
Книги по МРТ КТ на английском языке / Advanced Imaging of the Abdomen - Jovitas Skucas
.pdf
1035
ABDOMINAL VASCULATURE
A retrospective study of renovascular hypertension patients who underwent percutaneous transluminal renal angioplasty, percutaneous transluminal renal artery stent placement, or renal arterial bypass grafting found similar procedure success rates (91% to 98%), but complication rates were 13% for angioplasty, 16% for stent placement, and 38% for bypass grafting (114); of interest is that the number of antihypertensive medications returned to baseline in all patients by 12 months. In the Dutch Renal Artery Stenosis Intervention Cooperative Study Group, patients with hypertension and atherosclerotic renal artery stenosis were randomly assigned to either undergo percutaneous transluminal renal angioplasty or receive drug therapy (115); 3 months later no significant differences were evident in either systolic or diastolic blood pressures in the two groups.
Surgical Bypass
The initial therapy for renal artery stenosis consisted of aortorenal bypass surgery, which then was replaced by hepatorenal, splenorenal and other bypass, patch angioplasty, and renal artery endarterectomy. Retrospective surgical studies from the late 1980s and 1990s established standards for postoperative 5-year survival of 52% to 65% or higher and improvements in hypertension and renal insufficiency, and most current authors compare
their angioplasty and stenting results against these standards. These comparisons are difficult to place in proper perspective because surgical studies tended to contain “sicker” patients with their correspondingly greater morbidity and mortality. Currently surgical therapy in many institutions is limited to a few specific indications, including failed angioplasty and stenting.
Angioplasty
Percutaneous transluminal revascularization consists of balloon angioplasty, at times supplemented by stenting. If necessary in high-risk patients, carbon dioxide is used as a primary intravascular contrast agent during angioplasty, supplemental when necessary by iodinated contrast.
Angiography is generally used initially to evaluate the success of angioplasty; Doppler US is used by some investigators to assess patency during subsequent follow-up. Roughly half of patients with underlying fibromuscular dysplasia who undergo successful angioplasty are cured and most of the rest are improved, results far superior to those achieved in patients with atherosclerotic disease (Fig. 17.27).
Percutaneous angioplasty appears less appropriate for the relatively uncommon ostial stenosis. A randomized, prospective study compared transluminal angioplasty alone and angioplasty
A  B
B
Figure 17.27. Fibromuscular dysplasia in a young woman with severe hypertension. A: Renal arteriogram reveals an irregular, segmented right renal artery stenosis (arrow). B: Arteriogram after angioplasty shows an essentially normal renal artery. (Courtesy of Oscar Gutierrez, M.D., University of Chile, Santiago, Chile.)

1036
with stent placement in patients with ostial atherosclerotic renal artery stenosis (116); the primary success rate, defined as a <50% residual stenosis, of angioplasty alone was 57% compared with 88% for stenting. The 6-month postprocedure primary patency rate was 29% for angioplasty alone, and 71% for stenting. Among patients undergoing angioplasty alone, 29% required secondary stenting for angioplasty failure, with these patients then having a success rate similar to those with primary stenting. The authors concluded that primary stenting is a superior procedure for patients with ostial stenosis.
Stenoses can be dilated in patients with renovascular hypertension due to nonspecific aortoarteritis (Takayasu’s disease); the restenosis rate, evident by recurrence of hypertension and restenosis, found at angiography, was 16% at 22 months (117).
Complete renal artery occlusions have been successfully revascularized. Sufficient data are not available to draw firm conclusions about angioplasty for this indication.
Angioplasty complications include renal artery dissection and rupture, embolization of atheromatous fragments, and artery thrombosis. Even including complications due to femoral angiography, angioplasty is associated with fewer complications than surgical correction.
Doppler US detection of residual renal artery stenosis one day after percutaneous revascularization of atherosclerotic renal arteries appears to be a predictor for future restenosis (118).
Stenting
Currently, percutaneously implanted renal artery stents have a role in stenoses that are not amenable to angioplasty, those due to failed therapy, and in a setting of ostial stenosis. Percutaneous renal artery stent insertion has been performed for renal artery involvement in aortic dissection (119). Problems with dissection and residual stenosis are largely overcome with stenting. A review of published studies of stent placement up to 1998, comparing results of renal arterial stent placement and renal percutaneous transluminal angioplasty, concluded that stenting is technically superior and clinically comparable to angioplasty (120); stenting achieves a lower restenosis rate (17%) than
ADVANCED IMAGING OF THE ABDOMEN
angioplasty (26%). The complication rates are similar, but stenting results in fewer patients with a postprocedure residual stenosis. The importance of a postprocedure residual stenosis is its association with a significantly higher rate of eventual restenosis.
In a follow-up study of consecutive patients, renal arterial stent placement did not significantly improve primary patency of proximal and truncal renal arterial stenoses over that achieved by balloon percutaneous transluminal angioplasty (121); stents did, however, improve patency of ostial stenoses.
In general, stenting for angioplasty failure does relieve stenosis, but hypertension is cured only in half or fewer patients; likewise, arrest of clinical renal failure occurs only in about half or so of patients after stenting.
Renal artery stent obstruction can be evaluated with contrast enhanced MRI.
Acute complications of renal artery stent placement include renal artery thrombosis, renal artery emboli, cholesterol embolization to lower limbs, and femoral hematoma.
Renal Ablation/Embolization
Occasionally percutaneous transcatheter renal ablation is considered in patients with uncontrolled hypertension or nephrotic syndrome. A long-term improvement in hypertension and nephrotic syndrome can be achieved with this procedure, often with a lower morbidity and mortality than for comparable surgical nephrectomy.
