifications suggests dissection, but this appearance is mimicked by calcifications within a thrombus and an intramural hematoma. A thrombosed false lumen does not enhance with contrast during an early phase; for reasons unknown, occasionally a false lumen enhances on latephase images.
Intramural Ulcer/Hematoma
A penetrating atherosclerotic ulcer-like defect is detected in some patients with extensive atherosclerosis, an entity probably distinct from a dissecting aneurysm. The spectrum of findings ranges from an asymptomatic ulcer penetrating through lamina elastica and forming an intramural hematoma, to intramural dissection, aneurysm formation, and even aortic rupture. The evidence suggests that a hematoma, caused by bleeding from vasa vasorum, is the inciting event in this condition, weakening the aortic wall sufficiently to lead to a dissection.
Follow-up CT of some of these ulcerintramural hematomas reveals the hematoma regressing in size with time (23); some of these patients, however, have ulcer-like cavities that tend to evolve into saccular aneurysms. In others, an intramural hematoma without a patent false lumen is associated with intramural dissection; nonenhanced CT of these focal hematomas often shows a hyperdense rim. A typical appearance is a focal outpouching in a region involved by atherosclerosis and surrounded by a thickened, hyperdense aortic wall that does not enhance with contrast. Aortic intramural hematomas probably need to be followed with imaging.
Transesophageal US identifies an intramural hematoma as a homogeneous aortic wall thickening with intraluminal displacement of intimal calcification.
Hematomas appear hyperintense on T1and T2-weighted MR images.
Mycotic Aneurysm
An abdominal aneurysm in a patient with fever, abdominal or back pain, and leukocytosis sug-

987
ABDOMINAL VASCULATURE
gests a mycotic etiology, a life-threatening condition. Aneurysm wall tissue and blood often yield positive blood cultures for the involved organism. Complicating matters in the elderly, some atherosclerotic aneurysms become infected. These aneurysms tend to change size faster than atherosclerotic aneurysms.
Less common organisms encountered include
Salmonella enteritides and other Salmonella species, Yersinia enterocolitica, and even a hydatid origin. Syphilitic aneurysms are mostly of historical interest. Rare reports describe Mycobacterium bovis infected aortic aneurysms after intravesical bacillus Calmette-Guérin therapy for bladder cancer, even to the point of aneurysm rupture. Some aneurysms develop from an adjacent spinal abscess.
Most of these aneurysms are saccular. Early-stage CT findings consist of a hazy aortic wall outline, evolving into a periaortic infiltrate and paraaortic fluid. The false lumen is often thrombosed. An increase in surrounding fat density can suggest the diagnosis, although similar findings are seen with other causes of extraperitoneal fibrosis or hemorrhage. Aortic wall gas is only an occasional finding.
Complications
Rupture
The sudden onset of severe pain is the hallmark of a ruptured abdominal aortic aneurysm. The risk of rupture is directly related to aneurysm size. Rupture continues to be associated with high morbidity and mortality. It is fatal without surgical repair and is considered a surgical emergency. Rupture is unpredictable and can occur during a radiologic examination. Patients with a leaking aneurysm are often hypotensive and hemodynamically unstable. A ruptured aortic aneurysm occasionally occludes the inferior vena cava. A chronic ruptured but contained abdominal aortic aneurysm can result in extensive anterior lumbar spine erosions, findings readily detected with imaging.
A hemodynamically stable patient suspected of harboring a ruptured abdominal aortic aneurysm usually undergoes a CT study. The aneurysm is recognized and the extraperitoneal hemorrhage identified as isodense or hyperdense regions. Less often detected is an actual aortic wall defect. Extravasation of contrast signifies active bleeding.
The CT crescent sign, consisting of a hyperdense curvilinear structure within a mural thrombus or aneurysm wall, is found in larger aortic aneurysms and often signifies an aneurysm at risk of rupture or one already ruptured. This sign appears to be caused by hemorrhage within a thrombus or in the aneurysmal wall. It is more common in ruptured aneurysms, and if detected in an unruptured aneurysm, suggests impending rupture. A periluminal halo, on the other hand, consisting of a hypodense internal structure within a thrombus around the lumen, is found in roughly 10% of both nonruptured and ruptured aneurysms and does not have a connotation similar to that of the crescent sign.
In a Dutch study of consecutive patients admitted with a ruptured abdominal aortic aneurysm, an US diagnosis was consistent with rupture in only 51% (24).
