
- •Contents
- •Preface to the Third Edition
- •About the Authors
- •How to Use Herbal Medicines
- •Introduction
- •General References
- •Agnus Castus
- •Agrimony
- •Alfalfa
- •Aloe Vera
- •Aloes
- •Angelica
- •Aniseed
- •Apricot
- •Arnica
- •Artichoke
- •Asafoetida
- •Avens
- •Bayberry
- •Bilberry
- •Bloodroot
- •Blue Flag
- •Bogbean
- •Boldo
- •Boneset
- •Borage
- •Broom
- •Buchu
- •Burdock
- •Burnet
- •Butterbur
- •Calamus
- •Calendula
- •Capsicum
- •Cascara
- •Cassia
- •Cat’s Claw
- •Celandine, Greater
- •Celery
- •Centaury
- •Cereus
- •Chamomile, German
- •Chamomile, Roman
- •Chaparral
- •Cinnamon
- •Clivers
- •Clove
- •Cohosh, Black
- •Cohosh, Blue
- •Cola
- •Coltsfoot
- •Comfrey
- •Corn Silk
- •Couchgrass
- •Cowslip
- •Cranberry
- •Damiana
- •Dandelion
- •Devil’s Claw
- •Drosera
- •Echinacea
- •Elder
- •Elecampane
- •Ephedra
- •Eucalyptus
- •Euphorbia
- •Evening Primrose
- •Eyebright
- •False Unicorn
- •Fenugreek
- •Feverfew
- •Figwort
- •Frangula
- •Fucus
- •Fumitory
- •Garlic
- •Gentian
- •Ginger
- •Ginkgo
- •Ginseng, Eleutherococcus
- •Ginseng, Panax
- •Golden Seal
- •Gravel Root
- •Ground Ivy
- •Guaiacum
- •Hawthorn
- •Holy Thistle
- •Hops
- •Horehound, Black
- •Horehound, White
- •Horse-chestnut
- •Horseradish
- •Hydrangea
- •Hydrocotyle
- •Ispaghula
- •Jamaica Dogwood
- •Java Tea
- •Juniper
- •Kava
- •Lady’s Slipper
- •Lemon Verbena
- •Liferoot
- •Lime Flower
- •Liquorice
- •Lobelia
- •Marshmallow
- •Meadowsweet
- •Melissa
- •Milk Thistle
- •Mistletoe
- •Motherwort
- •Myrrh
- •Nettle
- •Parsley
- •Parsley Piert
- •Passionflower
- •Pennyroyal
- •Pilewort
- •Plantain
- •Pleurisy Root
- •Pokeroot
- •Poplar
- •Prickly Ash, Northern
- •Prickly Ash, Southern
- •Pulsatilla
- •Quassia
- •Queen’s Delight
- •Raspberry
- •Red Clover
- •Rhodiola
- •Rhubarb
- •Rosemary
- •Sage
- •Sarsaparilla
- •Sassafras
- •Saw Palmetto
- •Scullcap
- •Senega
- •Senna
- •Shepherd’s Purse
- •Skunk Cabbage
- •Slippery Elm
- •Squill
- •St John’s Wort
- •Stone Root
- •Tansy
- •Thyme
- •Uva-Ursi
- •Valerian
- •Vervain
- •Wild Carrot
- •Wild Lettuce
- •Willow
- •Witch Hazel
- •Yarrow
- •Yellow Dock
- •Yucca
- •1 Potential Drug–Herb Interactions
- •4 Preparations Directory
- •5 Suppliers Directory
- •Index
Ground Ivy
Summary and Pharmaceutical Comment
The chemistry of ground ivy is well studied. Documented pharmacological activities for constituents of ground ivy support some of the herbal uses, although there is a lack of clinical research assessing the effects of ground ivy and rigorous randomised controlled clinical trials are required. In view of the lack of safety and toxicity data, and the reported cytotoxic activity of ursolic acid, excessive use of ground ivy
Gand use during pregnancy and lactation should be avoided.
Species (Family)
Glechoma hederacea L. (Labiatae)
Synonym(s)
Nepeta hederacea (L.) Trevis, N. glechoma Benth.
