
Книги по МРТ КТ на английском языке / Advanced Imaging of the Abdomen - Jovitas Skucas
.pdf
1005
ABDOMINAL VASCULATURE
thirds that of the portal vein; hepatic artery flow increases significantly with temporary occlusion of the portal vein. Theoretically, percutaneous Doppler US should be able to measure liver and portal venous blood flow; in practice, blood flow measurements vary considerably.
Hepatic venous pressure gradients are obtained by hepatic vein catheterization and portal blood flow velocity and portal vein congestion index measured by duplex Doppler US. No significant correlation exists between venous pressure gradients and Doppler measurements in patients with cirrhosis and portal hypertension, but a significant linear correlation is evident if patients with patent paraumbilical veins are excluded (61).
Duplex portal vein US in patients before TIPS diagnosed portal hypertension with a sensitivity and specificity of 80% if a portal vein diameter >1.25cm or portal vein velocity <21cm/sec were detected, but US could not grade hypertension (62). On the other hand, in cirrhotic patients portal blood velocity does not differentiate between those with or without endoscopic evidence of esophageal varices or congestive gastropathy and, in general. Doppler US cannot identify those cirrhotic patients at risk for upper gastrointestinal bleeding.
Phase-contrast MRA has been used to measure portal blood flow. In general, MRA results correlate with data obtained by Doppler US. Phase contrast MRA also provides azygos vein flow changes during the cardiac cycle; flow rates are considerably greater in patients with chronic liver disease and portal hypertension than in healthy individuals. Potentially, such information aids in evaluating progression of diffuse liver disease.
An endoscopic balloon technique measures esophageal variceal pressure. This pressure correlates only partly with portal venous pressure, probably due to the presence of other collateral channels.
Etiology and Pathogenesis
Occasionally portal hypertension is associated primarily with increased portal blood flow, such as an arterioportal fistula, but most often it develops due to increased resistance to portal venous blood flow. A rare malignant vascular intrahepatic tumor leads to portal hyperten-
sion; these tumors result in increased portal blood flow due to shunting, intrahepatic portal obstruction caused by massive tumor growth,or both. Occasionally portal hypertension is idiopathic (Banti’s syndrome).
Increased Flow
Portal hypertension due to increased blood flow is most often a result of a systemic artery-to- portal vein shunt, either intraor extrahepatic in location. These shunts, or fistulas, extend to the splenic vein, superior mesenteric vein, or portal vein, including its intrahepatic branches. They can be congenital or acquired. A congenital artery-to-portal vein fistula should be suspected with infantile portal hypertension. At times portal hypertension manifests clinically only years after a fistula is established.
Increased Resistance
Table 17.1 lists some of the causes of portal hypertension due primarily to increased resistance to portal blood flow. Of interest is that portal venous pressure tends to be slightly elevated in some disorders not usually associated with portal hypertension. Thus portal pressure is increased in patients with biliary obstruction, decreasing after biliary decompression. Rarer causes of prehepatic portal venous obstruction include adenopathy at the liver hilum.A number of patients with abdominal tuberculosis and periportal adenopathy have portal hypertension (63). Similarly, periportal lymphoid infiltration in lymphoma or leukemia can result in portal hypertension.
With presinusoidal intrahepatic obstruction the hepatic vein wedged pressure is normal. Thus patients with hepatic schistosomiasis have hyperkinetic systemic and splanchnic circulations but a normal hepatic venous pressure gradient and hepatic blood flow; in those with esophageal varices a normal hepatic venous pressure gradient is indicative of presinusoidal portal hypertension. Of note is that portal hypertension in schistosomiasis patients is not due to fibrosis only but is multifactorial; hemodynamic values are not significantly different between patients with and without liver fibrosis. A rare cause of portal hypertension is portal vein invasion by an intrahepatic peripheral cholangiocarcinoma.