A Tc-99m–red blood cell study will occasionally suggest rupture of an aortic aneurysm.
Fistula
Vascular fistulas in general are discussed later (see Vascular Fistulas).
A rare ruptured atherosclerotic aortic aneurysm results in an aortocaval fistula. Surprisingly, some of these patients are relatively asymptomatic. These fistulas can be detected by CT. Early caval contrast enhancement is diagnostic. Multislice CT with overlapping slices improves anatomic depiction of these fistulas.
An aortoduodenal fistula and resultant massive gastrointestinal hemorrhage is a recognized complication after aortic aneurysm repair but is quite rare in the absence of prior surgery. An occasional small aneurysm communicates with a cavity and adjacent duodenum (25); the diagnosis can be suggested by CT.
Mural Thrombus
Mural thrombi are common in an aortic aneurysm. These thrombi tend to be soft and loosely attached to the aortic wall; as a result, they are a source of distal emboli. These thrombi do not strengthen the wall of an aneurysm. In time, calcifications develop within a thrombus.

988
Mural thrombi can be evaluated and characterized by MRI. Thrombi range from hyperintense signal on T1and T2-weighted images for an unorganized thrombus, an inhomogeneous signal intensity for a partially organized thrombus, and hypointense signal on both T1and T2weighted images for an organized thrombus.
A mural thrombus can be suspected with radionuclide aortic angiography. A photondeficient region along the aneurysm wall corresponds to a mural thrombus.
Therapy
Surgical
Indications for surgical repair of an abdominal aortic aneurysm vary somewhat among surgeons. Because of the risk of rupture in aneurysms >5cm in diameter, one recommendation is to consider elective surgery in fit patients with an aneurysm >4.5cm in diameter. Other indications include significant occlusive disease requiring repair, pain, or a suspected mycotic etiology.
An open, elective aortic abdominal aneurysm repair carries a mortality of about 2% to 4%, which is in contrasts to a mortality of about 80% for emergent repair of a ruptured aneurysm.
Endovascular
The introduction of endoprostheses has markedly changed aortic aneurysm surgery. Instead of an abdominal incision, vessel clamping, and associated major blood loss, the procedure is performed via a femoral artery approach. Instead of direct aneurysm inspection, oversawing of feeding vessels and surgical repair, both initial planning and repair are performed under imaging control. Preoperative CT, often with 3D reconstruction, is used for aneurysm evaluation and to select an appropriate prosthesis, although 3D MRA is assuming an increasing role in preoperative management of these patients. At times arteriography is helpful. C-arm fluoroscopic guidance is used for prosthesis insertion to bridge the aneurysm. If needed, a bifurcation graft or an aorto-uni-iliac graft is inserted. Such percutaneously inserted and deployed grafts have had a variable success rate. Long-term success rates are not available and the borderline between surgical correction
ADVANCED IMAGING OF THE ABDOMEN
and percutaneous stent therapy is poorly defined. Patients with a long life expectancy and low surgical risk continue to undergo surgical repair. Also, infected aneurysms remain in the surgeon’s domain.
Endovascular aneurysm repair performed using stent-grafts in patients considered too high risk for conventional repair resulted in complete aneurysm exclusion (based on CT criteria) in 88% (26). Deployment and complete aneurysm exclusion with covered stents is more successful with tube grafts than with bifurcation grafts. Surgical correction is necessary for migrating grafts. Nevertheless, a number of authors have commented that their morbidity rates compare favorably with those of open surgery in these high-risk patients.
A limitation of a transfemoral approach is that the iliac arteries must be accessible and relatively free of major atheromatous disease. A major reason for failure of stent-graft insertion is excessive iliac artery tortuosity or arteriosclerosis, with rates of failure dependent on patient selection factors and operator experience.
Covered stent-grafts have been successfully inserted in patients with aortic aneurysms close to renal artery orifices, with the proximal uncovered stent portion placed across one or both renal artery orifices. Stent-grafts have been deployed across saccular aneurysms.
Complications after initial stent-graft insertion are common, and secondary intervention is often necessary. The significance of leakage outside the graft is not clear.
Postoperative Findings
Postoperative imaging appearances differ after various surgical repairs and after percutaneous endoprosthesis insertion. Some of the complications encountered also differ. Knowledge of specific therapeutic technique used is necessary for postoperative imaging evaluation.