Transcatheter embolization should be considered in the hypertensive patient with an accessory renal artery or a branch artery stenosis not amenable to usual therapy and if the involved artery supplies only a small renal segment. Eventual scarring and some loss of renal function will occur.
Renal Artery Aneurysm
Renal artery aneurysms can be subdivided into true aneurysms,dissecting aneurysms,arteritisrelated aneurysms, and simply aneurysmal dilatation. True aneurysms are saccular, extraparenchymal, and located at bifurcations.

1037
ABDOMINAL VASCULATURE
Several etiologies account for these aneurysms: elastic tissue degeneration, possibly on a congenital basis, and atherosclerosis predominate, with an occasional one being secondary to trauma. The prevalence of renal artery aneurysms increases with age, with only a rare one found in children. They are a rare cause of hypertension; in some patients an aneurysm manifests with pain or hematuria, although most patients are asymptomatic.
Small intrarenal aneurysms develop in patients with polyarteritis nodosa, Wegener’s granulomatosis, and lupus erythematosus. Dilated renal arteries and aneurysms occur in Behçet’s disease.
An occasional true aneurysm ruptures, at times resulting in a loss of that kidney. Similar to rupture of a splenic artery aneurysm, rupture of a renal artery aneurysm during pregnancy carries a high maternal and fetal mortality. Repair of a known aneurysm should be considered prior to conception. Rupture is not necessarily related to hypertension. Calcifications in an aneurysm do not protect against rupture. Some ruptures evolve into an arteriovenous fistula.
Ring-like calcifications are detected with conventional radiography in less than a third of renal artery aneurysms.
Renal artery aneurysms are readily detected with arteriography. Most are also identified during arterial phase helical CT; precontrast and delayed postcontrast CT images show an isodense mass similar to a neoplasm. A majority are saccular in shape, with a minority fusiform or dissecting. Imaging also detects an occasional thrombosed aneurysm.
Some renal artery aneurysms dissect spontaneously or dissect secondary to trauma, including catheter manipulation. A minority of dissections occur bilaterally. Patients with a dissecting aneurysm can develop hypertension.
Arteriography should be diagnostic of a dissecting aneurysm. At times urography reveals delayed contrast excretion, although often this study is normal.
Renal artery aneurysms have been treated with an ex vivo surgical technique and autotransplantation. Of note is that follow-up of aneurysmectomy reveals a better cure rate of hypertension than in patients with renal artery stenosis. Before ascribing hypertension to an
aneurysm, however, a stenosis in another artery should be excluded.
Renal Vein Occlusion
Adults
Renal vein thrombosis is often associated with an underlying renal cell carcinoma or inferior vena caval thrombosis. Less common factors are dehydration, coagulopathy, nephrotic syndrome, pancreatitis and other retroperitoneal inflammation, systemic lupus erythematosus, intrinsic renal disorders such as glomerulonephritis, and as an occasional complication of acute pyelonephritis. The prevalence of thrombosis is sufficiently high in nephrotic syndrome to warrant screening these patients for renal vein thrombosis.
Few symptoms are evident in adults if thrombosis develops gradually. On a chronic basis collaterals (sometimes called renal varices) develop, the kidney is no longer enlarged, and venous flow returns to the renal hilum. A complication of renal vein thrombosis is pulmonary emboli.
Renal vein thrombosis with the thrombus extending into the inferior vena cava can be misdiagnosed as a renal cell carcinoma. Precontrast CT of a renal vein thrombus revealed a hyperdense tumor.
With acute thrombosis, contrast-enhanced CT detects renal enlargement and a thrombuscontaining enlarged renal vein. The venous wall tends to enhance with contrast. Corticomedullary differentiation is prolonged and a nephrogram delayed. Extraperitoneal hemorrhage is not an uncommon association.
Ultrasonography detects an enlarged, hypoechoic kidney. Initially a thrombus is anechoic. A thrombus is better detected in the shorter right renal vein than in the left. Doppler US findings have been disappointing. With some thrombi, Doppler US does not detect renal vein flow, although often the obstruction is incomplete and flow is present, albeit slow. Normal arterial Doppler US does not exclude venous thrombosis.
Scant literature exists on the use of MRI for renal vein thrombosis, although based on results from other vascular sites MR should be well suited in this setting.

1038
ADVANCED IMAGING OF THE ABDOMEN
Neonates
Predisposing factors for renal vein thrombosis in the neonate are dehydration, sepsis, maternal diabetes, polycythemia, and umbilical vein catheter manipulation and the rare hereditary thrombophilia. Thrombosis can be limited to small renal veins or extend into the main renal vein or even inferior vena cava. Clinically, these neonates develop hematuria or hypertension.
Gray-scale US is usually the first imaging modality employed. A transient early finding of an intrarenal vein thrombus is a linear echogenic structure. With sufficiently extensive involvement the affected kidney enlarges and assumes a heterogenous appearance. Ultrasonography should also detect any associated vena caval thrombus. Some neonates also have adrenal hemorrhage. Color Doppler US suggests a lack of flow in the intrarenal veins and reversal of arterial diastolic flow. Contrast enhanced MRI reveals persistent cortical enhancement and a lack of medullary enhancement.
Sequelae of renal vein thrombosis in a neonate range from an atrophic kidney, to focal scarring, to the kidney reverting to an essentially normal appearance.
