
- •Preface
- •Acknowledgments
- •Introduction
- •Cardiac Tissue Engineering
- •Objectives and Scopes
- •Organization of the Monograph
- •Bibliography
- •Introduction
- •The Heart and Cardiac Muscle Structure
- •Myocardial Infarction and Heart Failure
- •Congenital Heart Defects
- •Endogenous Myocardial Regeneration
- •Potential Therapeutic Targets and Strategies to Induce Myocardial Regeneration
- •Bibliography
- •Introduction
- •Human Embryonic Stem Cells
- •Induced Pluripotent Stem Cells
- •Direct Reprogramming of Differentiated Somatic Cells
- •Cardiac Stem/Progenitor Cells
- •Summary and Conclusions
- •Bibliography
- •Introduction
- •Basic Biomaterial Design Criteria
- •Biomaterial Classification
- •Natural Proteins
- •Natural Polysaccharides
- •Synthetic Peptides and Polymers
- •Basic Scaffold Fabrication Forms
- •Hydrogels
- •Macroporous Scaffolds
- •Summary and Conclusions
- •Bibliography
- •Biomaterials as Vehicles for Stem Cell Delivery and Retention in the Infarct
- •Introduction
- •Stem Cell Delivery by Biomaterials
- •Cardiac Stem/Progenitor Cells
- •Clinical Trials
- •Summary and Conclusions
- •Bibliography
- •Introduction
- •Myocardial Tissue Grafts Created in Preformed Implantable Scaffolds
- •Summary and Conclusions
- •Bibliography
- •Introduction
- •Bioreactor Cultivation of Engineered Cardiac Tissue
- •Mass Transfer in 3D Cultures
- •Bioreactor as a Solution for Mass Transfer Challenge
- •Perfusion Bioreactors
- •Inductive Stimulation Patterns in Cardiac Tissue Engineering
- •Mechanotransduction and Physical/Mechanical Stimuli
- •Mechanical Stimulation Induced by Magnetic Field
- •Electrical Stimulation
- •Summary and Conclusions
- •Bibliography
- •Introduction
- •Prevascularization of the Patch by Incorporating Endothelial Cells (ECs)
- •The Body as a Bioreactor for Patch Vascularization
- •Summary and Conclusions
- •Bibliography
- •Introduction
- •Decellularized ECM
- •Injectable Biomaterials
- •Injectable hydrogels based on natural or synthetic polymers
- •Injectable Decellularized ECM Matrices
- •Mechanism of Biomaterial Effects on Cardiac Repair
- •Immunomodulation of the Macrophages by Liposomes for Infarct Repair
- •Inflammation, Apoptosis, and Macrophage Response after MI
- •Summary and Conclusions
- •Bibliography
- •Introduction
- •Evolution of Bioactive Material Approach for Myocardial Regeneration
- •Bioactive Molecules for Myocardial Regeneration and Repair
- •Injectable Systems
- •Sulfation of Alginate Hydrogels and Analysis of Binding
- •Injectable Affinity-Binding Alginate Biomaterial
- •Summary and Conclusions
- •Bibliography

