
Книги по МРТ КТ на английском языке / Advanced Imaging of the Abdomen - Jovitas Skucas
.pdf
1015
ABDOMINAL VASCULATURE
teric vein shows that propranolol in cirrhotics does reduce shunting; this response to propranolol appears to depend on the severity of liver disease.
Esophageal and gastric variceal sclerotherapy or variceal ligation helps control variceal bleeding but, theoretically at least, should not reduce portal hypertension. Nevertheless, portal venograms performed before and after variceal ligation reveal that although in a majority of patients portal pressure does increase, in a minority the pressure decreases, presumably due to the opening of other major collaterals.
Surgical Therapy
The ideal therapy of portal hypertension due to cirrhosis is liver transplantation, a procedure both complex and controversial.
Any abdominal surgery in a patient with varices is fraught with bleeding complications. Preoperative CTA, including 3D reconstruction, is very useful in outlining collateral vascular channels, especially in unusual locations, prior to shunting.
A portocaval shunt is performed either end- to-side or side-to-side. The former diverts all portal blood away from the liver. Some surgeons prefer a mesocaval interposition shunt using a graft between the superior mesenteric vein and vena cava, but a distal splenorenal shunt (Warren shunt) is performed more often (Fig. 17.17). The number of these procedures has decreased considerably since the advent of TIPS.
Shunt stenosis or occlusion should be suspected if recurrent variceal bleeding occurs after a surgically constructed shunt. Doppler US evaluates the patency of these shunts in most patients by detecting flow in both limbs and through the anastomosis. Percutaneous transcatheter angioplasty, and if necessary stent insertion, is worthwhile if shunt stenosis or occlusion is detected, but keep in mind that angioplasty of stenotic surgical shunts carries a risk of encephalopathy.
The Sugiura procedure consists of esophageal transection and esophagogastric devascularization, with a splenectomy also included by some surgeons. Hepatic function tends to worsen immediately postoperatively after a modified Sugiura operation but then improves. In patients with previous variceal bleeding, a
Figure 17.17. Effect of splenorenal shunt. Splenic vein (SV) and part of superior mesenteric vein (SMV) blood are shunted into the left renal vein (RV). Hepatopetal flow is still maintained in the portal vein (PV) and intrahepatic branches, in spite of a patent paraumbilical vein (P). With further increase in intrahepatic resistance portal vein flow will eventually reverse.
modified Sugiura procedure results in somewhat greater survival rate than a portosystemic shunt. Esophageal transection does not cure esophageal varices, and in most patients they recur in time. New collaterals are also common at other sites.
Transjugular Intrahepatic Portosystemic
Shunting (TIPS)
Clinical Aspects: One reason why surgical portosystemic shunting is not performed more often is difficulty in predicting which patients will progress with their hepatic failure or develop significant encephalopathy. An orthotopic liver transplantation, on the other hand, although having its own morbidity and mortality, is not associated with subsequent hepatic failure or encephalopathy. In a setting of an acute variceal bleed, however, liver transplantation is often impractical and it is in this setting that TIPS evolved as a viable alternative to surgical portosystemic shunting. From a practical point of view, TIPS is less invasive than a surgical portosystemic shunt.
A relatively high prevalence of portal vein thrombosis is found in patients with portal

1016
hypertension. It thus seems prudent to search for portal vein thrombosis, such as with contrast-enhanced CT, prior to TIPS. A finding of an extensive portal vein, splenic vein, and superior mesenteric vein thrombus renders TIPS meaningless.
Currently, TIPS is the therapy of choice for portal hypertension–associated complications in most patients. It decompresses the portal system by creating a side-to-side portosystemic anastomosis. It decreases portal hypertension without the associated mortality and morbidity of a laparotomy, but at the same time introduces its own complications. The current primary indications for TIPS consist of acute variceal hemorrhage not amenable to medical management, prevention of recurrent variceal bleeding, and refractory ascites due to portal hypertension. Less common indications include BuddChiari syndrome and cirrhotic hydrothorax. In patients with end-stage cirrhosis, TIPS gains time, and once the patient is stable an elective liver transplantation can be performed. In patients with portal hypertension-associated colopathy, TIPS controls bleeding from angiodysplasia-like colonic lesions. It is effective in high-risk patients with continued bleeding from esophagogastric varices despite sclerotherapy or failure of surgical shunting. Massive bleeding from peristomal ileal conduit varices has been successfully treated with TIPS (75). In a number of patients with varices and a malignancy, TIPS aids subsequent surgery. Thus control of esophagogastric varices allows transcatheter hepatic segmental artery chemoembolization of a hepatocellular carcinoma. Similarly, TIPS in a patient with esophageal varices and an esophageal carcinoma decreases portal venous pressure and lessens the risk of hemorrhage during subsequent carcinoma therapy.