1006
Table 17.1. Causes of portal hypertension (due to increased vascular resistance)
Prehepatic
Portal venous system thrombosis
Extrinsic portal compression or tumor invasion Congenital atresia or thrombosis
Intrahepatic Presinusoidal
Conditions leading to diffuse hepatic fibrosis Gaucher’s disease type 1
Polycystic kidney disease and hepatic fibrosis Mixed connective tissue (collagen vascular)
disease
Primary biliary cirrhosis Schistosomiasis
Postsinusoidal Laënnec’s cirrhosis
Tumor infiltration, such as by a hepatocellular carcinoma
Amyloidosis
Systemic lupus erythematosus Hepatitis and hepatic failure
Diffuse intrahepatic portal venous system thrombosis
Distal biliary obstruction
Biliary atresia patients post-portoenterostomy (Kasai operation)
After renal transplantation Systemic mastocytosis Idiopathic
Posthepatic
Budd-Chiari syndrome
Congestive (right) heart failure
Inferior vena caval obstruction
Blood flow velocity decreases in a number of liver diseases, and duplex Doppler US measurements of blood flow are useful in evaluating disease progression. Increased intrahepatic resistance to flow in patients with chronic liver disease is due not only to intrahepatic morphologic changes but also to a dynamic constriction of the intrahepatic portal drainage bed, believed to be due to decreased synthesis of nitrous oxide in the intrahepatic circulation. Complicating this issue, patients with portal hypertension have increased portal blood flow due to splanchnic arteriolar vasodilation. Endothelins and poorly understood neural and humoral regulation, in part mediated by vasodilators, appear to play a role in increasing intrahepatic vascular resistance. Plasma endothelins, potent systemic and portal vasoconstrictors, are elevated in
ADVANCED IMAGING OF THE ABDOMEN
patients with bilharzial and postviral chronic liver diseases with portal hypertension; a positive correlation exists between plasma endothelin levels and portal vein diameter. On a systemic basis, peripheral arterial vasodilation and an increase in cardiac output ensue in patients with chronic liver disease.
Patients with mixed connective tissue disease (collagen vascular disease) can develop sufficient periportal fibrosis to cause portal hypertension and esophageal varices. Patients with advanced Gaucher’s disease type I and a noncirrhotic liver develop portal hypertension. These patients have extensive confluent central hepatic fibrosis, which presumably is responsible for their portal hypertension (64), although Gaucher’s cells compressing liver sinusoids and thus increasing resistance to flow is probably also a factor. Extensive perisinusoidal amyloid infiltration will also lead to portal hypertension.
Liver involvement in cystic fibrosis increases with age.With increasing survival,some of these individuals develop biliary cirrhosis and eventual portal hypertension. A liver transplantation is a viable option provided that adequate pulmonary function has been maintained.
A rare patient with sclerosing peritonitis and extensive liver capsule fibrosis develops portal hypertension even with a patent portal vein; the capsule fibrosis presumably prevents hepatomegaly and any liver disease leads to a sufficient increase in intrahepatic pressure to compress intrahepatic portal vein and hepatic vein branches and results in portal hypertension. Some patients with autosomal dominant polycystic kidney disease and extensive hepatic fibrosis also develop portal hypertension; in some, distortion of intrahepatic portal vein branches by extensive hepatic cysts is sufficient to produce portal hypertension.
An association of portal hypertension and pulmonary hypertension exists in patients with underlying liver cirrhosis and those with mixed connective tissue disease. The pulmonary findings are similar to those found in primary pulmonary hypertension.
In long-term surviving neonates with biliary atresia who undergo hepatic portoenterostomy (Kasai operation), about half develop portal hypertension (65); the incidence of subsequent portal hypertension is significantly lower in those with a serum bilirubin <2mg/dL at 3 months postsurgery than in those with a biliru-

1007
ABDOMINAL VASCULATURE
bin level >2mg/dL. Among those developing portal hypertension, esophageal varices were discovered between 11 months and 5 years of age in more than 70% of children, while thrombocytopenia tended to develop at a slightly older age.
The etiology of Banti’s disease, consisting of anemia, splenomegaly, and portal hypertension, is unclear. Portal hypertension is considered to be idiopathic. A rare patient develops portal hypertension several years after renal transplantation; their portal venous pressure decreases and esophageal varices clear after splenectomy.