Delayed aneurysm rupture after endovascular repair is rare.
Normal Imaging
Serial postoperative contrast-enhanced CT or MR identifies most major complications after surgical repair, including bleeding, false aneurysm formation, vessel occlusion, and most

989
ABDOMINAL VASCULATURE
A 
 B
B
Figure 17.6. Abdominal aortic aneurysm repair. A 3D reconstruction (A) and maximum intensity projection (MIP) reconstruction
(B) identify relationship of aorta to adjacent structures. (Courtesy of David Waldman, M.D., University of Rochester.)
fistulas (Fig. 17.6). With an end-to-end anastomosis, the native aneurysm wall is wrapped around a graft. Postoperative fluid is common between a graft and native aorta and is detected with CT or MR. Perigraft fluid in some patients persists for several months. This fluid is gradually reabsorbed; increasing amounts of fluid with time suggest infection.
Postoperative surveillance includes measurement of residual aneurysm size. A measure of maximum residual aneurysm diameter using CT angiography is common, although aneurysm volume is probably more accurate. Magnetic resonance imaging is limited in the immediate postoperative period if metal components are present. Covered nickel titanium stent-grafts used for abdominal aortic aneurysm repair have been safely imaged by 1.5-T MRI, with no ferromagnetism or heating detected (27). In an in vitro contrast-MR study of nitinol, tantalum, stainless steel, and cobalt alloy stents, only the latter stent resulted in a signal void inside the lumen (28); MR of some of the stents produces an artificial diameter narrowing.
A CT scan 24 to 48 hours after graft placement should reveal any partial or complete thrombosis of the aneurysmal sac; initially patent channels tend to close subsequently, but can recur. A mottled appearance within the aneurysmal sac is not uncommon. Maximum
intensity projections rendered from helical CT a week or so after aortic stent graft placement is useful to evaluate stent deformity or stent angulation; MIP also aids in detecting renal artery occlusion, leaks and thrombi. Computed tomography criteria of successful endovascular repair consist of a decrease or unchanged size aneurysmal sac without the presence of perigraft channels; the latter are often associated with subsequent aneurysm enlargement.
Eventual fibrosis surrounding the surgical site appears hypointense with both T1and T2-weighted images.
Some evidence suggests that MRA is as sensitive as CTA in detecting endoleaks (29); it also avoids iodinated contrast agents Eventual fibrosis surrounding the surgical site appears hypointense both with T1and T2-weighted images.
No firm guidelines are established for longterm follow-up of asymptomatic patients after endovascular repair. Preand postcontrast CT scans annually appear reasonable. Any symptoms or known complication require more frequent follow-up.
Bleeding/Leak
Precontrast CT is useful in detecting an acute postoperative hematoma; a recent bleed is slightly hyperdense to the surrounding struc-

990
tures. Postcontrast CT readily detects graft occlusion. A new false aneurysm enhances postcontrast, unless it is thrombosed.
Leakage adjacent to a prosthesis is considered a perigraft leak, while leakage along an aneurysm border is a retrograde leak. Leaks are uncommon in the thrombosed portion of an aneurysm; rather, continued patency of feeding vessels, such as the lumbar and inferior mesenteric arteries, the median sacral artery, and occasionally even the urethral and testicular arteries plays a role in the pathogenesis of leaks after stent-graft repair (30). Inferior mesenteric artery leaks are ventral to the prosthesis and lumbar or median sacral artery leaks are dorsolateral in location. For most leaks CT can thus establish its origin. Perigraft leak rates vary considerably and depend, in part, on the extent of the initial aneurysm. As the number of patent feeding vessels increases, the leak rate also increases, reaching 60% when more than six lumbar arteries are patent (31).A rare contained leak developing after aneurysectomy is sufficiently extensive to result in inferior vena cava compression.
Computed tomography angiography appears reliable in detecting perigraft leakage. The sensitivities and specificities for detecting leakage were 63% and 77% for conventional angiography and 92% and 90% for CTA, respectively (32). An occasional side-branch endoleak is not detected by CT but is identified by duplex US. Preliminary evidence suggests that contrast enhanced US also detects endoleaks after endovascular aneurysm repair, even detecting some missed by CTA (33).