One subgroup of patients consists of those in whom endoscopic sclerotherapy for acute variceal bleeding fails and TIPS is requested on an emergency basis. These are high-risk patients. One study reported a 30-day mortality of 50% in emergency TIPS patients compared to 7% for elective TIPS (76). TIPS may not be justified in patients with uncontrolled acute variceal bleeding and advanced liver disease, sepsis and multiorgan failure.
Current evidence suggests that preoperative TIPS does not directly affect subsequent liver
ADVANCED IMAGING OF THE ABDOMEN
transplantation; TIPS neither hinders nor facilitates surgery, nor influences postoperative survival. Subsequent transplantation operative time and transfusion requirements do not differ from those without TIPS. Malpositioned shunts, on the other hand, do interfere with subsequent orthotopic liver transplantation. They interfere with cross-clamping at the usual vascular sites during liver transplantation and prolong surgery and in such a situation the transplant team should be made aware of a shunt malposition.
Although the experience with TIPS has been limited, it is feasible and appears as safe in children as in adults, although it is technically more difficult in children and takes longer. Only limited experience is available in infants and younger children.
Secondary hypersplenism is commonly associated with portal hypertension and these patients often have leukopenia and thrombocytopenia; TIPS tends to improve secondary hypersplenism.
Technique: Prior to performing TIPS, interventional radiologists prefer to outline the hepatic vascular anatomy, determine the portal venous blood flow direction, and detect any underlying collateral shunts. These factors can be evaluated by several imaging techniques. Overall, MRI appears to provide more useful information than CT or US.
In experienced hands a TIPS shunt is installed in approximately 2 hours. It is performed using conventional angiographic techniques of angioplasty and a transjugular venous approach, the hepatic veins are catheterized and used to create an intrahepatic shunt between a portal vein branch and a systemic hepatic vein. It is a side-to-side portocaval shunt (Fig. 17.18). Color Doppler US during the procedure aids in selecting the most appropriate veins for puncture. If necessary, transjugular cholangiography defines underlying biliary anatomy. Postprocedure Doppler US evaluates shunt patency.
During TIPS, the catheter and guidewire pass through the right atrium, and thus cardiac arrhythmias are to be expected. Even in patients with no known underlying heart or electrolyte abnormality, nonsustained ventricular tachycardias are common.
An ideal shunt diameter is one that maintains the patient’s liver function, preprocedure varices or ascites resolves, and no hepatic

1017
ABDOMINAL VASCULATURE
Figure 17.18. Creation of an intrahepatic portosystemic shunt. A mesh stent is shown being inserted through a catheter and over a guidewire. IVC, inferior vena cava; PV, portal vein.
encephalopathy develops. A too-small shunt does not reduce the portosystemic pressure gradient enough to correct an underlying abnormality (Fig. 17.19). An overgenerous shunt, on the other hand, risks hepatic encephalopathy. Also, larger-caliber Wallstents have decreased radial strength and yield poorer results. The
optimal shunt diameter is estimated before the procedure from the patient’s hepatic reserve, presence of encephalopathy, and potential for bleeding.
Results: A transjugular intrahepatic portosystemic shunt is one of the most difficult intervention procedures performed, and thus the success rates and complications depend considerably on operator skill and experience. In most studies the success rate in achieving a patent portosystemic shunt with TIPS ranges over 90%. Numerous studies point to TIPS as being effective therapy for portal hypertension and a successful technique in managing variceal bleeding or (less often) intractable ascites. Several randomized studies suggest that in a setting of variceal bleeding patient survival is similar in those undergoing TIPS and those undergoing endoscopic variceal therapy. On a short-term basis TIPS appears to be equally effective in controlling both gastric fundal and esophageal variceal bleeding. Reintervention is eventually required in a majority of patients, with most reinterventions occurring during the first year after TIPS.