Renal and Ureteric Varices
Not all venous engorgement is due to renal vein obstruction. Portal hypertension has led to intrarenal segmental venous engorgement (renal varices). Rarely, intrarenal varices are idiopathic. Renal varices can lead to recurrent hematuria.
Computed tomography detects tubular contrast-enhancing perineal structures. Color Doppler US should detect these varices. Selective renal phlebography is diagnostic. Of note is that renal arteriography can miss these venous structures.
Ureteric varices are uncommon. Occasionally engorged gonadal veins lead to extrinsic compression and result in a characteristic serpiginous appearance. Somewhat similar findings are seen with dilation of a ureteral artery, generally in association with renal artery stenosis where the ureteral artery provides a collateral blood supply to the kidney.
Vascular Fistulas
A fistula involving the aorta can be either aortovascular or aortoenteric. Fistulas associated with aortic aneurysms were discussed earlier (see Aorta),as were portosystemic shunts and fistulas (see Collateral Veins).
Aortocaval Fistula
Most aortocaval fistulas are related to aortic aneurysms and resultant surgical correction. These patients tend to be elderly and in poor health. A rare malignant fibrous histiocytoma at the aortic bifurcation led to a pseudoaneurysm which evolved into an iliocaval fistula (122).
In a patient with oliguric renal failure and a suspected aortocaval fistula, angiography using carbon dioxide is an alternate diagnostic test. Aortocaval fistulas have been embolized with coils, cyanoacrylate, and other materials.
Aortoenteric Fistulas
The most common cause of an aortoesophageal fistula is a thoracic aortic aneurysm. Esophageal perforation by an ingested foreign body is less common.
Most aortoduodenal fistulas develop in a setting of prior aortic aneurysm repair. Some are related to prior radiotherapy to this region. Rarely, duodenal tuberculosis or even a brucellar aortic aneurysm evolves into an aortoduodenal fistula.
A fistulous tract between an aortic graft and duodenum can be detected by CTA. If performed while the patient is bleeding, a temporal increase occurs in duodenal contrast agent accumulation (123).
Cavoenteric Fistulas
Most cavoenteric fistulas are secondary to trauma, tumor invasion, or prior radiotherapy. Thus a cavo-duodenal fistula developed after a right nephrectomy and radiotherapy for a urothelial tumor 20 months earlier (124). Anecdotal reports describe migrating caval filters resulting in duodenal perforation and a caroduodenal fistula (124).

1039
ABDOMINAL VASCULATURE
Arterioportal Fistula
Clinically, arterioportal fistulas manifest through gastrointestinal bleeding, ascites, heart failure, or even diarrhea, findings induced by resultant portal hypertension, ischemia, or vascular erosions.
Dynamic MRA reveals arterioportal shunts as early focal regions of contrast enhancement (Fig. 17.28). Splenic arteriography is diagnostic.
Treatment of these fistulas is not clear. Some believe that embolization is the therapy of choice for acquired intrahepatic arterioportal fistulas but that extrahepatic ones should be corrected surgically. Successful transarterial embolization using coils and detachable balloons has been performed for these often highflow fistulas (Fig. 17.29).
Arteriovenous Fistula
An arteriovenous fistula is an abnormal communication that bypasses the usual capillary circulation.A congenital fistula usually has multiple connections between an artery and vein. Most acquired fistulas are traumatic in origin. Tumors eroding into vessels also result in fistulas. The most common acquired origin is from the hepatic artery.
Splenic arteriovenous fistulas are either congenital or acquired. The less common congenital ones tend to be intrasplenic in location and their imaging appearance mimics a hemangioma. Acquired fistulas are often associated
with atherosclerosis and trauma; an occasional one is related to pregnancy. They are identified by duplex Doppler US and arteriography.
Renal arteriovenous fistulas range from congenital to acquired. Some of these congenital fistulas do not manifest until adulthood. Among acquired ones are those secondary to prior vascular instrumentation or surgical manipulation, although many are idiopathic. Gross hematuria is the usual presentation with a renal fistula. Hypertension develops in some patients. Larger fistulas are associated with an enlarged feeding artery and draining vein. Loss of adjacent renal parenchyma and a resultant scar are evident with some fistulas. Similar to other sites, renal arteriovenous fistulas are detected by Doppler US. Renal arteriography defines the extent of a fistula and allows selective embolization.
At times scintigraphy of a vascular fistula suggests a hypervascular neoplasm.
Many congenital arteriovenous fistulas are difficult to treat with surgery, and recurrence is common.
Arterioureteral fistula
Arterioureteric fistulas are rare. Most are complications of vascular surgery, with atherosclerotic disease, chronic ureteral stenting or prior pelvic radiotherapy occasionally having a role. Clinical presentation consists of hematuria, often intermittent, ranging from mild to massive bleeding. Most aortoureteric fistulas are related to aortic prosthetic graft infections.
A  B
B
Figure 17.28. Hepatic artery to portal vein fistula. Early (A) and later (B) phase hepatic arteriograms reveal early filling of the portal vein (arrows). (Courtesy of David Lee, M.D., University of Rochester.)

1040
ADVANCED IMAGING OF THE ABDOMEN
A  B
B
C 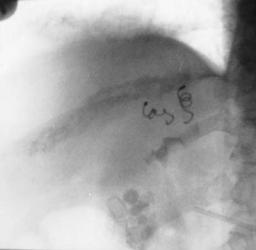
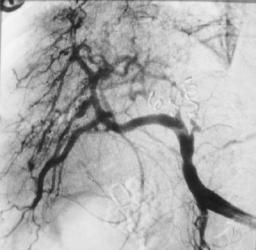 D
D
Figure 17.29. Congenital intrahepatic arterioportal fistula in a patient with hypertension. A,B: Hepatic arteriogram reveals portal vein filling (arrow) during arterial phase. C: Left and midright hepatic branches supplying the shunt are embolized with coil. D: Postembolization arteriogram reveals blocked arterial branches and no portal vein filling. (Courtesy of Oscar Gutierrez, M.D., University of Chile, Santiago, Chile.)