Most leaks can be treated successfully with additional stent-grafts. Some leaks are amenable to coil embolization; embolization results in aneurysmal sac thrombosis. Others can be sealed off with balloon dilation at the end of stent insertion. Still others disappear spontaneously. At times rather creative embolization is helpful. Thus endoleaks due to retrograde flow in the inferior mesenteric artery have been treated by selective superior mesenteric artery catheterization and embolization through the middle colic artery (34).
Infection/Fistula
Fever, leukocytosis, and anemia suggest graft infection as a complication of an aortic
ADVANCED IMAGING OF THE ABDOMEN
aneurysm or aortic bypass surgery. While graft infection is not common, it is associated with a high morbidity and mortality. An extraperitoneal lymphocele can develop after aortic reconstruction. It can become infected.
Some of the initial work with CT detection of graft infection suggested a sensitivity and specificity approaching 100%, but later studies tempered such enthusiasm, and the current belief is that CT detects mostly advanced infections.
Gas adjacent to a graft is identified by CT shortly after surgery in many patients even without infection. Gas developing several weeks or longer after surgery, on the other hand, implies infection or an aortoenteric fistula. Thus in a patient with anemia or gastrointestinal bleeding of unknown cause (sentinel bleed) and a remote history of aortic aneurysm repair, the presence of soft tissue gas, no matter how little, is presumptive evidence of graft infection and often serves as a harbinger for a future major bleed. A CT finding of periprosthetic wrap thickening, an inhomogeneous appearance and thickening of adjacent bowel wall and valvulae conniventes are common with an infection but are nonspecific findings. Perigraft fluid can persist for several months after surgery, although fluid collections for longer periods of time suggest an infection. If necessary, image-guided fluid can be obtained for culture. Some infectious organisms are notoriously difficult to culture, and prolonged incubation is necessary.
Some infections progress to an aortoenteric fistula, with the third and fourth parts of the duodenum most often involved. Direct signs of an aortoenteric fistula are not common; these include either IV contrast extravasating into bowel lumen or orally administered contrast leaking into soft tissues surrounding a graft.
One of the limitations of MR is an inability to detect small amounts of gas in soft tissues. However, MR differentiates persistent inflammation and fluid in an abscess from a hematoma. Fluid appears hypoto isointense on T1and hyperintense on T2-weighted images, while a persistent hematoma is hyperintense with both sequences, although these findings vary, depending on age.
Indium-111–white blood cell (WBC) scintigraphy is useful as a primary test for suspected infections or as an adjunct to ambiguous CT

991
ABDOMINAL VASCULATURE
results. Gallium scanning, or more recently, Tc-99m–hexamethylpropyleneamine oxime (HMPAO)–labeled leukocytes appear to have a role in suggesting an infection but have had limited application. An occasional test is false positive; thus an occasional patient with a noninfected pseudoaneurysm will have uptake of Tc-99m-HMPAO–labeled leukocytes.
Primary percutaneous drainage appears reasonable in patients with aortic graft infection and a fluid collection, although some of these patients later require removal of their infected prosthetic grafts.
Other Findings
Postoperative pseudoaneurysms develop both with and without an underlying infection. Iliac artery tortuosity predisposes to iliac artery injury during percutaneous endoprosthesis insertion. An extreme complication of a pseudoaneurysm is blowout of an aortic stump.
In a setting of a pseudoaneurysm, aortography provides a roadmap for future repair, while CT is superior in evaluating surrounding infection.
Ureteric stenosis, periureteritis, and ureteric compression by a false aneurysm are some of the complications of aortic surgery (35); some of these complications manifest only years later. Endovascular repair can also result in a periaortitis and ureteral obstruction (36). At times a portion of a self-expanding stent covers a renal artery; evidence of renovascular compromise, however, is not common.
In a patient with postoperative hematuria the surgical graft coursed through the bladder (37); presumably an intravesical tunnel was created during the original graft insertion.
Aortitis
Nonspecific aortoarteritis, or Takayasu’s arteritis,is a panarteritis of unknown etiology. Patient age at onset ranges from pediatrics to old age. In a collection of 31 patients with Takayasu’s arteritis, 45% had aortic aneurysms (38); of note is that aortic wall thickening was detected on CT in several of these aneurysms, aneurysms increased rapidly in size, and ruptured during follow-up.
Patients with Takayasu’s arteritis develop visceral artery stenoses. Although angioplasty and
stent insertion usually have an immediate benefit, these patients suffer from a high rate of restenosis.