A meta-analysis of 11 randomized clinical trials containing 750 patients comparing TIPS with endoscopic therapy found that TIPS markedly reduces the risk of rebleeding but at
A B
Figure 17.19. A: Transjugular intrahepatic portosystemic shunt (TIPS) insertion in a patient with portal hypertension. B: Angiogram after shunting reveals back-flow into the splenic vein (arrow) and coronary vein (arrowheads). (Courtesy of David Waldman, M.D., University of Rochester.)

1018
an increased risk of encephalopathy and without affecting survival (77); TIPS dysfunction developed in 55% of these patients. Survival, however, appears similar in both groups.
Published TIPS mortality rates depend on patient status pre-TIPS. The most frequent cause of death beyond the acute period is progression of liver failure. Mortality rates correlate with a higher Child’s classification.
Long-term follow-up reveals a decrease in blood urea nitrogen, an increase in albumin, and a return of bilirubin to initial levels or lower; surviving patients have an improved quality of life. The progression of acute liver failure is not common after successful TIPS, although in some patients the effects of TIPS on liver function are unpredictable. One measure of liver metabolic function is the arterial ketone body ratio, defined as arterial acetoacetate level divided by b-hydroxybutyrate level. This arterial ketone body ratio appears to have prognostic implications; among patients the 30-day post-TIPS mortality in those with a pre-TIPS ratio £0.5 was 75%, a rate higher than the 14% rate in patients with a pre-TIPS ratio >0.5 (76).
The results of TIPS performed primarily for ascites are discussed in Chapter 14; TIPS appears effective in reducing ascites and improving renal function in patients with functional or organic renal disease (78).
One unrelated outcome is that esophageal motor function tends to improve after TIPS without inducing any gastroesophageal reflux. An occasional protein-losing enteropathy associated with portal hypertension also resolves after TIPS.
Transjugular intrahepatic portosystemic shunting has a limited effect if a large spontaneous portosystemic shunt already exists. If necessary, embolization is an option when such a portosystemic shunt is discovered. Also, occasionally complications dictate TIPS shunt obliteration, which can be performed.
Transjugular portosystemic shunting results in systemic hemodynamic changes, with the major long-term hemodynamic alteration being an increase in pulmonary vascular resistance. Right atrial and pulmonary artery pressures increase, cardiac output increases, and peripheral resistance decreases. In patients with an elevated systemic venous pressure, reduction of
ADVANCED IMAGING OF THE ABDOMEN
the portosystemic pressure gradient after TIPS increases right atrial pressure further and accentuates any underlying congestive failure.
As expected, TIPS induces an immediate decrease in portal pressure and an increase in portal blood flow. A typical pre-TIPS portocaval pressure gradient of about 20 to 22mmHg decreases to <10mmHg. Compensatory hepatic artery flow increases, but no overall splanchnic and liver perfusion changes occur. Although the portocaval pressure gradient decreases after TIPS, eventually the pressure again increases. Patients developing ascites usually have a portocaval pressure gradient >12mmHg.
One alternative to Doppler US follow-up is Tc-99m–diethylenetriamine pentaacetic acid (DTPA) liver perfusion scintigraphy. Relative arterial and portal liver inflow can be calculated from a biphasic time-activity curve. Tech- netium-99m–macroaggregated albumin (MAA) injected into the portal veins evaluates portosystemic shunting by comparing counts in the lungs and liver; the amount of shunting correlates inversely with portosystemic pressure gradient. The clinical application of such scintigraphy remains to be established.
Shunt Stenosis/Occlusion: The most common TIPS complication is shunt stenosis, often requiring revision. Why do shunt stenoses develop? Autopsy study of three livers containing four TIPS tracts revealed shortand midterm TIPS occlusions to be caused by thrombi associated with hepatocyte necrosis and bile leakage, while long-term stenoses consisted of a combination of pseudointimal hyperplasia and hepatocyte ingrowth (79). Biopsies in patients with a shunt stenosis tend to reveal bile within an organising thrombus; the degree of stenosis appears related to amount of bile leakage. A rare shunt thrombosis extends proximally to involve the portal, splenic, and mesenteric veins.
Shunt stenosis or occlusion is suspected either with routine radiologic screening or when the patient develops recurrent symptoms; it is this complication that limits TIPS as an optimal long-term solution for portal hypertension. The prevalence of shunt stenosis or occlusion appears independent of a patient’s Child-Pugh class.
Anticoagulation appears to aid early shunt patency but probably does not affect patency on a long-term basis.