In some studies of noncirrhotic portal hypertension an idiopathic etiology continues to be a prominent feature.
Clinical Aspects
Portal hypertension is assessed by measuring hepatic venous pressure gradient, a technique that is the gold standard in planning subsequent hemodynamic therapy. Nevertheless, portal hypertension is often assumed to be present if portal vein collaterals are detected. Thus the presence of distal esophageal varices is generally taken to be presumptive evidence of portal hypertension. Splenomegaly may or may not be present in adults, although splenomegaly is more common in children.
A typical scenario with Laënnec’s cirrhosis is an increase in intrahepatic resistance to portal blood flow, opening of collateral vessels, and a decrease in portal blood flow to the liver.A compensatory increase in hepatic artery blood flow develops, but due to postsinusoidal obstruction some of this arterial blood is diverted to the portal vein and eventually portal vein blood flow reverses direction (hepatofugal flow). Doppler US in patients with cirrhosis reveals a significant decrease in portal flow with a worsening Child’s grade of cirrhosis; patients with ascites and encephalopathy also have a significantly lower portal blood flow rate compared to those without these abnormalities.
Some patients with portal hypertension develop large esophageal varices, while others have small varices or none. Likewise, the prevalence of nonesophageal portosystemic collaterals varies. Some develop both large esophageal varices and nonesophageal portosystemic collaterals.
Physical exercise in patients with liver cirrhosis and portal hypertension increases portal pressure and reduces hepatic blood flow and thus appears to increase risk of variceal bleeding.
Splenic artery occlusion will mask underlying portal hypertension.
A portosystemic shunt reverses gastropathy in most patients with noncirrhotic portal hypertension; in these patients it is presumably venous congestion that causes gastropathy, realizing that gastric mucosal capillary dilation does not signify portal hypertension.
Imaging
The thoracic duct caliber increases in cirrhosis. No direct relationship is apparent between the degree of portal hypertension and the caliber of this duct. The distal end of this duct in the left supraclavicular region can be visualized with US in most patients.
CT is insensitive in detecting hepatofugal flow in the main portal vein in patients with cirrhosis. On the other hand, a main portal vein diameter of <1cm is highly specific for hepatofugal flow (66).
Doppler US provides a measure of hepatic artery and portal vein pulsatility. Normally the hepatic artery has pulsatile flow, but portal venous blood is nonpulsatile with only minor cardiac and respiratory effects. A significant increase in hepatic artery pulsatility occurs in patients with end-stage liver disease. The hepatic veins, on the other hand, are dampened in cirrhosis and their appearance approaches that of the portal vein.
Detection of portosystemic collateral vessels is often used as proof for portal hypertension (Fig. 17.12). A collateral vein within the ligamentum teres is relatively common and is detectable with Doppler US. One should keep in mind, however, that in a minority of normal individuals Doppler US detects blood flow in a paraumbilical vein; velocity increases in portal hypertension and flow extends anterior to the liver surface, a finding not seen normally. Inferior vena cava dilation is also often found in patients with cirrhosis and portal hypertension.
In a setting of portal hypertension, contrastenhanced 3D MRA is currently the imaging modality of choice in evaluating portal blood flow and portal vein anatomy. Subtracting the

1008
Figure 17.12. Major pathways of portosystemic venous shunting in portal hypertension. E, esophageal varices; P, paraumbilical veins; R, rectal veins; S, splenorenal veins. Reversal of flow occurs in the coronary vein (C) and inferior mesenteric vein (I).
arterial phase from venous phase data aids in visualizing blood flow patterns and shunts using several viewing projections.
Discrepancies exist between Doppler US and contrast-enhanced MRA in assessing portal vein anatomy. Detection of portal vein patency, especially of intrahepatic portal vein branches, is more accurate with MRA. Splenorenal shunts and varices are better detected with MRA.
Portal Vein Obstruction/Thrombosis
A rare cause of portal hypertension is partial obstruction by a portal vein web or membrane. The etiology of these webs is not clear.
