
Книги по МРТ КТ на английском языке / Advanced Imaging of the Abdomen - Jovitas Skucas
.pdf
1025
ABDOMINAL VASCULATURE
Imaging
Imaging findings differ between acute and chronic liver outflow obstruction. Hepatomegaly, especially with caudate lobe enlargement and central liver contrast enhancement are common with acute obstruction. Left lobe, and especially caudate lobe hypertrophy and an irregular liver surface develop on a more chronic basis. Liver enhancement varies in chronic obstruction depending on degree of subcapsular collaterals and portal blood stasis. Homogeneous enhancement is found with less severe chronic obstruction, probably due to extensive collateral drainage.
The gold standard in diagnosing Budd-Chiari syndrome is inferior cavography and selective hepatic venography, yet the diagnosis can often be suspected with CT, US, MRI, or scintigraphy. Occasionally the condition is misdiagnosed as an infiltrating tumor. In particular, portal vein blood flow abnormalities are common in Budd-Chiari syndrome, and a misdiagnosis of primary portal hypertension should be avoided.
Computed tomography in patients with acute Budd-Chiari syndrome reveals hepatomegaly, a heterogeneous hypodense liver showing decreased central postcontrast enhancement, and marked ascites. Caudate lobe enlargement is common even with an acute onset, at times compressing the adjacent inferior vena cava. Veins draining the caudate lobe dilate. An irregular liver outline develops, but the liver is not nodular as found in cirrhosis.
Ultrasonography suggests Budd-Chiari syndrome if gray-scale US identifies hepatic veins and Doppler US detects either no blood flow or a reversal of flow. Likewise, the syndrome should be suspected if both gray-scale and Doppler US fail to identify hepatic veins. Normal flow in the inferior vena cava varies with respirations and cardiac cycle, but in some patients with Budd-Chiari syndrome and partial vena caval obstruction either reversed or continuous caval flow is detected by Doppler US. Still, the specificity with US is low.
Not only does the caudal lobe enlarge in Budd-Chiari syndrome, but the caudate vein also becomes prominent. In fact, US detection of a caudate vein equal to or >3mm in diameter, in the appropriate clinical setting, should suggest Budd-Chiari syndrome (93).
Both morphological and perfusion abnormalities are defined with MR angiography. The MR postcontrast appearance varies depending on chronicity. Acute Budd-Chiari syndrome results in early homogeneous enhancement of an enlarged caudate lobe and heterogeneously decreased enhancement of the rest of the liver; central liver portions enhance considerably more than the periphery. Magnetic resonance imaging identifies ascites, major hepatic vein thrombi, and alternate venous pathways; it detects either hepatic vein thrombosis or simply the absence of hepatic venous flow (94).
During the subacute obstruction phase, postcontrast CT enhancement differences between the central and peripheral liver become less pronounced than during the acute phase. Computed tomography of chronic Budd-Chiari syndrome results in caudate lobe and usually left lobe hypertrophy and varying degrees of right lobe atrophy, with the hypertrophic regions having heterogeneous postcontrast enhancement. Ascites is not as prominent as with an acute syndrome. Computed tomography arterial portography reveals similar heterogeneous liver contrast enhancement in a setting of vascular congestion.
Fibrosis ensues during the chronic phase, identified by its hypointense signal on both T1and T2-weighted images. Major venous thrombosis is not a prominent feature of chronic Budd-Chiari syndrome; instead, detected are extensive collaterals, caudate lobe enlargement, and regenerative nodules in less affected portions of the liver. These nodules are hyperintense on T1and isoto hypointense on T2-weighted images and enhance during the arterial phase. Collateral vessels are best identified during the portal vein phase.
Inferior vena caval involvement ranges from obstruction by a thrombus, to a web resulting in partial obstruction, to the vena cava being compressed by an enlarged caudate lobe. Occasionally vena cavography and hepatic venography fail to identify the full extent of venous outflow obstruction; in such a setting percutaneous transhepatic venography appears useful to define the proximal and distal ends of an occlusion.