1019
ABDOMINAL VASCULATURE
Recurrent portal hypertension due to stent thrombosis, stenosis, or stent retraction develops during the first year in over half of patients. Portography and portal manometry serve as gold standards in detecting and characterizing shunt stenoses, but, being invasive, are used only when other imaging is noncontributory. Endoscopic detection of varices is a less sensitive method, but appears superior to Doppler US. Although hepatic vein stenosis appears to be relatively common after TIPS, it is rarely symptomatic.
Ultrasonography is often used in evaluating TIPS shunt patency, although CTA shows considerable promise in detecting most shunt dysfunctions; CTA achieved a 92% sensitivity and 77% specificity in detecting hemodynamically significant TIPS abnormalities (80). Although these values are similar to the results obtained with US, CTA eliminates a subjective bias inherent in performing US through an often narrow acoustic window; 3D multiplanar reconstruction CTA is very helpful.
The degree of stenosis beyond which revision is necessary is not clear. At times lumen reduction of even 10% requires intervention due to a high portosystemic gradient; these mild stenoses are generally due to intimal hyperplasia.
Doppler US of portal venous flow reveals considerable variability in repeat measurements, and published studies are not always comparable. In spite of publications such as “The Inaccuracy of Duplex Ultrasonography in Predicting Patency of Transjugular Intrahepatic Portosystemic Shunts” (81) in the gastroenterology literature, most investigators believe that duplex US identifies flow within a shunt. Typical blood velocities within patent, well-functioning shunts are within the 120 to 200cm/sec range, often associated with a reversal of intrahepatic portal vein flow. Portal vein velocity increases from about 20cm/sec to 40cm/sec. In a thrombosed shunt Doppler US does not detect flow. In the practical sense, the issue is more complex because a shunt Doppler signal is not detected even from some patent shunts.
Either a Doppler US measurement of maximum peak velocity of 50cm/sec or less within the shunt or a change from hepatofugal to hepatopetal portal venous flow indicates shunt stenosis. Using these criteria, Doppler US
identifies TIPS occlusion and confirmed patency in over 95% patients. A choice of 50cm/sec shunt peak velocity is, of course, not absolute. An assumption of a greater Doppler US shunt peak velocity threshold improves the specificity of detecting normal shunt function. One further refinement is the use of either an increase or decrease in shunt peak velocity of more than 40 or 50cm/sec from the initial postTIPS baseline value as a cutoff criterion; such a change in shunt peak velocity appears to be a more sensitive sign of shunt stenosis than simply a low flow state. Also, blood flow in the right anterior portal vein becoming hepatopetal after TIPS is indirect evidence of shunt stenosis or occlusion.
Doppler US shunt velocity varies between the portal vein end and hepatic vein end. Thus in patients with functioning TIPS, median shunt velocity was 60cm/sec at the portal vein end and increased to 82cm/sec at the hepatic vein end (82); velocity is reduced in patients with compromised shunts. The use of the IV US signal enhancer Levovist improves color and flow signals (compared to pre-Levovist) at the portal vein shunt end in only 24% of studies but improved hepatic vein shunt end (83); more stenoses are identified with Levovist, with most stenoses being located at the hepatic vein shunt end evaluation of both shunt ends. Evaluation should be performed by Doppler US.
In a number of institutions Doppler US has replaced angiography as the post-TIPS screening modality. Yet published sensitivities and specificities of Doppler US in detecting TIPS stenosis or occlusion are difficult to place in proper perspective. Some of the studies conflict. Several more optimistic studies report almost 100% sensitivity and specificity in detecting stenosis or occlusion. Some authors ascribe Doppler US false-positive or false-negative diagnoses to technical factors, including the type of stent used. Thus, in one study a shunt velocity of <60cm/sec achieved a sensitivity of only 25% and specificity of 93% in detecting shunt stenosis (84); a high sensitivity (90%) could only be achieved at the expense of poor specificity (<33%). Some studies suggest that shunt or portal vein Doppler US velocities correlate poorly with portal pressure.
In summary, most patent shunts have a velocity >70cm/sec and hepatofugal flow in the liver portal vein branches; in general, midshunt
1020
velocities of <60cm/sec should suggest shunt dysfunction. It should be kept in mind, however, that Doppler US rarely identifies the cause of shunt stenosis.