Clinical Aspects
Infection, neoplasm, and a hypercoagulable state are associated with portal vein thrombosis. Blood stasis due to decreased flow presumably plays a role in thrombosis developing in a setting of portal hypertension. The reverse is also true, namely, portal vein thrombosis leads to portal hypertension. At times splenic vein thrombosis extends into the portal vein.
ADVANCED IMAGING OF THE ABDOMEN
The most common cause of massive gastrointestinal bleeding in children is from portal hypertension–induced varices secondary to portal vein thrombosis. Splenomegaly is common in children with portal vein thrombosis. Their liver is normal. In most children thrombosis is considered to be idiopathic, although prior umbilical vein catheterization or omphalitis play a role. Prior umbilical sepsis, even at birth, should be suspected in the child or young adult who develops portal hypertension with no obvious underlying cause. In an interesting US study of 100 neonates with umbilical vein catheterization, clinically silent portal venous thrombosis was detected in 43% (67); follow-up US revealed complete or partial resolution in only about half, with a correlation found between initial thrombus size and subsequent clot resolution. Significant risk factors for thrombosis were catheterization for >6 days and blood transfusion.
Some of the conditions associated with portal vein thrombosis are listed in Table 17.2. Patients
Table 17.2. Conditions associated with portal vein thrombosis
Children
Idiopathic
Prior umbilical vein catheterization
Prior omphalitis
Homocystinuria
Adults Idiopathic Inflammation
Pancreatitis Ascending cholangitis Ulcerative colitis Crohn’s disease Adjacent abscess
Tuberculosis involving porta hepatis lymph nodes Primary hepatic actinomycosis
Penetrating peptic ulcer Neoplasm
Pancreatic and liver carcinomas Gastric carcinoma
Bladder carcinoma Posttherapy
After hepatocellular carcinoma therapy After splenectomy
Gastric variceal therapy Other
Hypercoagulation state Behçet’s disease Postpartum
Myeloproliferative syndromes

1009
ABDOMINAL VASCULATURE
with a hepatocellular carcinoma are prone to developing portal vein thrombosis, with the thrombus often consisting of tumor encroaching into portal vein branches rather than being nonneoplastic. Thrombi develop after hepatocellular carcinoma therapy with percutaneous ethanol injection. Fine-needle biopsy of a portal vein thrombus is useful in some patients with hepatocellular carcinoma to identify a neoplastic thrombus if results will influence patient management.
Portal, splenic, and superior mesenteric vein thrombosis is an occasional complication after splenectomy. Most of these patients are symptomatic and an occasional one even develops an acute abdomen. Some authors suspect that endoscopic variceal sclerotherapy predisposes to portal vein thrombosis, although controlled evidence is lacking.
Portal vein thrombosis should be suspected if a patient with Behçet’s disease develops splenomegaly. These patients often have cavernous transformation of the surrounding vessels. Patients with homocystinuria, a rare, inherited metabolic disease, are at risk for arterial and venous thromboemboli, including portal vein thrombosis.
In a setting of portal vein thrombosis, an anomalous insertion of the right gastric vein maintains hepatopedal blood flow if the insertion is at the portal vein bifurcation or intrahepatically (distal to the thrombus). Such an anomalous right gastric vein insertion is a pathway for TIPS placement.
Ultrasonography mass screenings can detect extrahepatic portal venous obstruction in asymptomatic patients.
Imaging
Portal vein thrombosis can be assessed with contrast-enhanced CT, gray-scale and Doppler US, contrast-enhanced MRI, and angiography (Fig. 17.13). Most benign thrombi do not widen the portal vein caliber, while a malignant thrombus often does. Also, blood flow (neovascularity) is present in about half of malignant thrombi but not in benign ones.
Contrast-enhanced CT detection of portal vein thrombosis approaches 100% by visualizing an intraluminal thrombus. These thrombi often extend into the splenic and superior mesenteric veins. Coronal or sagittal 3D recon-
struction often provides an overall view of these thrombi.