In the appropriate clinical setting, arteriography will define these fistulas, but some are initially not detected due to their intermittent bleeding. Some are associated with a pseudoaneurysm. Excretory urography and retrograde ureterography may simply show blood clots. Surgery is difficult in these patients. An empiric nephrectomy is considered in some patients with life-threatening bleeding, but it will miss a more distal site of bleeding. Arterial emboliza-
tion and endovascular stenting are immediate therapeutic options.
Arteriovenous Malformations
Most mesenteric arteriovenous malformations are a variant of an arterioportal fistula.
Angiography detects the larger arteriovenous malformations. Many are in the pelvis and present difficult management problems. Surgi-

1041
ABDOMINAL VASCULATURE
cal excision often is not feasible, while percutaneous embolization tends to be incomplete. Even if embolization is initially successful, invariably new channels develop and the malformation recurs. Ischemia of underlying vital structures is a complication.
Examination and
Surgical Complications
One of the complications of transcatheter arterial embolization is iatrogenic dissection of the involved artery and its branches. For example, using the celiac artery to embolize hepatocellular carcinomas, the two most common sites of dissection are the celiac artery and proper hepatic artery. Sequelae of these dissections range from complete vessel recanalization, to stenosis, to complete obstruction.
Inferior vena caval laceration and dissection occur secondary to passage of various catheters and wires.
Temporary bacteremia occurs during angiographic procedures; prevalence is greater during angioplasties than during diagnostic arteriographies.
References
1.Ernst O, Asnar V, Sergent G, et al. Comparing contrastenhanced breath-hold MR angiography and conventional angiography in the evaluation of mesenteric circulation. AJR 2000;174:433–439.
2.Lin J, Zhou KR, Chen ZW, Wang JH, Yan ZP, Wang YX. 3D contrast-enhanced MR portography and direct X-ray portography: a correlation study. Eur Radiol 2003;13:1277–1285.
3.Alfke H, Ishaque N, Froelich JJ, Klose KJ. [Magnetic resonance phlebography (MRP) of the abdomen and pelvis.] [German] Radiologe 1998;38:591–596.
4.Shiomi S, Sasaki N, Habu D, et al. Natural course of portal hemodynamics in patients with chronic liver diseases, evaluated by per-rectal portal scintigraphy with Tc-99m pertechnetate. J Gastroenterol 1998;3: 517–522.
5.Gattoni F, Dova S, Tonolini M, Uslenghi CM. [Study of the liver and the portal venous system with digital rotational angiography.] [Italian] Radiol Med 2001; 101:118–124.
6.Spinosa DJ, Matsumoto AH, Angle JF, et al. Safety of CO(2)- and gadodiamide-enhanced angiography for the evaluation and percutaneous treatment of renal artery stenosis in patients with chronic renal insufficiency. AJR 2001;176:1305–1311.
7.Nyman U, Elmståhl B, Leander P, Nilsson M, Golman K, Almén T. Gadolinium contrast media for DSA in Azotemia. Acta Radiol 2002;9(suppl. 2):S528– S530.
8.American College of Radiology: Manual on Contrast Media, 4th ed., Reston, Virginia, 1998.
9.European Society of Urogenital Radiology: Guidelines on Contrast Media, Version 4.0, available at www.esur.org, 2004.
10.Caridi JG, Hawkins IF Jr, Cho K, et al. CO2 splenoportography: preliminary results. AJR 2003;180:1375– 1378.
11.Quek SC, Tan L, Quek ST, Yip W, Aw M, Quak SH. Abdominal coarctation and Alagille syndrome. Pediatrics 2000;106:E9.
12.Schmid MR, Pfammatter T. Agenesis of the hepatic segment of the inferior vena cava with portal continuation. AJR 2001;177:120–122.
13.Nonent M, Larroche P, Forlodou P, Senecail B. Celiacbimesenteric trunk: anatomic and radiologic descrip- tion—case report. Radiology 2001;220:489–491.
14.Puig S, Stuhlinger HG, Domanovits H, et al. Posterior “Nutcracker” phenomenon in a patient with abdominal aortic aneurysm. Eur Radiol 2002;12 Suppl 3:S133– S135.
15.Brown MA, Hauschildt JP, Casola G, Gosink BB, Hoyt DB. Intravascular gas as an incidental finding at US after blunt abdominal trauma. Radiology 1999;210: 405–408.
16.Wong H, Gotway MB, Sasson AD, Jeffrey RB. Periaortic hematoma at diaphragmatic crura at helical CT: sign of blunt aortic injury in patients with mediastinal hematoma. Radiology 2004;231:185–189.
17.Serfaty JM, Chaabane L, Tabib A, Chevallier JM, Briguet A, Douek PC. Atherosclerotic plaques: classification and characterization with T2–weighted high-spatial- resolution MR imaging—an in vitro study. Radiology 2001;219:403–410.
18.Therasse E, Cote G, Oliva VL, et al. Infrarenal aortic stenosis: value of stent placement after percutaneous transluminal angioplasty failure. Radiology 2001;219: 655–662.