Thrombosis Leriche’s Syndrome
Acute abdominal aortic thrombosis is not common. Rather than being acute,some of these thrombotic occlusions present with renal failure or congestive heart failure, and the diagnosis is suspected from renal scintigraphy.
A rare cause of aortic occlusion was intraaortic growth of hydatid cysts (39); recurrent hydatid cysts developed after previous surgery for a paraspinal hydatid cyst.
Leriche originally described obstruction at the aortic bifurcation, but his name is now associated with symptoms due to infrarenal aortic obstruction. Varying degrees of claudication and impotence develop depending on the extent of atherosclerosis and collateral flow. Diminished femoral artery pulses are common.
Three-dimensional contrast-enhanced MRA using MIP and a rotated display in patients with Leriche’s syndrome located the level of aortic occlusion as juxtarenal, infrarenal but cranial to inferior mesenteric artery, or caudal to the inferior mesenteric artery (40). Collateral pathways and concomitant renal artery stenoses can often be detected. Although in theory IV DSA provides similar information, increased contrast conspicuity and a 3D rotational display make MRA superior, visualizing even small collaterals. Whether MRA image quality is superior to that of intraarterial DSA is debatable, but the lack of catheter manipulation and arterial injection makes MRA a simpler study.
Inferior Vena Cava
Obstruction
Thrombosis
Most inferior vena caval thrombi originate in an adjacent vein and spread centrally. Thus a lower extremity venous thrombus can extend superiorly, or a renal malignancy, especially originating in the right kidney, not uncommonly invades and obstructs the inferior vena cava. Caval thrombosis is a complication of Crohn’s disease, systemic lupus erythematosus, and
992
Behçet’s syndrome. In these settings caval obstruction should be suspected if hepatosplenomegaly, ascites, and lower extremity dependent edema develop.
Imaging detects a thrombus as an intraluminal filling defect or simply as lack of caval filling with contrast (Fig. 17.7). A primary thrombus and a neoplastic thrombus have a similar imaging appearance and can be differentiated only if thrombus neovascularity or other evidence of a neoplasm is detected. Noncontrast CT reveals an acute thrombus to be isodense or slightly hyperdense to blood. With age, a thrombus gradually becomes hypodense. The involved vessel diameter tends to be expanded focally, regardless of etiology. Gas within a thrombus is rare and suggests infection. Calcifications develop in some chronic thrombi.
With incomplete obstruction, postcontrast CT identifies most thrombi as a hypodense tumor surrounded by contrast-opacified blood. A bland thrombus does not enhance postcontrast, while a tumor thrombus does. Complicating the issue is the occasional bland thrombus attached to a tumor thrombus. Still, caution is needed to differentiate a thrombus from incomplete mixing of opacified and nonopacified blood and a resultant transient artifact.
ADVANCED IMAGING OF THE ABDOMEN
Collateral vessels are common with obstruction of the inferior vena cava. Lower extremity radionuclide venography reveals collateral flow; an occasional patient has diffuse hepatic uptake of the radionuclide. With superior vena caval obstruction, the azygos vein, hemiazygos vein, internal mammary veins, vertebral venous plexus, and lateral thoracic and some superficial thoracoabdominal veins enlarge and become collateral vessels. Because of these collaterals, after contrast injection into an upper extremity CT can reveal enhancement of a liver segment or the inferior vena cava. Early and dense contrast enhancement of liver segment IV occurs due to segmental liver perfusion from epigastric and paraumbilical veins; such a pseudolesion in segment IV is a potential pitfall seen occasionally during both arterial portography and helical CT.