Established portal vein thrombosis often results in a hypodense liver on precontrast CT scans, presumably secondary to fat accumulation. Segmental atrophy develops in an involved segment. The proportion of blood supplied by the hepatic artery increases and as a result postcontrast liver CT shows increased enhancement during the late arterial phase and decreased enhancement during the venous phase. Segmental intrahepatic portal vein thrombi result in transient wedge-shaped parenchymal defects showing increased enhancement during the arterial phase.
Postcontrast CT reveals a benign thrombus as a tubular low-density intraluminal tumor. At times enhancing collateral vessels are evident (cavernous transformation). A thrombus in a nonoccluded portal vein is seen as a nonenhancing tumor surrounded by contrast enhanced venous blood. Mesenteric edema and mesenteric varices are evident in some patients even without a thrombus extending into the superior mesenteric vein. On a chronic basis, CT identifies a cord-like sclerotic portal vein or portal vein calcifications.
Some of these patients develop arterioportal shunts, seen as segmental intrahepatic portal vein enhancement during arterial CT phase.
Controversy surrounds the role of US. Gray-scale US reveals a portal vein thrombus as an intraluminal tumor or abnormal intraluminal echoes. A thrombus ranges from focal to diffuse. Gray-scale US does not detect an anechoic clot, and Doppler US is necessary for these.With complete extrahepatic portal thrombosis, Doppler US reveals an absent portal vein lumen except for a hyperechoic band from which no flow is detected. Usually the site of obstruction can be established. Doppler US evaluates residual blood flow in patients with partial obstruction. Ultrasonography is more problematic in differentiating a benign from a malignant portal vein thrombus in a setting of cirrhosis. Doppler US detection of pulsatile flow in the thrombus is rather specific in diagnosing a malignant thrombus but at the expense of a lower sensitivity, although published results vary considerably. In general, the presence of pulsatile arterial flow, detected by Doppler US, is assumed by some to obviate a need for percutaneous biopsy of the thrombus to estab-

1010
ADVANCED IMAGING OF THE ABDOMEN
A  B
B
C 
 D
D
Figure 17.13. Portal vein thrombosis in a man with cirrhosis. A 3D magnetic resonance angiography (MRA) coronal maximum intensity projection (A) and coronal portal venous phase image (B) identify partial portal (1), splenic (2) and superior mesenteric (3) vein thromboses. C: DSA intraarterial splenoportography shows a collateral portal vessel (arrow). D: Mesenteric portography show portal vein thrombosis (small arrow), and retrograde flow is identified in the inferior mesenteric vein (large arrow). For full evaluation both MRA and DSA were necessary in this patient. (Source: Kreft B, Strunk H, Flacke S, et al. Detection of thrombosis in the portal venous system: comparison of contrast-enhanced MR angiography with intraarterial digital subtraction angiography. Radiology 2000;216:86–92, with permission from the Radiological Society of North America.)
lish a definitive diagnosis. Continuous flow, on the other hand, is seen with both benign and malignant thrombi. With slow portal vein blood flow Doppler US reveals no flow, but no thrombus is detected by gray-scale US. In
patients with portal vein thrombosis Doppler US reveals a significantly lower mean hepatic artery resistive index than controls; such a lower resistive index is a secondary sign of portal vein thrombosis.

1011
ABDOMINAL VASCULATURE
Published studies favor MR over US. MRI detects more occlusion or encasement of smaller portal vein branches than US, although occasionally the reverse is true. In general, MRI provides additional information over US in preoperative assessment of the portal venous system and MRA achieves a sensitivity and specificity similar to intraarterial DSA in assessing portal venous system patency or thrombosis in patients with portal hypertension (68). In fact, contrast enhanced 3-D MRA is emerging as the method of choice for studying the portal venous system in patients with portal hypertension, and thus potentially could replace DSA for this application.
Flowing blood within the portal vein appears as a void on both T1and T2-weighted MR images. A thrombus appears similar to intraluminal soft tissue, although slow flow has a similar appearance (Fig. 17.14). On contrastenhanced MR the normally hyperintense intraluminal blood is replaced by a hypointense thrombus. At times enhanced collateral circula-
tion is identified. Any enhancing vessels within the thrombus suggest malignant infiltration.