19.Biederer J, Link J, Steffens JC, Fronius M, Heller M. [Contrast media-enhanced 3D MR angiography before endovascular treatment of aneurysm in the abdominal aorta, iliac artery and peripheral vessels.] [German] Rofo Fortschr Geb Rontgenstr Neuen Bildgeb Verfahr 2000;172:985–991.
20.Macari M, Israel GM, Berman P, et al. Infrarenal abdominal aortic aneurysms at multi-detector row CT angiography: intravascular enhancement without a timing acquisition. Radiology 2001;220:519–523.
21.LePage MA, Quint LE, Sonnad SS, Deeb GM, Williams DM. Aortic dissection: CT features that distinguish true lumen from false lumen. AJR 2001;177:207– 211.
22Hansmann HJ, Dobert N, Kucherer H, Richter GM. [Various spiral CT protocols and their significance in the diagnosis of aortic dissections: results of a prospective study.] [German] Rofo Fortschr Geb Rontgenstr Neuen Bildgeb Verfahr 2000;172:879–887.
23.Quint LE, Williams DM, Francis IR, et al. Ulcerlike lesions of the aorta: imaging features and natural history. Radiology 2001;218:719–723.

1042
ADVANCED IMAGING OF THE ABDOMEN
24.Akkersdijk GJ, van Bockel JH. Ruptured abdominal aortic aneurysm: initial misdiagnosis and the effect on treatment. Eur J Surg 1998;164:29–34.
25.Kapadia BJ, Agarwal M, de Silva RD. Primary aortoduodenal fistulas in minimally aneurysmal aortas: imaging diagnosis. Abdom Imaging 2000;25:51–54.
26.Chuter TA, Gordon RL, Reilly LM, et al. Abdominal aortic aneurysm in high-risk patients: shortto inter- mediate-term results of endovascular repair. Radiology 1999;210:361–365.
27.Engellau L, Olsrud J, Brockstedt S, et al. MR evaluation ex vivo and in vivo of a covered stent-graft for abdominal aortic aneurysms: ferromagnetism, heating, artifacts, and velocity mapping. J Magn Reson Imaging 2000;12:112–121.
28.Lenhart M,Volk M, Manke C, et al. Stent appearance at contrast-enhanced MR angiography: in vitro examination with 14 stents. Radiology 2000;217:173–178.
29.Cejna M, Loewe C, Schoder M, et al. MR angiography vs CT angiography in the follow-up of nitinol stent grafts in endoluminally treated aortic aneurysms. Eur Radiol 2002;12:2443–2450.
30.Gorich J,Rilinger N,Sokiranski R,et al. Endoleaks after endovascular repair of aortic aneurysm: are they predictable? Initial results. Radiology 2001;218:477–480.
31.Fan CM, Rafferty EA, Geller SC, et al. Endovascular stent-graft in abdominal aortic aneurysms: the relationship between patent vessels that arise from the aneurysmal sac and early endoleak. Radiology 2001; 218:176–182.
32.Armerding MD, Rubin GD, Beaulieu CF, et al. Aortic aneurysmal disease: assessment of stent-graft treat- ment-CT versus conventional angiography. Radiology 2000;215:138–146.
33.Napoli V, Bargellini I, Sardella SG, et al. Abdominal aortic aneurysm: contrast-enhanced US for missed endoleaks after endoluminal repair. Radiology 2004;233:217–225.
34.Baum RA, Carpenter JP, Tuite CM, et al. Diagnosis and treatment of inferior mesenteric arterial endoleaks after endovascular repair of abdominal aortic aneurysms. Radiology 2000;215:409–413.
35.Clemens L, Bernardini S, Chabannes E, Debiere F, Bittard H. [Ureteral lesions after surgery of the aortic bifurcation. Report of 6 cases.] [French] Prog Urol 2000;10:1156–1160.
36.Simons PC, van Overhagen H, Bruijninckx CM, Kropman RF, Kuijpers KC. Periaortitis with ureteral obstruction after endovascular repair of an abdominal aortic aneurysm. AJR 2002;179:118–120.
37.Kluge A, Bachmann G,Weimar B, Rau WS. Inconspicuous path of an aortic bypass straight through the urinary bladder. AJR 1999;173:246.
38.Sueyoshi E, Sakamoto I, Hayashi K. Aortic aneurysms in patients with Takayasu’s arteritis: CT evaluation. AJR 2000;175:1727–1733.
39.Men S, Yucesoy C, Edguer TR, Hekimoglu B. Intraaortic growth of hydatid cysts causing occlusion of the aorta and of both iliac arteries: case report. Radiology 1999;213:192–1944.
40.Ruehm SG, Weishaupt D, Debatin JF. Contrastenhanced MR angiography in patients with aortic occlusion (Leriche syndrome). J Magn Reson Imaging 2000;11:401–410.
41.Wildberger JE, Schmitz-Rode T, Reffelmann T, Siewert E, Hubner D, Gunther RW. [Percutaneous transjugular thrombectomy in iliocaval thrombosis—initial experience with a newly developed 12F balloon sheath.] [German] Rofo Fortschr Geb Rontgenstr Neuen Bildgeb Verfahr 2000;172:651–655.
42.Le Bouedec G, Bailly C, Penault-Llorca F, Fonck Y, Dauplat J. [Intravascular leiomyomatosis of uterine origin. A case of pseudo-metastatic cavo-cardial thrombus.] [Review] [French] Presse Med 1999;28: 1463–1465.
43.Mingoli A, Sapienza P, Cavallaro A, et al. The effect of extent of caval resection in the treatment of inferior vena cava leiomyosarcoma. Anticancer Res 1997;17: 3877–3881.