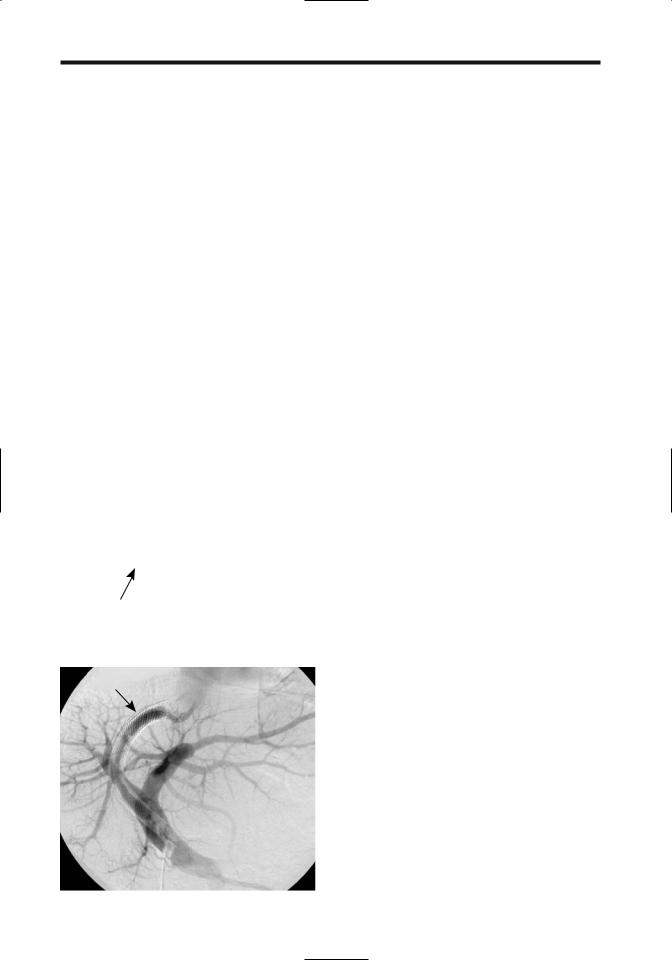
Once a stenosis is suspected and is confirmed by venography, percutaneous transluminal angioplasty is the primary therapy to reestablish shunt patency, with multiple interventions necessary in some patients (Fig. 17.20). Patients treated by dilation tend to develop a restenosis, but those managed by insertion of new shunts tend to remain patent.
A rheolytic thrombectomy catheter is designed to remove thrombi by fragmentation and suction. It uses multiple retrograde highspeed fluid jets to create a recirculating vortex that fragments the adjacent thrombi, and the
ADVANCED IMAGING OF THE ABDOMEN
thrombi are then evacuated through an aspiration lumen. It can recanalize an acutely occluded TIPS stent.
Other Complications: Other complications include intraperitoneal hemorrhage, hematoma, hemobilia, accelerated liver failure, hepatic vein stenosis, and infection. Less commonly encountered are perforation through the liver capsule, stent migration with portal vein perforation and portal vein dissection, thrombosis, and laceration. Occasionally a stent migrates; some of these stents have been successfully retrieved. Inadvertent hepatic artery puncture has resulted in fatal hemorrhage. Likewise, right atrial and aortic perforation during stent placement can lead to hemopericardium and cardiac tamponade.
A 
 B
B
|
Figure 17.20. Occluded TIPS. A: Contrast injected into the portal |
|
vein does not flow through the stent (arrow). B: Stent has been |
|
recanalized. Contrast and stent do not superimpose due to arti- |
|
fact. C: Partly occluded TIPS (arrow) in another patient is shown by |
|
portal vein injection. (Courtesy of David Waldman, M.D., University |
C |
of Rochester.) |

1021
ABDOMINAL VASCULATURE
Sepsis following TIPS is not common, and the significance of TIPS infections is poorly understood. Reported findings in patients believed to have a primary shunt infection include tender hepatomegaly, hypoxemia, septic pulmonary emboli, septic shock, and necrotizing fasciitis; blood cultures tend to be positive.
Hepatic encephalopathy develops in 15% to 30% of patients after TIPS, presumably due to their increased portosystemic shunting. It is more frequent during the first 3 months after TIPS, and a majority of these patients have a portocaval pressure gradient of <12mmHg. The prevalence of encephalopathy appears increased in patients undergoing TIPS for refractory ascites. Doppler US appears useful in predicting the risk of encephalopathy; encephalopathy developed in 83% of patients with Doppler US evidence of hepatofugal blood flow in both right and left portal vein branches, but encephalopathy occurred in only 12% of patients with hepatopetal blood flow (85). In most patients encephalopathy can be controlled medically. Symptomatic encephalopathy is a consideration for subsequent liver transplantation.