Magnetic resonance readily detects a caval thrombus. With SE sequences the vena cava contains a tumor rather than a signal void as seen with flowing blood. Slow-flowing blood, however, also results in loss of the caval signal void. With GRE sequences flowing blood appears hyperintense, and this technique appears more reliable in detecting a thrombus than a SE technique. In some patients incom-
A
B
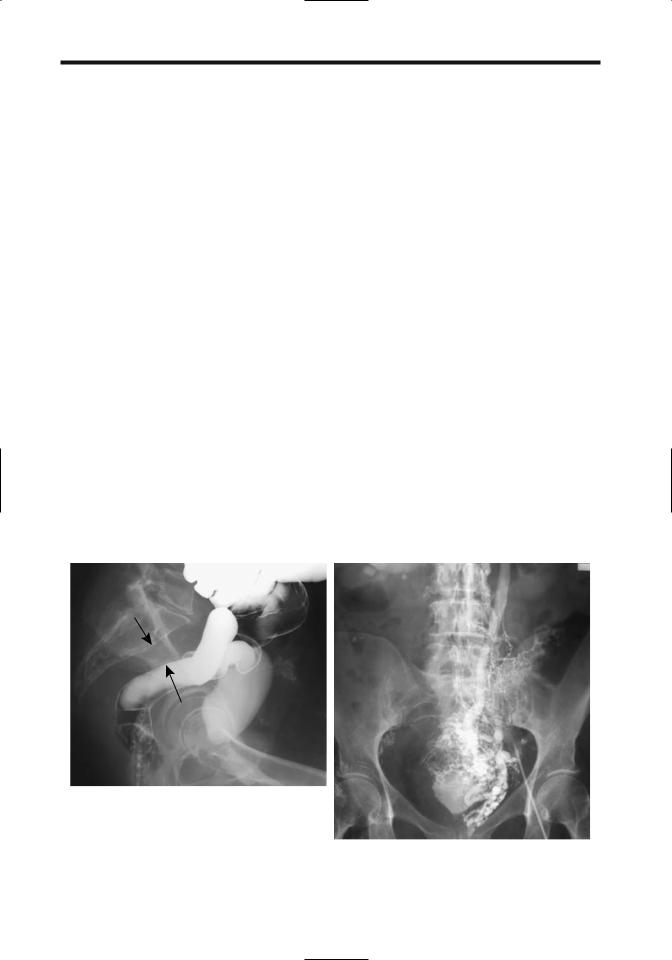
Figure 17.7. Idiopathic inferior vena caval obstruction. A: A lateral view from a barium enema and cystogram reveals widening of the presacral soft tissues (arrows), a common finding with caval obstruction. B: Venogram identifies extensive collaterals veins and confirms lack of vena cava filling.

993
ABDOMINAL VASCULATURE
plete obstruction simply results in hepatosplenomegaly and follows a relatively benign course, while others develop chronic liver disease and esophageal varices. Caudate lobe and left lobe hypertrophy, right lobe atrophy, and a nodular liver outline develop with intrahepatic inferior vena caval obstruction; peripheral linear or wedge-shaped hypodense defects are detected in some patients. Some chronic thrombi are associated with caval wall thickening; MR contrast enhancement extends from the vena cava into surrounding soft tissues, probably reflecting thrombophlebitis.
Occasionally a caval thrombus regresses spontaneously.
Membranous Obstruction
One of the causes of Budd-Chiari syndrome is inferior vena cava obstruction by a membrane (web). This condition is more common in East Asia than in the West and predominates in young adult males. Some of these patients also have hepatic vein membranes.
A congenital inferior vena cava web is rare. After therapy some of these webs restenose and require several dilations. Why an acquired web forms in some patients is not clear. A number of affected patients have suffered prior abdominal trauma. An underlying hypercoagulable state has been suggested; in fact, some patients with membranous obstruction have both an underlying hypercoagulable condition and also had recent trauma.
A membranous obstruction can be detected by either US or cavography but not by CT. Doppler US is useful in evaluating membranous obstruction of both the inferior vena cava and main hepatic veins.
With some membranous obstructions the hepatic veins act as an alternate pathway for blood flow. Thus intrahepatic collaterals can develop between inframembranous and supramembranous hepatic veins; because of these pathways, a Budd-Chiari syndrome does not develop.
Other Obstructions
A rare inferior vena caval obstruction is associated with a diaphragmatic hernia; liver herniation results in torsion and narrowing of the inferior vena cava.
Therapy
Most membranous obstructions are amenable to transfemoral balloon dilatation with excellent results. An occasional membrane, however, is relatively thick and resists balloon dilatation. Endoluminal recanalization and stent insertion are viable options in patients with chronic inferior vena caval obstruction
Unique percutaneous thrombectomy of floating iliocaval thrombi has been performed with an occluding balloon sheath. Using a transjugular access route, the sheath was positioned in the inferior vena cava, a balloon inflated to prevent central thrombi embolization, mechanical fragmentation performed through a working channel using a rotating basket, and residual thrombus fragments then aspirated (41).