44.Kelekis NL, Semelka RC, Hill ML, Meyers DC, Molina PL. Malignant fibrous histiocytoma of the inferior vena cava: appearances on contrast-enhanced spiral CT and MRI. Abdom Imaging 1996;21:461–463.
45.Gibo M, Murata S, Kuroki S. Pericaval fat collection mimicking an intracaval lesion on CT in patients with chronic liver disease. Abdom Imaging 2001;26:492– 495.
46.Dewald CL, Jensen CC, Park YH, et al.Vena cavography with CO(2) versus with iodinated contrast material for inferior vena cava filter placement: a prospective evaluation. Radiology 2000;216:752–757.
47.Savin MA, Panicker HK, Sadiq S, Albeer YA, Olson RE. Placement of vena cava filters: factors affecting technical success and immediate complications. AJR 2002;179:597–602.
48.Athanasoulis CA, Kaufman JA, Halpern EF, Waltman AC, Geller SC, Fan CM. Inferior vena caval filters: review of a 26–year single-center clinical experience. Radiology 2000;216:54–66.
49.Ha HK, Lee HJ, Yang SK, et al. Intestinal Behçet syndrome: CT features of patients with and patients without complications. Radiology 1998;209:449–454.
50.Park JH, Chung JW, Joh JH, et al. Aortic and arterial aneurysms in Behçet disease: management with stent- grafts—initial experience. Radiology 2001;220:745– 750.
51.Byun JY, Ha HK, Yu SY, et al. Computed tomography features of systemic lupus erythematosus in patients with acute abdominal pain: emphasis on ischemic bowel disease. Radiology 1999;211:203–209.
52.Kaushik S, Federle MP, Schur PH, Krishnan M, Silverman SG, Ros PR. Abdominal thrombotic and ischemic manifestations of the antiphospholipid antibody syndrome: CT findings in 42 patients. Radiology 2001;218:768–771.
53.Riddell AM, Khalili K. Sequential adrenal infarction without MRI-detectable hemorrhage in primary antiphospholipid-antibody syndrome. AJR 2004;183: 220–222.
54.Sharma MC, Deshpande V, Sharma R, Pal S, Sahni P. Giant cell phlebitis as a cause of large intestinal stricture. J Clin Gastroenterol 1998;27:79–81.
55.Lancrenon C, Delacour JL, Floriot C, et al. [Intestinal perforation due to cholesterol crystal embolism.] [French] Presse Med 2000;29:1982–1983.
56.Rand T, Weninger M, Kohlhauser C, et al. Effects of umbilical arterial catheterization on mesenteric hemodynamics. Pediatr Radiol 1996;26:435–438.

1043
ABDOMINAL VASCULATURE
57.Heiss U, Helms A, Tietje H, Mundinger A. [Aneurysm rupture of the ileocolic artery, multiple aneurysms, renal arteriovenous fistula and fatal aortic rupture in Ehlers-Danlos syndrome, subtype IV.] [German] Rofo Fortschr Geb Rontgenstr Neuen Bildgeb Verfahr 1999;170:608–610.
58.De Toma G, Plocco M, Nicolanti V, Cavallaro G, Amato D, Letizia C. [Arterial aneurysms associated with cystic hepato-renal disease.] [French] Presse Med 2000;29: 1559–1561.
59.Garaci FG, Gandini R, Romagnoli A, Fasoli F, Varrucciu V, Simonetti G. Hepatic artery pseudoaneurysm in von Willebrand’s disease. Eur Radiol 2003;13:1913–1915.
60.Scesa JL, Garcier JM, Privat C, et al. [Aneurysm of the duodeno-pancreatic arcades. Diagnostic imagery and therapeutic indications] [French] Presse Med 2000;29: 1115–1117.
61.Merkel C, Sacerdoti D, Bolognesi M, Bombonato G, Gatta A. Doppler sonography and hepatic vein catheterization in portal hypertension: assessment of agreement in evaluating severity and response to treatment. J Hepatol 1998;28:622–630.
62.Haag K, Rossle M, Ochs A, et al. Correlation of duplex sonography findings and portal pressure in 375 patients with portal hypertension. AJR 1999;172: 631–635.
63.Dutta U, Bhutani V, Nagi B, Singh K. Reversible portal hypertension due to tuberculosis. Indian J Gastroenterol 2000;19:136–137.
64.Lachmann RH, Wight DG, Lomas DJ, et al. Massive hepatic fibrosis in Gaucher’s disease: clinicopathological and radiological features. QJM 2000;93: 237–244.
65.Saeki M, Honna T, Nakano M, Kuroda T. [Portal hypertension after successful hepatic portoenterostomy in biliary atresia.] [Japanese] Nippon Geka Gakkai Zasshi 1996;97:648–652.
66.Bryce TJ, Yeh BM, Qayyum A, et al. CT signs of hepatofugal portal venous flow in patients with cirrhosis. AJR 2003;181:1629–1633.
67.Kim JH,Lee YS,Kim SH,Lee SK,Lim MK,Kim HS. Does umbilical vein catheterization lead to portal venous thrombosis? Prospective US evaluation in 100 neonates. Radiology 2001;219:645–650.
68.Kreft B, Strunk H, Flacke S, et al. Detection of thrombosis in the portal venous system: comparison of contrast-enhanced MR angiography with intraarterial digital subtraction angiography. Radiology 2000;216: 86–92.