With a simple obstruction the portal venous system is not affected, but an extensive obstruction results in flow reversal. Alternate venous

1026
ADVANCED IMAGING OF THE ABDOMEN
B
A
|
Figure 17.23. Patient with Budd-Chiari syndrome treated with |
|
TIPS. A: Inferior vena cavogram reveals a narrowed intrahepatic |
|
vena cava (arrow). B: Right hepatic vein injection show multiple |
|
occlusions (arrows). C: Successful TIPS (arrow) reveals good por- |
|
tosystemic flow. (Courtesy of Oscar Gutierrez, M.D., University of |
C |
Chile, Santiago, Chile.) |
pathways are common in chronic Budd-Chiari |
detected with Doppler US, should suggest the |
syndrome, although clinically apparent varices |
diagnosis. |
are not. Collateral vessels or partial recanaliza- |
Incomplete hepatic vein obstruction results |
tions often have a spider web–like drainage |
in a partial Budd-Chiari syndrome. In a patient |
pattern. Especially capsule-based collaterals |
with obstructed middle or left hepatic veins, the |
become more evident with subacute obstruc- |
affected liver segments appear hypodense on |
tion. Budd-Chiari syndrome in children is |
precontrast CT, but vary in appearance on post- |
associated with multiple intrahepatic venove- |
contrast images. The margins of involved seg- |
nous shunts; the presence of these shunts, |
ments correspond to intersegmental planes, and |

1027
ABDOMINAL VASCULATURE
such a finding should suggest a partial BuddChiari syndrome.
Therapy
Short stenotic segments are dilated with balloon angioplasty; shunt placement, TIPS, or thrombolytic therapy are employed with thrombosed segments. Therapy in some patients is surgical.
Hepatic Vein Thrombosis
The primary problem in patients with hepatic vein thrombosis is to control the underlying hematologic abnormalities; otherwise recurrent thrombosis is common. The type of therapy varies with the underlying lesion. Thus an intraluminal thrombus in an isolated hepatic vein is amenable to fibrinolytic infusion or balloon thrombectomy, more extensive obstruction is treated with venoplasty or stenting, and TIPS should be considered for diffuse intrahepatic venous obstruction.
Although angioplasty is safe and usually successful, recurrent stenosis is common. Therapy often involves several sessions. A stent placed across a stenotic hepatic vein segment reduces the pressure gradient. These stents should be monitored every 6 months or so because intimal fibrosis leads to lumen obliteration.
Transjugular intrahepatic portosystemic shunt placement is effective therapy for some patients with acute and even chronic BuddChiari syndrome (Fig. 17.23). Using a transjugular access, puncture from a hepatic vein stump is performed into an engorged intrahepatic vein. At times, puncture is feasible even in the absence of any patent residual hepatic vein, with a stylet perforating from the inferior vena cava through the liver into a portal vein; dilatation and stent placement then establish a venous communication. For unknown reasons stent occlusion is a common complication after TIPS placement for Budd-Chiari syndrome.
A percutaneous transhepatic approach is feasible to recanalize the hepatic veins but is not often employed.
Inferior Vena Cava Obstruction
Percutaneous transluminal angioplasty and stent placement is an option in patients with Budd-Chiari syndrome caused by intrahepatic
inferior vena caval obstruction. Percutaneous transluminal angioplasty alone results in about a 50% patency rate; stenting after primary percutaneous transluminal angioplasty decreases the restenosis rate considerably. A residual narrowing is often evident after stent insertion, but a stent keeps the vena cava patent and decreases the risk of Budd-Chiari syndrome recurrence.
Renal Vessels
Hypertension
Nonrenal Causes
Most hypertension is idiopathic. Identified causes can be subdivided into nonrenal, nonvascular renal, and renovascular. Nonrenal causes of hypertension include primary aldosteronism and the presence of a pheochromocytoma, Cushing’s syndrome, vasculitis, and other disorders. An unusual cause of nonrenal malignant hypertension was gallbladder hemobilia (95). A rare patient has several conditions responsible for hypertension.
Nonvascular Renal Causes
The final pathway for nonvascular renal causes of hypertension is increased renin production.
Nephroptosis is a rare cause of renal hypertension. Technetium-99m-renography identifies diminished perfusion and excretion on the affected side.
In general, renal cysts are not associated with hypertension. Patients with cysts, however, tend to have systolic and diastolic blood pressures significantly higher than those without cysts, either due to underlying renal disease that is responsible for both or due to renal artery compression caused by an expanding cyst, thus leading to increased renin release. Compression by an adjacent extrarenal tumor, typically an adrenal one, is occasionally implicated in unilateral renal ischemia and the resultant hypertension.