Occlusion of an intrahepatic portal vein branch results in a peripheral wedge-shaped hyperintense segment on immediate contrastenhanced MR due to a compensatory increased arterial blood supply. This segment gradually becomes isointense. One should keep in mind, however, that contrast-enhanced MRI reveals a signal-intensity decrease in some right (8%) and left (9%) portal vein branches and portal vein (6%) during the equilibrium phase (69); these flow artifacts tend to mimic a portal venous thrombosis.
Portal Vein Cavernous Transformation
Cavernous transformation represents formation of venous channels either within a thrombosed portal vein or in surrounding extraperitoneal tissues. Some fresh portal vein thrombi recanalize within days, but cavernous transformation of surrounding vessels, consist-
A 
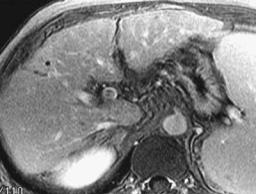 B
B
Figure 17.14. Portal vein artifact. A–C: Contrast-enhanced gradi- |
|
ent-recalled echo (GRE) MR images at equilibrium phase suggest |
|
portal vein thrombi (arrows). Postcontrast CT showed no thrombi |
|
(not shown). (Source: Nishibori H, Kanematsu M, Kondo H, Matsuo |
|
M, Hoshi H. Pseudothrombosis in the portal venous system: a |
|
potential pitfall with gadolinium-enhanced dynamic gradient- |
|
recalled echo imaging of the liver. J Magn Reson Imaging |
|
2000;12:763–768, with permission of Wiley-Liss, a subsidiary of |
C |
John Wiley & Sons.) |

1012
ing of a sponge-like mass of collateral vessels around the main portal vein, takes several weeks. Collateral circulation develops via two pathways: First, portosystemic shunting such as through a left gastric vein and these portosystemic collaterals usually imply that portal hypertension is present. Second, portoportal channels develop and extend into intrahepatic portal venous branches. Cavernous transformation is associated with such conditions as chronic pancreatitis, tumor infiltration, and intraabdominal sepsis.
In a Spanish study of patients with cavernous transformation, predisposing factors were omphalitis, echinococcal cyst, major abdominal surgery, cirrhosis, Sjögren’s syndrome, and idiopathic (70). A hydatid cyst in the porta hepatis can lead to cavernous transformation. The extent of collateralization varies considerably but tends to be more prominent with more chronic portal vein thrombi. Thus patients with portal vein thrombosis during childhood can eventually develop an extensive cavernous transformation involving adjacent structures. Many patients with congenital hepatic fibrosis have cavernous transformation of the portal vein.
Biliary veins (cystic and paracholedochal) represent an alternate blood flow pathway in cavernous portal vein transformation, and thus it is not uncommon to see numerous serpiginous extrinsic bile duct indentations. These bile duct varices can compress the extrahepatic bile ducts to the point of inducing biliary obstruction.
Of interest is that in most patients with cavernous transformation of the portal vein the pancreatic duct is smaller than normal, presumably due to pancreatic venous congestion.
In patients with cavernous transformation of the portal vein confirmed by angiography, color Doppler US detected the transformation in 93%, B-mode US in 64%, and contrast-enhanced CT in 50% of patients (71); color Doppler US was superior to B-mode US in identifying collateral channels.
Therapy
Treatment with heparin alone can result in recanalization of acute portal venous thrombosis. A number of case reports suggest that either intraportal plasminogen infusion or such therapy as plasminogen activator and urokinase
ADVANCED IMAGING OF THE ABDOMEN
through a transjugular intrahepatic catheter approach to the portal vein have dissolved thrombi.
Transjugular intrahepatic portosystemic shunting and thrombolysis appear to be viable therapy in patients with noncavernomatous portal vein thrombosis in order to increase portal output and restore portal blood flow; patent shunts can be achieved, although those with an initial complete thrombosis require more frequent shunt revisions than those with incomplete obstruction. Some of these patients continue to be symptomatic despite a functioning TIPS.