Part(s) Used
Herb
Pharmacopoeial and Other Monographs
BHC 1992(G6)
BHP 1996(G9)
Martindale 35th edition(G85)
Legal Category (Licensed Products)
GSL(G37)
Constituents
The following is compiled from several sources, including General Reference G6.
Amino acids Asparagic acid, glutamic acid, proline, tyrosine and valine.
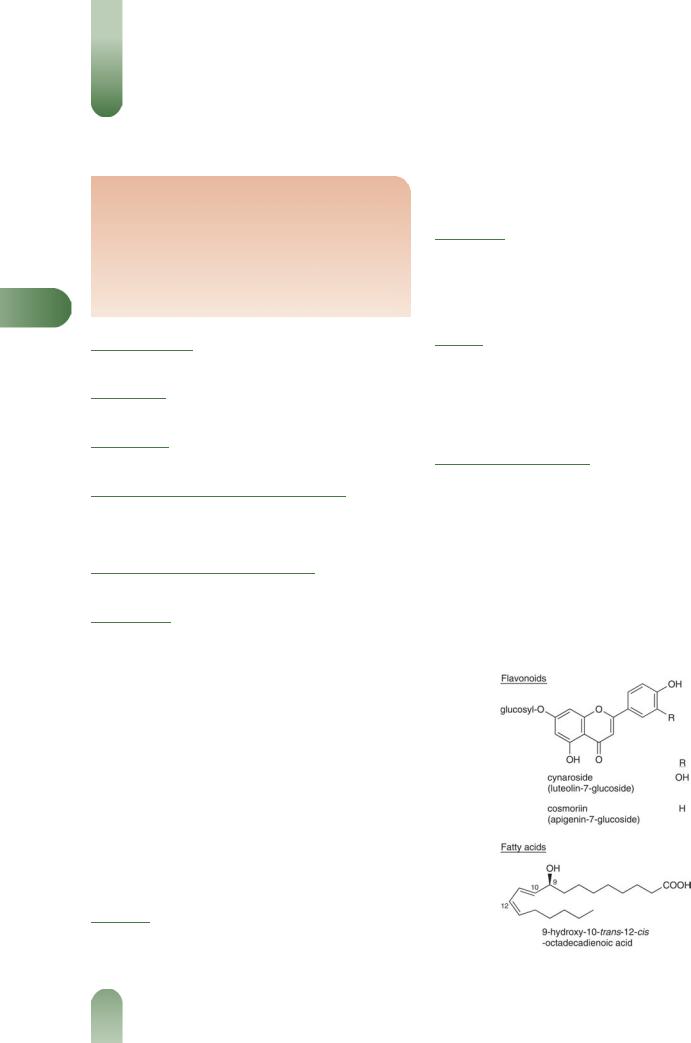
Flavonoids Flavonol glycosides (e.g. hyperoside, isoquercitrin, rutin) and flavone glycosides (e.g. luteolin diglucoside, cosmosyin, cynaroside and cosmoriin).(1)
Steroids b-Sitosterol.
Terpenoids Oleanolic acid, a-ursolic acid, b-ursolic acid.(2)
Volatile oils 0.03–0.06%. Various terpenoid components including p-cymene, linalool, limonene, menthone, a-pinene, b- pinene, pinocamphone, pulegone and terpineol; glechomafuran (a sesquiterpene).(3)
Other constituents Palmitic acid and other fatty acids, rosmarinic acid, succinic acid, bitter principle (glechomin), choline, gum, diterpene lactone (marrubiin), saponin, tannin and wax.
Food Use
Ground ivy is listed by the Council of Europe as a natural source of food flavouring (category N3). This category indicates that ground ivy can be added to foodstuffs in the traditionally accepted
manner, although there is insufficient information available for an adequate assessment of potential toxicity.(G16)
Herbal Use
Ground ivy is stated to possess mild expectorant, anticatarrhal, astringent, vulnerary, diuretic and stomachic properties. Traditionally, it has been used for bronchitis, tinnitus, diarrhoea,
haemorrhoids, cystitis, gastritis, and specifically for chronic
bronchial catarrh.(G6, G7, G8, G49, G64)
Dosage
Dosages for oral administration (adults) for traditional uses recommended in standard herbal reference texts are given below.