A thrombus or an atheromatous plaque embolizing distally can result in renal hypoperfusion and hypertension, or the involved renal parenchyma infarcts.
A number of glomerular and interstitial renal diseases result in hypertension. Occasionally ureteric obstruction, generally on an acute

1028
basis, leads to increased renin secretion and hypertension. Chronic pyelonephritis is an uncommon cause of hypertension.
Both renal cell carcinomas and Wilms’ tumors result in hypertension, either by vascular compression or by intrinsic renin production. Juxtaglomerular tumors also produce renin.
Hypertension after renal trauma is not common even with a renal artery thrombosis. Nevertheless, hypertension has developed after traumatic renal artery narrowing, extrinsic renal artery compression, an arteriovenous fistula, and a traumatic aneurysm. At times a segmental artery is involved. Occasionally a subcapsular hematoma or urinoma compresses the adjacent renal tissue, leads to ischemia, increases the renin production, and manifests by hypertension (Page kidney).
Renal Doppler US in women with pregnancyassociated hypertension reveals markedly prolonged interlobar artery acceleration times (96), suggesting that renal artery or segmental artery stenosis or vasospasm plays a role in this condition.
Hypertension after a renal transplant is not always due to renal artery stenosis. In some, it appears to be associated with rejection. In others, renin production by a native kidney plays a role.
Renal Artery Stenosis
The most common cause of renovascular hypertension is renal artery stenosis, with an occasional renal artery aneurysm or dissection being responsible. The true prevalence of renovascular hypertension is not known because not all patients with hypertension undergo a full diagnostic workup. Many patients with renal artery stenosis are asymptomatic and do not seek medical attention, thus introducing a bias in any statistical analysis. As a rough estimate, among all causes of hypertension, a renovascular etiology accounts for approximately 3% to 5%, with the prevalence of secondary causes being greater in children. Considerable emphasis is placed on renovascular causes because in many patients these are correctable.
ADVANCED IMAGING OF THE ABDOMEN
ogy in about 20% of patients and arteriosclerotic vascular disease in the rest. The primary lesion associated with renal artery narrowing in the elderly is atherosclerosis, while in a younger population fibromuscular hyperplasia predominates.
Fibromuscular Dysplasia
Fibromuscular dysplasia occurs most often in the renal arteries, followed by the carotid and iliac arteries. It is the second most common cause of renal artery stenosis involving the middle to distal portions of the renal artery. The etiology is not known. Fibromuscular dysplasia is often subdivided into medial, intimal, and adventitial (perimedial) dysplasia, with medial fibromuscular dysplasia being the most common. Typically, imaging detects multiple stenotic segments (Fig. 17.24). Rarely, medial dysplasia results in spontaneous arterial rupture and an extraperitoneal hematoma.
Medial dysplasia typically affects middleaged women and tends to be bilateral, consisting of single or multiple constrictions in the distal two thirds of the renal artery. Segmental renal branches are occasionally involved. A “string of beads” appearance is characteristic.
Intimal fibroplasia manifests as a smooth stenosis of varying length involving distal renal artery segments. Occasionally it has a web-like appearance. The proximal end of the renal artery is often diseased if the aorta is also involved.
Perimedial dysplasia also affects middle-aged women, tends to be unilateral, and involves the distal renal artery segments. Whether perimedial dysplasia and medial fibroplasia are different manifestations of the same process is conjecture.
Complete renal artery occlusion is rare with these dysplasias. Intimal and perimedial dysplasias are associated with vessel dissection and thrombosis—complications uncommon with medial dysplasia.
In distinction to atherosclerotic disease, fibromuscular dysplasia tends not to recur after therapy.
Atherosclerosis
Etiology
Among patients with renal artery stenoses, fibromuscular dysplasia is the underlying etiol-
Most renovascular hypertension is due to atherosclerosis, a progressive disease. The proximal one third of the renal artery and its ostium are

1029
ABDOMINAL VASCULATURE
A 
 B
B
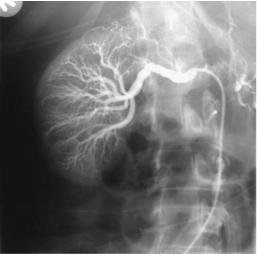
Figure 17.24. Fibromuscular dysplasia. A: Right renal arteriography reveals a slightly irregular main renal artery stricture and several mild strictures in the intrarenal arteries. (Courtesy of David Waldman, M.D., University of Rochester.) B: Fibromuscular dysplasia of right renal artery in another patient. (Courtesy of Oscar Gutierrez, M.D., University of Chile, Santiago, Chile.)
most often affected, and usually the adjacent aorta is also involved by atherosclerosis. Some patients also have segmental and diffuse intrarenal atherosclerosis. Bilateral renal artery involvement is common. An older patient population is affected than with fibromuscular dysplasia. This is a progressive and recurrent condition.