1H-MR-spectroscopy of brain metabolites is useful in liver transplantation to evaluate hepatic encephalopathy (86); whether such spectroscopy has a role after TIPS-associated hepatic encephalopathy is unclear.
A rare TIPS complication is liver infarction.
Portal Vein Stenting
Portal vein stenting is an option in patients with an unresectable pancreatic or biliary tumor invading the portal vein and leading to portal hypertension. These stents are inserted after percutaneous transhepatic portography evaluates the underlying anatomy. In 13 such patients, the mean portal venous pressure decreased significantly immediately after stent placement, but follow-up revealed increased risk of stent occlusion in those with initial splanchnic vein tumor involvement (87).
Portal Vein Aneurysm
Portal and splenic vein aneurysms, especially those extrahepatic in location, are rare. Most are associated with portal hypertension or an arterioportal fistula. Some are congenital in origin; others are related to previous trauma.
Most portal vein aneurysms are asymptomatic. They occur at any age. A number of these aneurysms gradually increase in size with time, often with progression of portal hypertension.
Some patients have both an arterioportal fistula and a portal vein aneurysm, an association that appears to be more than fortuitous. In some of these patients the aneurysm thromboses after percutaneous arterial embolization closes the fistula.
Portal vein aneurysms tend to be fusiform, while intrahepatic ones are mostly cystic. Unless thrombosed, US reveals these aneurysms to be anechoic. Contrast-enhanced CT or 3D power Doppler US should detect an aneurysm and identify any portosystemic fistulas. Doppler US shows turbulent flow in most. Portal venography is rarely necessary for diagnosis.
Portal Vein Gas
The most common etiology for portal venous gas is bowel ischemia; some of the other reported associations presumably have ischemia as a common pathway (Table 17.3). Some of the listed conditions, especially in pediatric patients, have a benign course. Some cystic fibrosis patients have both portal and systemic venous gas, and presumably gas is shunted from the portal circulation via the inferior mesenteric vein and hemorrhoidal veins into the inferior vena cava.
Table 17.3. Etiologies of portal venous gas (listed from more common to less common)
Bowel ischemia
Necrotizing enterocolitis in babies After colonoscopy
Trauma
With increased bowel intraluminal pressure due to lumen obstruction
After double-contrast barium enema in pediatric patients
Colonic diverticulitis
Endoscopic retrograde cholangiopancreatography and sphincterotomy
Associated with pneumatosis intestinalis in the pediatric age group
Intraabdominal infection with a gas-forming organism In a setting of hypertrophy pyloric stenosis
Perforated duodenal ulcer complicated by an abscess After a seizure
In a patient with cystic fibrosis with bowel obstruction

1022
Traditionally, conventional radiographs have identified portal gas. It appears as multiple, branching, linear lucencies in the liver and is familiar to radiologists. Conventional US, color Doppler flow imaging, and CT have a high sensitivity in detecting portal gas; of these modalities, CT appears most useful in suggesting an etiology. Doppler US reveals venous gas as turbulent flow with fleeting echoes. Gas bubbles result in a highly echogenic appearance, often transient in nature.
Portal Vein Tumors
Primary portal vein neoplasms are rare. An adjacent extrinsic carcinoma often invades the portal vein, encases it, and eventually obstructs. The most common is pancreatic carcinoma, with those originating in the body and tail of the pancreas invading the splenic vein, and pancreatic head cancers invading the portal vein. Similarly, metastases to the porta hepatis invade not only the portal vein but also adjacent bile ducts.
The role of CT and MR in preoperative staging of portal vein invasion is still evolving. Whether Doppler US is comparable to angiography or CT arterial portography in detecting portal vein encasement or occlusion is debatable.
Calcifications
Portal vein calcifications are rare, with the pertinent literature consisting mostly of case reports. One CT study, however, found an 11% prevalence of portal and mesenteric venous calcification in patients with advanced cirrhosis (88). Most patients are adults with longstanding portal hypertension.
Some portal vein thrombi calcify. These calcifications appear as linear or mottled densities corresponding to the portal vein path.
Portal vein calcifications are better identified with CT than with conventional radiography. They should not be confused with intrapancreatic calcifications, atherosclerosis, or aneurysmal calcifications.