Dried herb 2–4 g as an infusion three times daily.(G6, G7)
Liquid extract 2–4 mL (1 : 1 in 25% alcohol) three times
daily.(G6, G7)
Pharmacological Actions
In vitro and animal studies
In vivo anti-inflammatory activity has been reported for an ethanolic extract of ground ivy, which was stated to exhibit a moderate inhibition (27%) of carrageenan-induced rat paw oedema.(4)
Ursolic acid analogues, 2aand 2b-hydroxyursolic acid, have
been documented to provide significant ulcer-protective activity in mice.(5)
The astringent activity documented for ground ivy has been attributed to rosmarinic acid, a polyphenolic acid.(6)
Glechomin and marrubiin are stated to be bitter principles, and
a-terpineol is known to be an antiseptic component of volatile
oils.(G48, G49)
Figure 1 Selected constituents of ground ivy.
342

Figure 2 Ground ivy (Glechoma hederacea).
Anti-inflammatory and astringent properties are generally associated with flavonoids and tannins, respectively. Anti-inflam- matory properties have been documented for rosmarinic acid (see Rosemary).
In vitro antiviral activity against the Epstein–Barr virus has been documented for ursolic acid.(7) Both oleanolic and ursolic acids were found to inhibit tumour production by TPA in mouse skin, with activity comparable to that of retinoic acid, a known tumour-promoter inhibitor.(7)
Significant cytotoxic activity has also been reported for ursolic acid in lymphocytic leukaemia (P-388, L-1210) and human lung carcinoma (A-549), and marginal activity in KB cells, human colon (HCT-8), and mammary (MCF-7) tumour cells.(8)
Clinical studies
There is a lack of clinical research assessing the effects of ground ivy and rigorous randomised controlled clinical trials are required.
Side-effects, Toxicity
There is a lack of clinical safety and toxicity data for ground ivy and further investigation of these aspects is required.
Poisoning in cattle and horses has been documented in Eastern Europe.(9) Symptoms include accelerated weak pulse, difficulty in breathing, conjunctival haemorrhage, elevated temperature, dizziness, spleen enlargement, dilation of the caecum, and gastroenteritis revealed at post-mortem. Antitumour and cytotoxic activities have been reported for oleanolic and ursolic acids (see Pharmacological Actions, In vitro and animal studies).
Ground ivy volatile oil contains many terpenoids and terpenerich volatile oils are irritant to the gastrointestinal tract and kidneys. Pulegone is an irritant, hepatotoxic, and abortifacient principle of the volatile oil of pennyroyal. However, in comparison with pennyroyal the overall yield of volatile oil is much less (0.03– 0.06% in ground ivy and 1–2% in pennyroyal).
Ground Ivy |
343 |
Figure 3 Ground ivy – dried drug substance (herb). |
G |
|
Contra-indications, Warnings
Ground ivy is contra-indicated in epilepsy(G7) although no rationale for this statement has been found. Excessive doses may be irritant to the gastrointestinal mucosa and should be avoided by individuals with existing renal disease.
Drug interactions None documented. However, the potential for preparations of ground ivy to interact with other medicines administered concurrently, particularly those with similar or opposing effects, should be considered.
Pregnancy and lactation The safety of ground ivy has not been established. In view of the lack of toxicity data and the possible irritant and abortifacient action of the volatile oil, the use of ground ivy during pregnancy and lactation should be avoided.
Preparations
Proprietary multi-ingredient preparations
UK: Gerard House Water Relief Tablets; Water Naturtabs.
References
1Zieba J. Isolation and identification of flavonoids from Glechoma hederacea. Pol J Pharmacol Pharm 1973; 25: 593–597.
2 Zieba J. Isolation and identification of nonheteroside triterpenoids from Glechoma hederacea. Pol J Pharmacol Pharm 1973; 25: 587–592.
3Stahl E, Datta SN. New sesquiterpenoids of the ground ivy (Glechoma hederacea). Justus Liebigs Ann Chem 1972; 757: 23–32.
4 Mascolo N et al. Biological screening of Italian medicinal plants for anti-inflammatory activity. Phytother Res 1987; 1: 28–31.
5Okuyama E et al. Isolation and identification of ursolic acid-related compounds as the principles of Glechoma hederacea having an
antiulcerogenic activity. Shoyakugaku Zasshi 1983; 37: 52–55.