Typical presentations include hypertension, renal ischemia, parenchymal loss, and eventual renal failure.As a rough estimate, approximately a 60% stenosis of a main renal artery is necessary to detect renal atrophy with imaging.
Other Etiologies
Renal artery hypoplasia is a developmental anomaly most often found in children and young adults with renovascular hypertension. Renal artery origins are involved.
Takayasu’s arteritis, a rare cause of renal artery stenosis and hypertension,results in arterial wall thickening.
A rare patient with neurofibromatosis develops renal artery stenosis and hypertension.
Also rare is acute renal artery occlusion due to necrotizing vasculitis.
Renal artery stenosis is not uncommon after renal transplantation. This topic is discussed in Chapter 10.
Clinical
Sufficient renal artery stenosis leads to decreased renal perfusion, activation and release of renin and angiotensin II, and increased renal prostaglandin, and via a multifactorial feedback mechanism results in hypertension. Especially in long-established hypertension, plasma renin values and related clinical tests serve little purpose in differentiating renovascular from essential hypertension. Clinically, renovascular hypertension manifests similar to essential hypertension.
Progression of severity of renal artery stenosis is common, yet in patients with fibromuscular dysplasia renal perfusion correlates inversely with the degree of stenosis, a finding not seen in patients with atherosclerosis. Rare exceptions to stenosis progression do occur, and renal artery stenosis regresses.
Occasionally seen is the patient with a renal cell carcinoma with an incidentally discovered contralateral renal artery stenosis. Although a simple nephrectomy can be performed, in such a setting the stenosis can be expected to progress, even to occlusion. In this specific setting of renal cell carcinoma and contralateral renal artery stenosis, prophylactic percutaneous transluminal angioplasty of the stenosis appears to be of benefit.

1030
A not uncommon finding is both renal artery disease and infrarenal aortic disease; although simultaneous aortic and renal revascularization can be performed, at times percutaneous transluminal renal angioplasty is preferred prior to surgical revascularization, especially in highrisk patients.
Detection
The first problem encountered is defining the degree of renal artery stenosis. Using angiograms performed with a DSA technique in consecutive hypertensive patients, three experienced radiologists could not distinguish between 50% and 60% stenosis or between 60% and 70% stenosis (97); the authors concluded that statements about degree of renal artery stenosis are not realistic or clinically significant when using DSA. Nevertheless, many authors do use DSA results as their gold standard.
The imaging definition of renal artery stenosis is obviously arbitrary. Most investigators use a lumen narrowing of >50% as a cutoff point, with a minority adopting 60% narrowing as their limit. A pressure gradient across a stenotic segment >10mmHg is generally also considered significant. The use of a miniaturized pressure guidewire revealed that a 50% stenosis results in a mean pressure gradient of 22mmHg (98).
The definition of an ostial stenosis is also arbitrary; some investigators define an ostial stenosis as any stenosis within 5mm of the renal artery origin, while others use a 10-mm length adjacent to an atherosclerotic aorta. Regardless of definition, detecting an ostial stenosis is not always clear-cut; most renal arteries originate either anterolateral or posterolateral from the aorta; a completely lateral origin is found only in a minority. In some patients CT angiography identifies an ostial lesion, but DSA shows it to be truncal in location.
In patients with chronic hypertension due to renal artery stenosis, the end-stage finding detected with most imaging modalities is a small, smooth kidney without urinary obstruction. These changes, however, are not always present; also, stenosis of an accessory renal artery rarely leads to a small kidney. A more basic question is whether accessory artery stenosis results in hypertension—some indirect
ADVANCED IMAGING OF THE ABDOMEN
findings suggest that accessory renal arteries are not a direct cause of hypertension (99).