162 10. BIOMATERIAL-BASED CONTROLLED DELIVERY OF BIOACTIVE MOLECULES
&
a |
b |
** |
** |
* |
* |
*
*
Figure 10.7: Sequential delivery of VEGF, PDGF-BB and TGF-β1 induce angiogenesis and vessel maturation in affinity-binding alginate scaffolds after subcutaneous implantation in rats. C. Blood vessel size and maturation in the implanted scaffolds as judged by (a) percentage of area occupied by blood vessels determined on lectin-stained sections, and (b) percentage of matured vessels, i.e., the ratio of α- SMA-positive vessel density to lectin-positive vessel density × 100. Alg-S/Alg-Triple and Alg-Triple are alginate-sulfate/alginate and alginate scaffolds, respectively, loaded with triple factors (VEGF, PDGF-BB and TGF-β1). Alg-S/Alg is alginate-sulfate/alginate scaffold with no factors. Empty bars - Alg-S/Alg; grey bars - Alg-Triple; black bars - Alg-S/Alg-Triple. ( )p < 0.05, ( )p < 0.01. Values represent the mean and standard deviation. Reprinted with permission from [49].
cells, also showed beneficial results, possibly due to the sustained presentation of the included growth factors and their prolonged activity at the infarct zone.
10.6.4 INJECTABLE AFFINITY-BINDING ALGINATE BIOMATERIAL
Features of the System
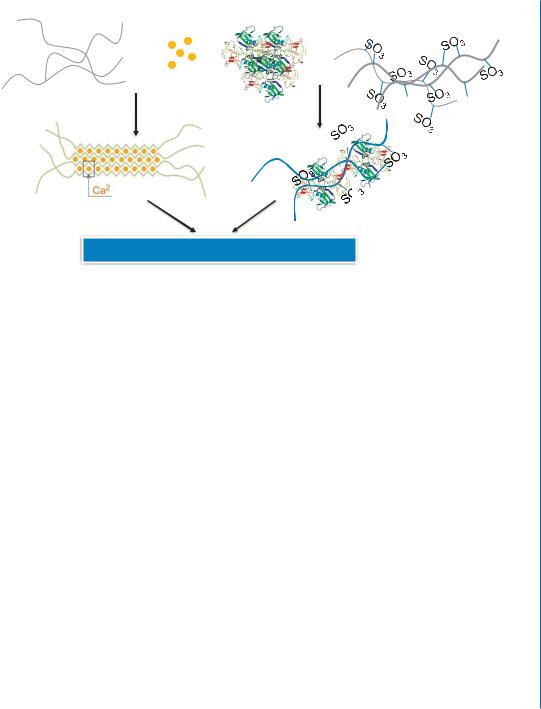
To enhance the clinical applicability of the affinity-binding alginate system for myocardial repair, it was fabricated in an injectable form. This has been achieved by mixing the alginate-sulfate/growth factor bioconjugates with partially cross-linked alginate solution, whose preparation and properties are described in Section 9.5 (Fig. 10.8). The preparation of such a system is simple and rapid ( 2 hours), compared to the far more elaborative processes described above for the fabrication of delivery systems for multiple growth factors.
The injectable affinity-binding alginate system was shown to be easily delivered into the infarcted heart where it created a hydrogel in situ, capable of the sustained delivery of growth factors. As such the resultant system has dual functions: 1) it may confer temporary tissue support and replace damaged ECM, together with temporary mechanical stabilization of the infarct, as previously shown
10.6. AFFINITY-BINDING ALGINATE BIOMATERIAL 163
$OJLQDWH &DOFLXP *URZWK IDFWRU $OJLQDWH VXOIDWH
|
|
62 |
62 |
|
|||
+RPRJHQL]DWLRQ |
,QFXEDWLRQ |
62 |
62 |
|
|
 62 62
62 62
622 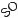 62
62
3DUWLDOO\ FURVV OLQNHG K\GURJHO |
%LRFRQMXJDWHV RI *) DOJLQDWH VXOIDWH |
|
$IILQLW\ ELQGLQJ DOJLQDWH K\GURJHO
Figure 10.8: Schematic representation of the preparation methodology of injectable affinity-binding alginate system.
for this unique alginate biomaterial; 2) it should control the release and presentation of multiple growth factors. In the next section, we present several animal studies, using different disease models and different combination of growth factors, which provide proof-of-concept for the efficacy of the injectable affinity-binding alginate system.
Dual Growth Factor Release and Tissue Retention at the Infarct
Among the known available bioactive molecules, insulin-like growth factor-1 (IGF-1) and hepatocyte growth factor (HGF) are well known as potent cardiovascular-protective proteins, affecting several major aspects of myocardial regeneration. IGF-1 is a strong antiapoptotic factor in different cell types, including cardiomyocytes [10, 57, 58, 59]. Due to its marked cardioprotection effect, IGF-1 administration can improve cardiac function after MI [6]. HGF is a strong proangiogenic and antifibrotic factor [30, 60, 61, 62, 63]. In addition, HGF, together with IGF-1, induced resident cardiac stem cell migration and activation, that led to new myocardium formation in dogs [64, 65]. Due to their established and complimentary beneficial effects on infarcted myocardium, these proteins were chosen as bioactive components of the injectable delivery system. Strong affinity binding of both proteins to alginate-sulfate has been previously confirmed (Table 10.2) [48].
The release profile of the proteins from affinity-binding alginate hydrogel revealed a sequential factor delivery pattern with a greater amount of IGF-1 initially being released to the external medium until cessation on day 3, while HGF continued to be released and accumulated in the medium (Fig. 10.9) [51].
164 10. BIOMATERIAL-BASED CONTROLLED DELIVERY OF BIOACTIVE MOLECULES
Figure 10.9: Dual IGF-1/HGF release pattern from affinity-binding alginate hydrogel. A. In vitro release from the dual factor-loaded system. B. The dual factor release profile coincides with the sequence of reparative processes after MI. Faster released IGF-1 could provide a strong pro-survival signal at early stages, while slower released HGF could reduce fibrosis and induce angiogenesis at later stages. Reprinted with permission from [51].