69.Nishibori H, Kanematsu M, Kondo H, Matsuo M, Hoshi H. Pseudothrombosis in the portal venous system: a potential pitfall with gadolinium-enhanced dynamic gradient-recalled echo imaging of the liver. J Magn Reson Imaging 2000;12:763–768.
70.Cosme Jimenez A, Barrio Andres J, Bujanda Fernandez de Pierola L, et al. Clinical characteristics of nonneoplastic cavernomatous transformation of the portal vein at a Gastroenterology Service in Spain. Rev Esp Enferm Dig 2000;92:448–457.
71.Ueno N, Sasaki A, Tomiyama T, Tano S, Kimura K. Color Doppler ultrasonography in the diagnosis of cavernous transformation of the portal vein. J Clin Ultrasound 1997;25:227–233.
72.Matsumoto A, Kitamoto M, Imamura M, et al. Threedimensional portography using multislice helical CT is clinically useful for management of gastric fundic varices. AJR 2001;176:899–905.
73.Tanoue S, Kiyosue H, Komatsu E, Hori Y, Maeda T, Mori H. Symptomatic intrahepatic portosystemic venous shunt: embolization with an alternative approach. AJR 2003;181:71–78.
74.Bernard B, Lebrec D, Mathurin P, Opolon P, Poynard T. Propranolol and sclerotherapy in the prevention of gastrointestinal rebleeding in patients with cirrhosis: a meta-analysis. J Hepatol 1997;26:312–324.
75.Medina CA, Caridi JG, Wajsman Z. Massive bleeding from ileal conduit peristomal varices: successful treatment with the transjugular intrahepatic portosystemic shunt. J Urol 1998;159:200–201.
76.Terasaki M, Patel NH, Helton WS, et al. Effects of transjugular intrahepatic portosystemic shunts on hepatic metabolic function determined with serial monitoring of arterial ketone bodies. J Vasc Interv Radiol 1998;9: 129–135.
77.Luca A, D’Amico G, La Galla R, Midiri M, Morabito A, Pagliaro L. TIPS for prevention of recurrent bleeding in patients with cirrhosis: meta-analysis of randomized clinical trials. Radiology 1999;212:411–421.
78.Waggershauser T, Muller-Schunk S, Holl J, Reiser M. [TIPS in patients with therapy refractory ascites and kidney dysfunction.] [German] Radiologe 2001;41: 891–894.
79.Terayama N, Matsui O, Kadoya M, et al. Transjugular intrahepatic portosystemic shunt: histologic and immunohistochemical study of autopsy cases. Cardiovasc Intervent Radiol 1997;20:457–461.
80.Chopra S, Dodd GD 3rd, Chintapalli KN, et al. Transjugular intrahepatic portosystemic shunt: accuracy of helical CT angiography in the detection of shunt abnormalities. Radiology 2000;215:115–122.
81.Owens CA, Bartolone C, Warner DL, et al. The inaccuracy of duplex ultrasonography in predicting patency of transjugular intrahepatic portosystemic shunts. Gastroenterology 1998;114:975–980.
82.Libicher M, Radeleff B, Madler U, et al. [After-care of TIPSS patients. Comparison between color Doppler ultrasound and portography.] [German] Radiologe 1998;38:370–377.
83.Leutloff UC, Richter GM, Libicher M, et al. [Follow-up of TIPSS by color-coded duplex sonography using an ultrasonic signal enhancer. First results.] [German] Radiologe 1999;39:1072–1077.
84.Murphy TP, Beecham RP, Kim HM, Webb MS, Scola F. Long-term follow-up after TIPS: use of Doppler velocity criteria for detecting elevation of the portosystemic gradient. J Vasc Interv Radiol 1998;9:275–281.
85.Kimura M, Sato M, Kawai N, et al. [Evaluation of hepatic encephalopathy and portal hemodynamics by Doppler ultrasonography after a transjugular intrahepatic portosystemic shunt.] [Japanese] Nippon Igaku Hoshasen Gakkai Zasshi 1997;57:233–237.
86.Naegele T, Grodd W, Viebahn R, et al. MR imaging and (1)H spectroscopy of brain metabolites in hepatic encephalopathy: time-course of renormalization after liver transplantation. Radiology 2000;216:683–691.
87.Yamakado K, Nakatsuka A, Tanaka N, et al. Portal venous stent placement in patients with pancreatic and

1044
ADVANCED IMAGING OF THE ABDOMEN
biliary neoplasms invading portal veins and causing portal hypertension: initial experience. Radiology 2001;220:150–156.
88.Verma V, Cronin DC 2nd, Dachman AH Portal and mesenteric venous calcification in patients with advanced cirrhosis. AJR 2001;176:489–492.
89.Choi D, Choo SW, Lim JH, Lee SJ, Do YS, Choo IW. Opacification of the intrahepatic portal veins during CT hepatic arteriography. J Comput Assist Tomogr 2001;25:218–224.
90.Aldrighetti L, Ferla G. Portal vein graft rectal evacuation after Whipple procedure. The Fabrizio’s disease. Hepatogastroenterology 1996;43:1638–1639.
91.El Fortia M, Bendaoud M, Taema S, et al. Segmental portal hypertension due to a splenic Echinococcus cyst. Eur J Ultrasound 2000;11:21–23.
92.Andres E, Pflumio F, Knab MC, et al. [Splenic thrombosis and celiac disease: a fortuitous association?] [French] Presse Med 2000;29:1933–1934.
93.Bargallo X, Gilabert R, Nicolau C, Garcia-Pagan JC, Bosch J, Bru C. Sonography of the caudate vein: value in diagnosing Budd-Chiari syndrome. AJR 2003;181: 1641–1645.