Comparison Studies
Urography is not sensitive in detecting corrective renal artery stenosis as a cause of renovascular hypertension and is not used for this purpose.Aside from an end-stage small, smooth kidney, main renal artery stenosis results in a delayed nephrogram. Likewise, US and scintigraphy, although used at times for screening, are inferior to CTA and MRA in detecting renal artery stenosis in patients with suspected renovascular hypertension (100).
In general, CTA renal artery image quality is worse than intraarterial DSA but is better than intravenous DSA. Also, MRA sensitivity in detecting at least 50% stenoses was higher for MRA (100%) than US (79%), but specificities were similar (101). Although some studies suggest that MRA and DSA are equally effective in detecting significant main renal artery stenoses, MRA and conventional angiography can diverge both by overestimating and underestimating the degree of stenosis by more than 10% (relative to angiography).
Some imaging studies include detection of accessory renal arteries while others do not, thus introducing another variable in study comparisons.
Accessory Renal Artery Detection
Accessory renal arteries are found in about 40% of patients. Renal arteries are end arteries, and the acute occlusion of an accessory renal artery leads to segmental renal infarction. Thus knowledge of renal volume perfused by an accessory artery is of some importance prior to surgical procedures such as an aortic stent or graft implantation over this segment. The perfused vascular bed of an accessory renal artery can be obtained from CT performed with selective accessory renal artery angiography.
A limitation of noninvasive techniques is that some accessory renal arteries are not detected. Several studies have concluded that CTA does not visualize about 20% to 25% of accessory renal arteries, although other studies indicate that CTA does not detect <10%.

1031
ABDOMINAL VASCULATURE
Only a minority of accessory renal artery stenoses are detected by US. A not untypical conclusion is that duplex Doppler US detects only about 5% of accessory renal arteries seen at catheter angiography (102).
In general, more accessory renal arteries are detected with MRA than with color Doppler US (101). Comparison of CTA and MRA yields conflicting results, with some studies suggesting that MRA is inferior both in detecting accessory renal arteries and in grading renal artery stenoses. One should keep in mind, however, that MR results are very technique dependent, and results vary accordingly. A realistic estimate is that MRA identifies 75% to 90% of accessory renal arteries.
Computed Tomography
Traditionally, conventional angiography has been the gold standard in evaluating renal artery stenosis. In a number of institutions arterial-phase helical CT has replaced renal angiography. Arterial phase 3D reconstruction images outline a stenosis in most patients. A number of studies have concluded that CTA has essentially the same accuracy for detecting renal artery stenosis ≥50% as DSA, although underand overestimation of stenosis grade are encountered. The sensitivities and specificities in detecting clinically significant stenoses of the main renal arteries are over 90%, but keep in mind that the accuracy of detecting a proximal renal artery stenosis is greater than in distal segments. As a further refinement, CTA with real-time interactive volume rendering appears faster and is more accurate in detecting renal artery stenoses than the use of other current techniques (103). Nevertheless, clinically significant renal artery stenosis, including stenosis of an accessory artery, can be missed by CTA.
Electron beam CT can determine renal volume, measure perfusion, and identify progressive changes in unilateral renal artery stenosis (104); initially symmetric timeattenuation curves are present, then asymmetric time-attenuation curves but with similar perfusion, and eventually asymmetric curves with impaired perfusion.
Evaluation of renal artery fibromuscular dysplasia presents its own unique problems. Computed tomography angiography in most hypertensive patients with renal artery fibro-
muscular dysplasia identifies most but not all lesions; arteriography remains the imaging modality of choice in the study of fibromuscular dysplasia.
Ultrasonography
Investigators are deeply divided on the use of Doppler US for evaluating possible renal artery stenosis. The accuracy of Doppler US is reasonable in patients with a tight stenosis, but this test appears to be of doubtful value in screening hypertensive patients where detection of a 50% to 70% stenosis is relevant.