Few surgical options exist for chronic portal vein thrombosis. Lysis and thrombus aspiration, portal vein stenting, and TIPS are potential options.
Collateral Veins
Portosystemic Shunts
Extrahepatic portosystemic shunts are rare in absence of portal hypertension and in such a setting a congenital shunt origin is likely. An extrahepatic shunt, together with intrahepatic portal venous hypoplasia, generally implies a congenital basis. Some of these shunts in patients without portal hypertension can be rather large. Some are associated with hepatic encephalopathy and after successful shunt embolization encephalopathy clears.
In portal hypertension, for significant collaterals to open, the portocaval pressure gradient needs to be >12mmHg. Initially, as develops, the portal vein caliber increases, but the caliber tends to decrease once shunts start to form.
Traditionally conventional angiographic portography was used to evaluate portosystemic shunts, but 3D helical CT portography appears to be equal to and at times even superior to angiography (72). Results of CT portography can be used to plan therapy.
Esophagogastric Shunts: Esophageal and gastric fundal varices represent the most common spontaneous portosystemic shunts. A common pathway is from the portal vein, through the left gastric vein (also called the coronary vein) and into the gastroesophageal veins (varices). A common drainage path for these paraesophageal varices is via veins around the aorta into the hemiazygous vein. An alternate path is via a vein located anterior to the

1013
ABDOMINAL VASCULATURE
inferior vena cava (precaval vein) and into the inferior vena cava.
Doppler US in the presence of these shunts reveals reversal of flow in the left gastric vein. A parallel pathway is from the splenic vein via the short gastric veins,which tend to dilate. Imaging detection of a dilated coronary vein implies either portal hypertension or splenic vein obstruction. The latter condition, of course, does not result in esophageal varices.
Splenomegaly is common in portal hypertension and the presence of esophageal varices, but little or no correlation exists between splenic size and size of esophageal varices.
Therapy and the resultant imaging of esophagogastric shunts are discussed in Chapters 1 and 2.
Intrahepatic Shunts: Spontaneous intrahepatic portal vein-to-systemic vein shunts are rare in a nondiseased liver. If present, aside from paraumbilical veins, they do not necessarily imply that portal hypertension is present; these shunts develop in either the right or left lobe and consist of either a single dilated vessel or multiple small veins. In the liver the left portal vein forms anastomoses with veins in the ligamentum terres and ligamentum venosum.
Gray-scale US reveals shunts as typical anechoic structures. Color Doppler US establishes flow patterns, such as bidirectional flow. Some shunts have a continuous flat portal vein flow pattern in both the shunt and the related hepatic vein. Computed tomography and MRI simply show abnormal vascular channels.
Patients with large intrahepatic portosystemic venous shunts develop hepatic encephalopathy. In symptomatic patients these shunts can be embolized using various coils and detachable balloons (73); retrograde transcaval obliteration is least invasive in treating simple portosystemic venous shunts.
Paraumbilical Collaterals: Paraumbilical veins are relatively common collaterals (Fig. 17.15). Rather than a single vein, it is usually a collection of veins close to the original obliterated paraumbilical vein. Located in the ligamentum teres, it connects the left portal vein and systemic abdominal wall collaterals and, when present, it is detected by US and the flow is established with Doppler US. Spontaneous formation of dilated umbilical veins should suggest portal hypertension (CruveilhierBaumgarten syndrome). Some patients develop a venous hum and a caput medusae. Occasion-
Figure 17.15. Effect of shunting via the paraumbilical vein (P). Hepatopetal portal vein (PV) flow is still maintained, but flow is reversed in the intrahepatic portal vein branches.
ally esophageal variceal sclerotherapy in a patient with idiopathic portal hypertension leads to portal vein thrombosis at the origin of the umbilical vein and the disappearance of the venous hum and dilated abdominal wall veins characteristic of Cruveilhier-Baumgarten syndrome.