94.Noone TC, Semelka RC, Siegelman ES, et al. BuddChiari syndrome: spectrum of appearances of acute, subacute, and chronic disease with magnetic resonance imaging. J Magn Reson Imaging 2000;11:44– 50.
95.Seguin P, Le Bouquin V, Campion JP, Malledant Y. [Hemobilia of gallbladder origin manifesting as malignant hypertension.] [French] Presse Med 1998;27:913.
96.Nakai A, Asakura H, Oya A, Yokota A, Koshino T, Araki T. Pulsed Doppler US findings of renal interlobar arteries in pregnancy-induced hypertension. Radiology 1999;213:423–428.
97.van Jaarsveld BC, Pieterman H, van Dijk LC, et al. Interobserver variability in the angiographic assessment of renal artery stenosis. DRASTIC study group. Dutch Renal Artery Stenosis Intervention Cooperative. J Hypertens 1999;17:1731–1736.
98.Gross CM, Kramer J, Weingartner O, et al. Determination of renal arterial stenosis severity: comparison of pressure gradient and vessel diameter. Radiology 2001;220:751–756.
99.Gupta A, Tello R. Accessory renal arteries are not related to hypertension risk: a review of MR angiography data. AJR 2004;182:1521–1524.
100.Boudewijn G, Vasbinder C, Nelemans PJ, et al. Diagnostic tests for renal artery stenosis in patients suspected of having renovascular hypertension: a meta-analysis. Ann Intern Med 2001;135:401–411.
101.De Cobelli F, Venturini M, Vanzulli A, et al. Renal arterial stenosis: prospective comparison of color Doppler US and breath-hold, three-dimensional, dynamic, gadolinium-enhanced MR angiography. Radiology 2000;214:373–380.
102.Nchimi A, Biquet JF, Brisbois D, et al. Duplex ultrasound as first-line screening test for patients suspected of renal artery stenosis: prospective evaluation in high-risk group. Eur Radiol 2003;13:1413–1419.
103.Johnson PT, Halpern EJ, Kuszyk BS, et al. Renal artery stenosis: CT angiography-comparison of real-time volume-rendering and maximum intensity projection algorithms. Radiology 1999;211:337–343.
104.Paul JF, Ugolini P, Sapoval M, Mousseaux E, Gaux JC. Unilateral renal artery stenosis: perfusion patterns with electron-beam dynamic CT—preliminary experience. Radiology 2001;221:261–265.
105.Radermacher J, Chavan A, Schaffer J, et al. Detection of significant renal artery stenosis with color Doppler sonography: combining extrarenal and intrarenal approaches to minimize technical failure. Clin Nephrol 2000;53:333–343.
106.Claudon M, Plouin PF, Baxter GM, Rohban T, Devos DM. Renal arteries in patients at risk of renal arterial stenosis: multicenter evaluation of the echo-enhancer SH U 508A at color and spectral Doppler US. Levovist Renal Artery Stenosis Study Group. Radiology 2000; 214:739–746.
107.Fain SB, King BF, Breen JF, Kruger DG, Riederer SJ. High-spatial-resolution contrast-enhanced MR angiography of the renal arteries: a prospective comparison with digital subtraction angiography. Radiology 2001;218:481–490.
108.Lee VS, Rofsky NM, Ton AT, Johnson G, Krinsky GA, Weinreb JC. Angiotensin-converting enzyme inhibitor-enhanced phase-contrast MR imaging to measure renal artery velocity waveforms in patients with suspected renovascular hypertension. AJR 2000;174:499–508.
109.Soulez G, Therasse E, Qanadli SD, et al. Prediction of clinical response after renal angioplasty: respective value of renal Doppler sonography and scintigraphy. AJR 2003;181:1029–1035.
110.Bongers V, Bakker J, Beutler JJ, Beek FJ, De Klerk JM. Assessment of renal artery stenosis: comparison of captopril renography and gadolinium-enhanced breath-hold MR angiography. Clin Radiol 2000;55: 346–353.
111.Radermacher J, Chavan A, Bleck J, et al. Use of Doppler ultrasonography to predict the outcome of therapy for renal-artery stenosis. N Engl J Med 2001;344:410– 417.
112.Soulez G, Therasse E, Qanadli SD, et al. Prediction of clinical response after renal angioplasty: respective value of renal Doppler sonography and scintigraphy. AJR 2003;181:1029–1035.
113.Plouin PF, Raynaud A, Elkohen M, Pannier-Moreau I, Battaglia C. [Non-surgical treatments of renal artery stenoses.] [Review] [French] Presse Med 1996;25: 725–730.
114.Xue F, Bettmann MA, Langdon DR, Wivell WA. Outcome and cost comparison of percutaneous transluminal renal angioplasty, renal arterial stent placement, and renal arterial bypass grafting. Radiology 1999;212:378–384.
115.van Jaarsveld BC, Krijnen P, Pieterman H, et al. The effect of balloon angioplasty on hypertension in atherosclerotic renal-artery stenosis. N Engl J Med 2000;342:1007–1014.
116.van de Ven PJ, Kaatee R, Beutler JJ, et al. Arterial stenting and balloon angioplasty in ostial atherosclerotic renovascular disease: a randomised trial. Lancet 1999;353:282–286.
117.Sharma S, Gupta H, Saxena A, et al. Results of renal angioplasty in nonspecific aortoarteritis (Takayasu disease). J Vasc Interv Radiol 1998;9:429– 435.