Initial Doppler US studies of renal artery stenosis were directed primarily toward the renal artery and relied on detecting an increased velocity in the stenotic segment, with mixed results. Emphasis then shifted to poststenotic changes in smaller intrarenal branches. Normal intrarenal arteries have a rapid systolic upstroke, while in a setting of renal artery stenosis the upstroke is dampened and time to peak systole is also dampened. Still, even this approach has led to mixed results. Although several studies show that intrarenal Doppler US will detect more severe stenoses (greater than about 70%), US misses less severe stenoses. The sensitivity and specificity for duplex US in detecting a ≥60% diameter main renal artery stenosis were 91% and 97%, respectively (102). In another prospective study, intraand extrarenal Doppler US achieved a sensitivity and specificity of 79% and 93%, respectively, in detecting at least a 50% renal artery narrowing (101), results similar to a number of other studies. Some investigators assumed a renal artery peak systolic velocity >100cm/sec or even >180cm/sec to represent significant renal artery stenosis; although the early work was promising, later studies achieved substantially worse results. Also, duplication of such parameters as acceleration time is inconsistent.
The sensitivity and specificity for detecting significant stenosis in a given vessel (including accessory arteries) in consecutive patients were 97% and 98%, respectively (105); these impressive results were reached by twofold use of Doppler US: a stenosis of >50% was presumed if the main renal artery maximal systolic velocity was >180cm/sec and velocity in the more distal segment was less than one quarter of the maximum velocity, or, in the absence of

1032
these criteria, stenosis was presumed if the intrarenal segmental artery acceleration time was >70msec. Arteriography was the gold standard.
The Renal Artery Stenosis Study Group from 14 European centers using the echo-enhancing agent Levovist for renal artery Doppler US found more diagnostic studies with enhanced Doppler US (84%) compared with nonenhanced Doppler US (64%) (106).
Magnetic Resonance
Magnetic resonance angiography is a promising noninvasive screening test in patients suspected of renal artery stenosis, and the indications for its use continue to expand. The results with MRA approach those of conventional angiography in detecting renal artery stenosis. In general, a normal MRA is highly predictive of normal arteries, although some stenoses are underand overestimated by MRA. Several consecutive data sets with imaging times of 7 seconds or so can be acquired during a single breath-hold; postgadolinium and 3D views are then evaluated. Introduction of MR phasecontrast flow measurements adds functional information. These studies are technically demanding, however, and require optimal contrast bolus timing. Also, most studies have addressed the detection of stenosis only in proximal renal artery segments.
Magnetic resonance angiography is considered especially valuable in patients with chronic renal failure; IV gadolinium in the doses used in MR is less nephrotoxic than iodinated contrast agents. Captopril renography is of limited value in these patients. Thus in an elderly hypertensive patient with suspected proximal renal artery stenosis, MRA can be the primary screening test and, if positive, the patient is referred directly for DSA and possible angioplasty. If necessary, even DSA and angioplasty are performed with gadolinium or carbon dioxide as contrast agents.
Can MRA visualize all renal artery ostial and truncal stenoses? Magnetic resonance angiography tends to overestimate stenosis grade. One constraint of 3D MRA is that a false ostial stenosis can be suggested due to loss of signal intensity at a renal artery origin. Nevertheless, 3D gradient-echo contrast enhanced MRA achieves a >90% sensitivity and specificity for correctly
ADVANCED IMAGING OF THE ABDOMEN
identifying a clinically relevant stenosis. A stenosis is identified as signal extinction, a finding based not only on flow turbulence but also on the MR parameters used. Image subtraction in 3D contrast enhanced reual artery MRA does improve study quality and aids in visualizing distal renal arteries. Gadolinium enhancement increases the apparent precontrast renal artery diameter, probably due to improved visualization of slow-flowing blood along the periphery.
One approach in providing both detailed renal artery evaluation and also abdominal aortic coverage is to first obtain high-resolution, small field-of-view 3D contrast-enhanced renal artery MRA, followed by large field-of-view 3D MRA of the aorta (107); such highresolution MRA yielded a sensitivity of 97% and specificity of 92% in detecting renal arterial stenosis.
The reliability of phase-contrast MR flow measurements to differentiate between hemodynamically significant renal artery stenosis and nonstenosed vessels is not clear; some have achieved high sensitivity and specificity, but others have found velocity waveforms from phase-contrast data to be inaccurate in predicting renal artery stenosis (108).
The role of MR angiography in fibromuscular hyperplasia is not well established.