Why some patients develop markedly dilated paraumbilical veins rather than other collaterals is not known. In general, those having hepatofugal paraumbilical flow greater than hepatopetal portal vein flow tend not to develop esophageal varices. Turbulent flow is identified in some patients.
A dilated paraumbilical vein most often drains via the inferior epigastric vein into the external iliac vein. Less common is drainage via the superficial epigastric vein into either the internal thoracic vein or saphenous vein, pathways identifiable with color Doppler US.
Other Collaterals: The azygos vein, which drains esophageal varices, dilates in a setting of portal hypertension and esophagogastric varices. Endoscopic US visualizes this vein; its caliber is increased and maximal azygous vein blood velocity is greater in patients with varices compared to controls. An occasional large splenic vein-to-azygos vein shunt is detected in a noncirrhotic patient, presumably on a congenital basis. Similarly, spontaneous left gastric vein to left renal vein shunts can occur, often in a portal hypertension setting.

1014
Less often encountered are gastroepiploic and splenorenal collaterals and shunting from the inferior mesenteric vein to the inferior hemorrhoidal veins (Fig. 17.16). A spontaneous shunt involving the right renal vein is rare. Likewise, portosystemic collaterals through duodenal and other extraperitoneal structures are uncommon.
Colonic variceal bleeding is rare, but at times is massive. Several studies suggest that colonic varices tend to become more prominent after successful sclerotherapy of esophageal varices and the resultant obliteration of coronaryazygous venous anastomoses.
The prevalence of mesenteric varices in portal hypertension is not known. Rupture of a mesenteric varix is a rare cause of hemoperitoneum. In patients with ascites and mesenteric varices, bleeding into the peritoneal cavity can follow large-volume paracentesis, possibly induced by the sudden decrease in intraperitoneal pressure due to fluid withdrawal.
In general, MRI detects more varices in more patients than does US.
In addition to endoscopic obliteration of esophagogastric varices (by sclerotherapy and other procedures), portosystemic shunts can be embolized. Such embolization generally helps control hepatic encephalopathy after the failure of medical management. In a setting of a large portosystemic shunt with considerable superior mesenteric venous blood flowing through the shunt, shunt obliteration tends to improve liver function.
ADVANCED IMAGING OF THE ABDOMEN
Therapy of Portal Hypertension
Medical Therapy
In a patient with cirrhosis who bled, what are the relative roles of propranolol and sclerotherapy in preventing rebleeding and on survival? A meta-analysis of nine randomized trials concluded that the mean percentage of patients free of variceal rebleeding was 39% in the propranolol group and 55% in the sclerotherapy group, but that adverse events were higher in the sclerotherapy group (74); the mean survival rate, however, did not differ significantly. In this subgroup of patients, although sclerotherapy is more effective than propranolol in preventing variceal rebleeding, the authors suggest that propranolol is the preferred therapy for preventing rebleeding.
Beta-blocking agents reduce portal venous pressure in cirrhotic patients, although the results are somewhat idiosyncratic. The goal is to reduce the portal pressure gradient below 12 mmHg. Beta-blockers do not achieve such pressure reductions in some patients, and their use is thus limited as prophylactic agents. A reduction in portal pressure appears to be potentiated by combining beta-blockers and isosorbide-5- mononitrate.
Both flow velocity and pulsatility index are obtained with Doppler US, although US is of limited value in discriminating good from poor responders to medical therapy (61).
Rectal Tc-99m–pertechnetate scintigraphy of collateral blood flow from the inferior mesen-
A 
 B
B
Figure 17.16. Retroperitoneal shunt. A: Postcontrast CT shows tortuous, dilated vessels adjacent to left kidney (arrows). B: Coronal maximum intensity projection CT portal venography detects a tortuous shunt (arrows) communicating with left renal vein. (Source: Kang HK, Jeong YY, Choi JH, et al. Three-D multi-detector row ct portal venography in evaluation of portosystemic collateral vessels in liver cirrhosis. RadioGraphics 2002;22:1053–1061, with permission from the Radiological Society of North America.)