6 Okuda T et al. The components of tannic activities in Labiatae plants. I. Rosmarinic acid from Labiatae plants in Japan. Yakugaku Zasshi 1986; 106: 1108–1111.
7Tokuda H et al. Inhibitory effects of ursolic and oleanolic acid on skin tumor promotion by 12-O-tetradecanoylphorbol-13-acetate. Cancer Lett 1986; 33: 279–285.
8 Lee K-H et al. The cytotoxic principles of Prunella vulgaris,
Psychotria serpens, and Hyptis capitata: Ursolic acid and related derivatives. Planta Med 1988; 54: 308.
9 MAFF. Poisonous Plants in Britain. London: HMSO, 1984: 139.
Guaiacum
Summary and Pharmaceutical Comment
Guaiacum is characterised by the resin fraction of the heartwood and much has been documented on the constituents (principally lignans) of the resin, although little is known regarding other constituents. No scientific information was found to justify the herbal use of guaiacum as an antirheumatic or anti-inflammatory agent. In view of the lack of toxicity data, excessive use of guaiacum and use during
Gpregnancy and lactation should be avoided.
Species (Family)
Guaiacum officinale L. (Zygophyllaceae)
Guaiacum sanctum L.
Synonym(s)
Guaiac, Guajacum, Guayacar, Lignum Vitae, Palo Santo
Part(s) Used
Resin obtained from the heartwood
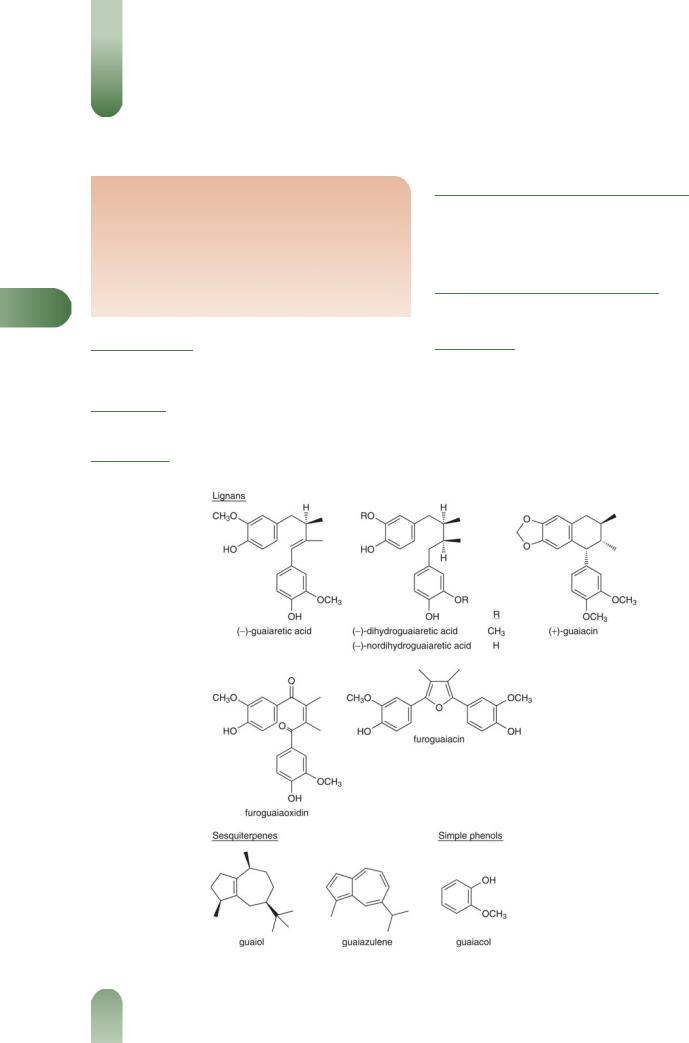
Figure 1 Selected constituents of guaiacum.
Pharmacopoeial and Other Monographs
BHC 1992(G6)
BHP 1996(G9)
Complete German Commission E(G3)
Martindale 35th edition(G85)
Legal Category (Licensed Products)
GSL(G37)
Constituents
The following is compiled from several sources, including General Reference G6.