Scintigraphy
The glomerular filtration rate is maintained at normal levels through arteriolar vasoconstriction induced by angiotensin II even in renal artery stenosis. Conversion of the inactive angiotensin I to angiotensin II is inhibited by captopril (angiotensin-converting enzyme inhibitor) administration. In a setting of renal artery stenosis, captopril administration results in a decreased glomerular filtration rate as measured with Tc-99m-DTPA or Tc-99m- mercaptoacetylglycilglycilglycine (MAG3). Captopril renography thus potentially detects underlying renal artery stenosis, although the reported sensitivities and specificities of captopril renography in detecting renal artery stenosis vary considerably. Earlier literature suggested that a distinct advantage of captopril renography was that it identified those patients who will most benefit from correction of renal artery stenosis, yet a more recent analysis
1033
ABDOMINAL VASCULATURE
concluded that “renal scintigraphy had no significant predictive value” after renal angioplasty (109).
Why some patients have false-negative captopril renography is puzzling, but the response to captopril decreases with poor renal function. Test accuracy tends to be compromised in a setting of renal failure and it is not applicable to a poorly functioning kidney. Because MAG3 is filtered by glomeruli and secreted by tubules, while DTPA is filtered by glomeruli, the former is preferred in those with renal failure. In one study captopril Tc-99m-MAG3 renography achieved a sensitivity and specificity of 85% and 71%, respectively, in detecting a stenosis >50% (110). Occasionally captopril scintigraphy findings of a mobile kidney mimic those seen with renal artery stenosis.
Scintigraphy provides little anatomic detail, but in a setting of a normal serum creatinine level a scintigraphic finding of impaired fractional flow to one kidney is an indication for further investigation. Iodine-131-hippuran renography can detect arterial luminal narrowing in a setting of unilateral renal artery stenosis where the nonstenotic kidney served as a “normal” landmark. In general, studies using Tc-99m are technically superior to I 131, and most institutions prefer the former.
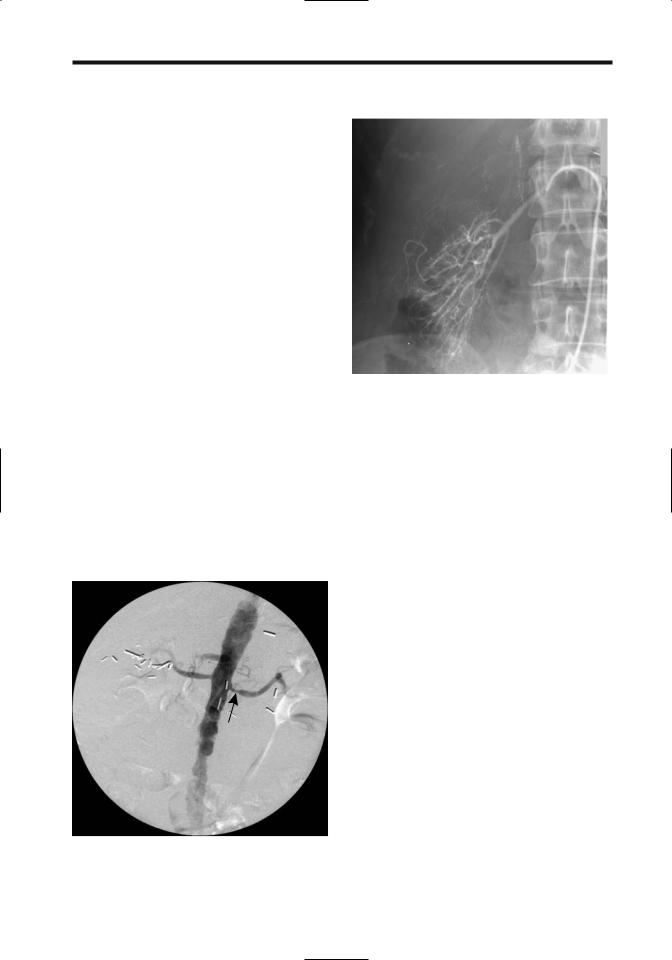
Figure 17.25. Left renal artery stenosis (arrow) in a hypertensive patient. A stent was later inserted. (Courtesy of David Waldman, M.D., University of Rochester.)
Figure 17.26. Renal arteriogram in a woman with renal failure, malignant hypertension and scleroderma reveals marked vasospasm and tortuosity of major and smaller intrarenal vessels. The distal intrarenal arteries are tapered. Vasospasm resulted in slow intrarenal flow and persistence of arterial filling. Similar changes are found both in renovascular hypertension and in scleroderma. (Courtesy of Oscar Gutierrez, M.D., University of Chile, Santiago, Chile.)