Other Findings
Not uncommonly segments of portal vein branches are opacified during CT hepatic arte-
ADVANCED IMAGING OF THE ABDOMEN
riography. These arterioportal shunts can be divided into intrahepatic and extrahepatic. Causes for intrahepatic shunting include tumors—mostly hepatocellular carcinomas, previous liver biopsy, and portal vein thrombosis, regardless of cause (89); extrahepatic causes include inflow through adjacent organ portal collateral circulations.
Portal and mesenteric vein septic thrombophlebitis is usually secondary to other foci of infection. Sigmoid diverticulitis, appendicitis, infected pancreatitis, and similar entities are generally responsible; they are discussed in their respective chapters. Computed tomography readily detects such thrombophlebitis.
A portal vein graft was inserted during a pancreatic resection and Whipple procedure (90); rectal evacuation of the portal vein graft occurred as a late complication, presumably induced by graft infection.
Splenic Vein
Splenic vein obstruction is often clinically silent, although in a minority of patients it results in splenic infarction or leads to gastrointestinal bleeding. The most common etiologies of splenic vein thrombosis are pancreatitis and pancreatic carcinoma. An occasional adjacent tumor, such as a pancreatic cystadenoma, compresses the splenic vein. Perihilar splenic varices secondary to a splenic hilar hydatid cyst compressed adjacent hilar vessels (91). An association of celiac disease and splenic venous thrombosis has been raised (92).
Without underlying liver disease, these patients tend to develop gastric varices without esophageal varices, although splenic vein thrombosis tends to extend and involve the portal vein. Occasionally gastric fundal varices are associated with colonic varices. In some patients the spleen becomes enlarged; in fact, splenic vein obstruction should be considered in the differential diagnosis in a patient with gastrointestinal bleeding occurring in a setting of unexplained splenomegaly.
Splenic vein occlusion is diagnosed by CT, conventional and endoscopic US, MRI, or angiography. Computed tomography reveals no splenic vein visualization but perigastric collateral veins are present; these are indirect signs, and an occasional obstruction detected

1023
ABDOMINAL VASCULATURE
by angiography is not identified by CT. At times splenic vein thrombosis reveals a hypodense splenic vein, collateral vessels, and a patent portal vein. Ultrasonography shows an echogenic thrombus in the splenic vein lumen.
Splenic vein aneurysms are rare. An occasional aneurysm regresses after resolution of splenomegaly.
Pancreatic pseudocysts occur in the general location of the splenic and portal veins. An occasional one erodes into the splenic vein.
Superior Mesenteric Vein
Superior mesenteric vein thrombosis is idiopathic, associated with an underlying malignancy, develops in hypercoagulable states, and occurs in a setting of pancreatitis, sigmoid diverticulitis, or appendicitis, and as a sequela after abdominal surgery such as a Whipple procedure or even a complicated appendectomy (Fig. 17.21). It occurs in young patients. Acute thrombosis, in the absence of collaterals, often progresses to intestinal infarction. A more chronic course leads to pain, diarrhea, and malabsorption.
The major issue with superior mesenteric vein thrombosis is that the clinical presentation is usually sufficiently vague and the diagnosis is initially overlooked. The mortality from this condition has not changed significantly over the
Figure 17.21. Superior mesenteric vein thrombosis after appendectomy. Contrast-enhanced CT reveals an enlarged vein, an intraluminal clot and an enhancing vein wall (arrow). (Source: Schmutz GR, Benko A, Billiard JS, Fournier L, Péron JM, FischPonsot C. Computed tomography of superior mesenteric vein thrombosis following appendectomy. Abdom Imaging 1998;23: 563–567, with permission from Springer-Verlag.)
Figure 17.22. Superior mesenteric vein thrombosis. Contrastenhanced CT shows an enlarged, enhancing superior mesenteric vein and an intraluminal clot (arrow). (Courtesy of Gérard Schmutz, M.D., Centre Hospitalier Universitaire, Caen, France.)
years. Complicating the picture is the frequent presence of other intraabdominal disease.
Occasionally an acute thrombus resolves spontaneously. In most patients, however, prompt diagnosis and therapy with thrombolytic, anticoagulant, antiplatelet, or antispasmodic agents is warranted to prevent complications and reduce mortality. At times selective superior mesenteric artery infusion of urokinase is helpful.