Resins 15–20%. Guaiaretic acid, dehydroguaiaretic acid, guaiacin, isoguaiacin, a-guaiaconic acid (lignans), furoguaiacin and its monomethyl ether, furoguaiacidin, tetrahydrofuroguaia- cin-A and tetrahydrofuroguaiacin-B (furano-lignans), furoguaia oxidin (enedione lignan).(1–4)
Steroids b-Sitosterol.
344

Terpenoids Saponins, oleanolic acid.(5, 6) Sesquiterpenes, including guaiol and guaiazulene.
Food Use
Guaiacum is listed by the Council of Europe as a natural source of food flavouring (category N2). This category indicates that guaiacum can be added to foodstuffs in small quantities, with a
possible limitation of an active principle (as yet unspecified) in the final product.(G16)
Herbal Use
Guaiacum is stated to possess antirheumatic, anti-inflammatory, diuretic, mild laxative and diaphoretic properties. Traditionally, it has been used for subacute rheumatism, prophylaxis against gout,
and specifically for chronic rheumatism and rheumatoid arthri-
tis.(G6, G7, G8, G64)
Dosage
Dosages for oral administration (adults) for traditional uses recommended in older standard herbal and/or pharmaceutical reference texts are given below.
Dried wood 1–2 g as a decoction three times daily.(G6, G7)
Liquid extract 1–2 mL (1 : 1 in 80% alcohol) three times
daily.(G6, G7)
Figure 2 Guaiacum (Guaiacum officinale).
Figure 3 Guaiacum – dried drug substance (wood).
Guaiacum |
345 |
Tincture of Guaiacum (BPC 1934) 2–4 mL.
Pharmacological Actions
In vitro and animal studies
None documented. Antimicrobial properties are associated with lignans and much has been documented for nordihydroguaiaretic acid, the principal lignan constituent in chaparral (see Chaparral).
Clinical studies |
|
There is a lack of clinical research assessing the effects of |
|
guaiacum and rigorous randomised controlled clinical trials are |
|
required. |
|
Side-effects, Toxicity |
G |
There is a lack of clinical safety and toxicity data for guaiacum and further investigation of these aspects is required.
Guaiacum resin has been reported to cause contact dermatitis.(G51) The resin is documented to be of low toxicity; the oral LD50 in rats is greater than 5 g/kg body weight.(G48)
Contra-indications, Warnings
It is recommended that guaiacum is avoided by individuals with hypersensitive, allergic or acute inflammatory conditions.(G49)
Drug interactions None documented. However, there is limited pharmacological information for guaiacum, and the potential for preparations of guaiacum to interact with other medicines administered concurrently should be considered.
Pregnancy and lactation The safety of guaiacum during pregnancy has not been established. In view of this, and the overall lack of pharmacological and toxicological data, the use of guaiacum during pregnancy and lactation should be avoided.
Preparations
Proprietary multi-ingredient preparations
Australia: Boswellia Compound; Guaiacum Complex. Switzerland: Pommade au Baume. UK: Gerard House Reumalex; Rheumatic Pain Relief; Rheumatic Pain Remedy; Rheumatic Pain.
References
1 King FE, Wilson JG. The chemistry of extractives from hardwoods. Part XXXVI. The lignans of Guaiacum officinale L. J Chem Soc 1964; 4011–4024.
2Kratochvil JF et al. Isolation and characterization of a-guaiaconic acid and the nature of guaiacum blue. Phytochemistry 1971; 10: 2529–3251.
3Majumder PL, Bhattacharyya M. Structure of furoguaiacidin: a new furanoid lignan of the heartwood of Guaiacum officinale L. Chem Ind
1974; 77–78.
4Majumder P, Bhattacharyya M. Furoguaiaoxidin – a new enedione lignan of Guaiacum officinale: a novel method of sequential introduction of alkoxy functions in the 3- and 4-methyl groups of 2,5-
diaryl-3,4-dimethylfurans. JCS Chem Commun 1975; 702–703.
5Ahmad VU et al. Officigenin, a new sapogenin of Guaiacum officinale. J Nat Prod 1984; 47: 977–982.
6Ahmad VU et al. Guaianin, a new saponin from Guaiacum officinale. J Nat Prod 1986; 49: 784–786.