Angiography
Digital subtraction angiography using IV contrast injection enjoyed brief popularity as a screening test for renal artery stenosis, but most centers have abandoned this technique for more modern imaging.
Direct injection angiography is the gold standard used in evaluating the main and accessory renal arteries (Fig. 17.25). Angiography is often preferred in patients with fibromuscular dysplasia. One should keep in mind that hypertension, in general, is associated with arterial vasospasm and tortuosity of intrarenal arteries (Fig. 17.26).
Carbon dioxide can be used in place of iodinated contrast to evaluate renal arteries during digital subtraction arteriography. In patients at risk for contrast-related nephrotoxicity or who are allergic to iodinated contrast, carbon dioxide arteriography appears to be a viable alternative.

1034
Venous Renin Assay
Selective renal vein assay for renin is not used either as a screening test or for detecting renovascular causes of hypertension. It is useful, however, to evaluate whether a detected renal artery stenosis is indeed a cause of a patient’s hypertension.
Therapy
Although renovascular hypertension often is controlled medically, in a setting of atherosclerotic renal artery stenosis medical therapy often does not prevent renal ischemia and resultant renal insufficiency and, in fact, accelerates parenchyma damage. Therapeutic modalities for renal artery stenosis include percutaneous transluminal angioplasty (balloon dilation), stenting, surgical correction and occasionally some other type of therapy. The primary indications for therapy of renal artery stenosis are hypertension refractory to medical therapy and renal ischemia manifesting by a decrease in renal function; one should keep in mind, however, that because renal artery stenosis exists does not mean that it is responsible for a patient’s hypertension or renal failure. Hypertension persisting after successful angioplasty or other therapy is often ascribed to underlying essential hypertension.
Overall therapeutic success is measured by two outcomes: Is hypertension cured and is renal function deterioration arrested? Different results are obtained in patients with fibromuscular dysplasia and atherosclerotic stenosis, and in an analysis of success rates these two patient populations should be separated. Some investigators distinguish ostial stenoses from other stenoses because success rates for ostial stenoses are better with stenting than with simple transluminal angioplasty. One should also keep in mind that hypertension can be caused by stenosis of an isolated accessory renal artery, but for renal function to be impaired generally both main renal arteries need to be involved.
An exception to direct interventional therapy is trauma-induced hypertension; once the underlying defect is identified and appears stable, a number of these patients are initially followed medically because the hypertension resolves spontaneously in some.
ADVANCED IMAGING OF THE ABDOMEN
When to suggest therapeutic intervention is controversial. Classic dogma is that medical therapy should be exhausted first, yet numerous studies have confirmed that outcomes of angiographic and surgical therapeutic are better if initial renal function is close to normal.
The cure rates for hypertension after reconstructive surgery, angioplasty, and stenting are low and are similar. The current trend in revascularization therapy is away from emphasis on cure of renovascular hypertension and a shift toward control of renal failure progression due to ischemia. One sign of successful revascularization is that serum creatinine levels do not worsen significantly in those with impaired renal function.
Preliminary evidence suggests that intrarenal resistance index identifies patients whose blood pressure or renal function improves after correction of renal artery stenosis. Among patients with a preprocedure resistance index (RI) of <80, mean arterial pressure decreased by at least 10% in 94% of patients, while in patients with a preprocedure RI of >80, pressure did not decrease in 97% (111); a preprocedure RI of >80 thus selects those patients with renal artery stenosis in whom therapy will not improve their hypertension. Captopril assisted Doppler US also appears useful in predicting outcome; multivariate analysis suggests that the best predictors of a favorable therapeutic outcome are a unilateral RI of <0.65 after captopril and a kidney longer than 93mm (112).
Therapy Comparisons
A French review of published therapies for renal artery stenosis found that most studies were retrospective and uncontrolled, and their success rates overestimated (113); hypertension cure rates after percutaneous angioplasty were 50% in patients with fibromuscular dysplasia but only 19% in those with an atherosclerotic stenosis, while in patients with progressive renal failure due to atherosclerotic disease, surgical revascularization improved renal function in 55% and angioplasty improved function in 41%. Some more pessimistic studies have reported that hypertension was cured only in about 5%, worsened also in about 5%, and renal insufficiency improved in about a third and continued to worsen in slightly fewer patients.