Tumor
Primary
Primary inferior vena caval malignancies are rare; they are readily confused with adjacent extraperitoneal tumors. Primary leiomyomas and sarcomas should be distinguished from secondary ones, the latter often representing intravenous leiomyomatosis from a uterine leiomyoma. Some of these women have had a previous hysterectomy for uterine leiomyomas. For example, cavocardiac leiomyomatosis was discovered in a woman who had a hysterectomy 16 years previously for a hemorrhagic fibroma (42); her present tumors were believed to be uterine in origin.
Leiomyosarcoma
The most common primary vena caval malignancy is a leiomyosarcoma. For some reason authors have an urge to publish their experience with vena cava leiomyosarcomas, but these extensive case reports belie the rarity of this condition.A number of these patients have been enrolled in the International Registry of Inferior Vena Cava (IVC) Leiomyosarcomas. Out of 218 patients in this study, over half developed tumor recurrence after radical resection (43). Recurrence consisted of local spread, distant metastases, or both.
Leiomyosarcomas range from an intraluminal tumor obstructing the inferior vena cava, to

994
a tumor extending primarily outside the caval wall, to a complex tumor having features of both; CT shows a lobulated well-defined heterogeneous tumor, while MRI reveals a tumor having an isointense signal on T1and an isoto hyperintense signal on T2-weighted images. Regions of hemorrhage result in a hyperintense T1-weighted signal. These tumors usually enhance markedly with contrast. With a complex appearance, both CT and MRI are useful in differentiating between a neoplasm and a simple thrombus. At times a tumor is so extensive that identification of the site of origin is not possible radiologically, surgically, or even pathologically.
Histiocytoma
Only a few inferior vena caval malignant fibrous histiocytomas have been reported. Contrastenhanced CT of one revealed a minimally enhancing intraluminal tumor expanding the lumen considerably, findings not seen with a simple thrombus (44); hyperand hypointense regions were identified on T1and T2-weighted sequences. Small serpiginous enhancing tubular structures on immediate postgadolinium SGE images were believed to represent feeding vessels.
Secondary
Extraperitoneal tumors readily compress the inferior vena cava or invade and progress to an intraluminal tumor thrombus. Invasion by renal cell carcinoma is familiar to most radiologists. Some of the more unusual invasive tumors include a liposarcoma and adrenal carcinoma. Intracaval extension of these tumors can be determined by transesophageal US.
Magnetic resonance imaging and MRA identify both intraluminal tumors and extrinsic caval compression by a tumor. Collateral vessels are also detected. Magnetic resonance imaging generally provides more information than CT.
Caval Fat
True intracaval fat-density tumors range from a lipoma to renal angiomyolipoma extending through a renal vein.
A fat-density intraluminal caval tumor is rare; a more common reason for such a CT appearance is juxtacaval fat, located medially at the
ADVANCED IMAGING OF THE ABDOMEN
hepatic vein level or slightly superior, artificially projecting into the caval lumen. This fat is contiguous to fat surrounding the subdiaphragmatic esophagus. Either coronal reconstruction images or contrast-enhanced images should suggest a correct diagnosis. Such fat is generally considered to be a normal variant, although it appears to be more common in patients with chronic liver disease (45).
Filters
Clinical
From initial open surgical placement, insertion of inferior vena cava filters has evolved into a percutaneous technique using an introducer sheath. Indications for inserting inferior vena cava filters have expanded from patients who had a pulmonary embolus and could not be anticoagulated to those at risk for emboli. Ease of filter insertion and reduced associated morbidity have expanded the indications further, although in a setting of a pulmonary embolus anticoagulation is generally the first step. Recurrent emboli in the face of adequate anticoagulation or a complication of anticoagulation, such as bleeding, indicates the need for filter insertion. Contraindications to anticoagulation include a recent hemorrhage, with other contraindications often relative and at times subjective.
A number of vena cava filters are available. They differ in their appearance, such as cone, basket,or net; in their construction material and thus radiodensity; and in whether they are readily removable. In broad terms, all filters trap most clots, but some pulmonary emboli are unavoidable. At times vena caval filters are inserted temporarily, such as in the presence of iliac vein or caval thrombi and or in the risk of embolization during thrombolysis.
Carbon dioxide is an alternate contrast agent for vena cavography during filter insertion when iodinated contrast agents are contraindicated or in those patients with renal insufficiency (46).
Complications
Complication rates for radiologic and surgical placement of inferior vena cava filters appear comparable although in one institution radiologists achieved a higher success rate and fewer