Computed tomography shortly after clot formation reveals a thrombus to have a higher density than does soft tissues. The superior mesenteric vein is often enlarged. Later, a characteristic CT appearance consists of a hypodense central thrombus surrounded by a higher density wall (Fig. 17.22). At times a thrombus extends into the portal vein. With incomplete lumen obstruction, contrast-enhanced blood surrounds a lower density thrombus. The vein wall enhances after contrast. Surrounding mesenteric edema is not uncommon.
Inferior Mesenteric and Pelvic Veins
Septic thrombophlebitis of the inferior mesenteric vein is most often secondary to sigmoid diverticulitis. It is readily detected with CT.
Contrast-enhanced MR venography using a blood-pool contrast agent outlines major pelvic veins and evaluates for pelvic vein thrombosis. Superimposed arterial structures can be minimized by a subtraction technique.

1024
Budd-Chiari Syndrome
Clinical
Budd-Chiari syndrome consists of obstruction to the venous outflow from the liver. The reported prevalence of Budd-Chiari syndrome varies throughout the world, in part due to different reporting criteria. The older literature considered only acute obstruction under BuddChiari syndrome, although currently the definition has been expanded to also include a more common chronic form. It is caused by hepatic vein obstruction or, less often, obstruction in the adjacent inferior vena cava (Table 17.4). In Japan, most patients with Budd-Chiari syndrome have a chronic form; the etiology is idiopathic in the majority, and over 90% have obstruction in the intrahepatic portion of the inferior vena cava. The most common direct underlying cause of Budd-Chiari syndrome in Western countries is either hepatic vein or inferior vena caval thrombosis. Patients developing a Budd-Chiari syndrome believed to be idio-
Table 17.4. Conditions associated with Budd-Chiari syndrome
Idiopathic
Vascular conditions Veno-occlusive disease
Inferior vena cava web or membrane
Inferior vena caval narrowing induced by right hemidiaphragm elevation
Behçet’s disease Polycythemia vera
Thrombotic thrombocytopenic purpura in a pregnant patient
Drug induced
Oral contraceptives
Neoplasms
Hepatocellular carcinoma
Inferior vena cava or hepatic vein sarcoma Adjacent neoplasm invading inferior vena cava
Pregnancy
Infection
Trauma
Other
Hypereosinophilic syndrome
Systemic lupus erythematosus
ADVANCED IMAGING OF THE ABDOMEN
pathic in origin should be investigated for an undetected latent coagulation disorder. Other causes include a web-like membranous obstruction of the inferior vena cava, a condition more common in the Orient. Trauma is a rare cause of Budd-Chiari syndrome. Major hepatic surgery with compromise of the hepatic veins or intrahepatic portion of the inferior vena cava is an occasional predisposing factor. A renal cell carcinoma, a rare adrenal neoplasm with inferior vena cava invasion, retroperitoneal sarcoma, and malignancies involving the inferior vena cava also result in Budd-Chiari syndrome. Hepatic vein thrombosis is a complication of Behçet’s disease, and Budd-Chiari syndrome should be suspected if hepatomegaly and ascites are detected in this disease. A rare cause of acute Budd-Chiari syndrome is percutaneous insertion of a transhepatic inferior vena cava catheter.
Regenerative liver nodules are more prevalent in chronic Budd-Chiari syndrome than expected. The question of whether this syndrome is linked to an increased risk for hepatocellular carcinoma has been raised. Complicating this question, some of these patients have underlying cirrhosis.
The onset of symptoms in Budd-Chiari syndrome ranges from acute to insidious. Initially liver dysfunction is mild, but gradually hepatomegaly, ascites, and complications of portal hypertension ensue. The presentation is complicated by associated portal vein, splenic vein, or superior mesenteric vein thrombosis, which also develop in some patients. A majority of these patients have limited therapeutic options and a poor prognosis.
From a clinical viewpoint it is useful to subdivide Budd-Chiari syndrome into acute and chronic presentations. From an anatomic viewpoint, a subdivision into a partial obstruction limited to thrombosis of one hepatic vein and a more complete obstruction involving more of the liver drainage is more informative. Complete obstruction of the hepatic veins is rare; drainage of the caudate lobe tends to be preserved unless the adjacent inferior vena cava is obstructed. Especially in chronic obstruction, accessory hepatic veins enlarge and become evident. At times the obstruction involves mostly smaller veins, with the main hepatic veins being patent.