10.6. AFFINITY-BINDING ALGINATE BIOMATERIAL 165
The sequential delivery of IGF-1 and HGF is suited for the proper execution of the reparative processes in the infarcted myocardium, to achieve a more favorable course of repair (Fig. 10.9). The faster released IGF-1 could provide an immediate strong pro-survival signal to rescue the functional myocardium and reduce cell apoptosis and loss after the initial ischemic event [59, 66, 67]. Processes required at later phases of repair, such as angiogenesis induction, more favorable ECM remodeling, and fibrosis reduction, can be mediated by the slower, yet continuous, release of HGF [68, 69, 70].
The released IGF-1 and HGF maintained their biological activities. Both proteins were shown to activate their respective intracellular signaling pathways (phosphorylation of AKT and ERK1/2 protein kinases for IGF-1 and HGF, respectively) and prevent cardiac cell death in an oxidative stress model [50, 51].
Increased retention of therapeutic proteins over time is one of the main attributes of a successful therapy. As mentioned, the infarct after the initial ischemic event represents a very hostile environment, where extensive protein degradation takes place as part of inflammation and ECM remodeling-induced enzymatic responses [71, 72]. Thus, we chose an acute MI and immediate post-MI injection as a model for testing the efficacy and impact of our delivery system on protein retention. HGF delivery from the affinity-binding alginate solution resulted in much greater retention and bioavailability of the factor in myocardial tissue after acute MI, as measured using anti-human HGF-specific ELISA. In contrast, soluble HGF administered by bolus injection was rapidly eliminated from the infarct [50] (Fig. 10.10).
The greater retention of HGF when delivered from the affinity-binding system is attributed to the strong, yet reversible, binding of the factor to alginate-sulfate. The in situ gelation of alginate forms a reservoir for the HGF/alginate-sulfate bioconjugates, thus providing an additional barrier for HGF diffusion and release.
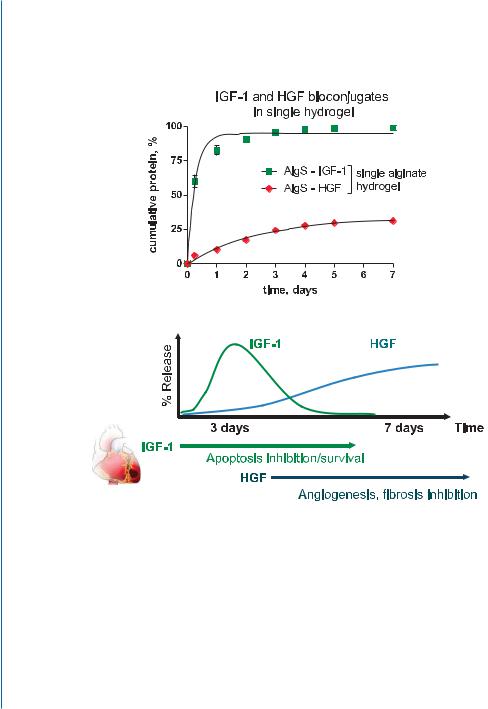
Based on the collected data (bioconjugation and growth factor protection, sustained release and increased protein retention in vivo), the activity of the growth factors delivered by the in situ formed hydrogel with the affinity module could be the manifestation of three main processes and their combinations (Fig. 10.11): 1) the proteins are released at a rate determined by their equilibrium binding constants to alginate-sulfate and the initial loaded concentration of the individual components (affinity-binding mechanism); 2) the bioconjugates of alginate-sulfate and the factors are presented in a natural way to their respective cellular receptors, thus improving their activation and signaling, similar to their native interaction with heparan-sulfate [73]; and 3) the bioconjugates are released with time due to hydrogel dissolution, due to a decrease in calcium concentration (Fig. 10.11).

166 10. BIOMATERIAL-BASED CONTROLLED DELIVERY OF BIOACTIVE MOLECULES
$

+*) SJ PJ WLVVXH






|
|
6DOLQH +*) |
|
$IILQLW\ ELQGLQJ DOJLQDWH +*) |
|
1RQ PRGLILHG DOJLQDWH +*) |
|
|
|
|
|
|
|
WLPH KRXUV
%
3DUDPHWHU |
6DOLQH +*) |
$IILQLW\ ELQGLQJ DOJ |
1RQ PRGLILHG DOJ ± |
|
|
|
+*) |
+*) |
|
$8& ± PHDVXUH RI |
|
|
|
|
ELRDYDLODELOLW\ SJ×K PJ WLVVXH |
||||
|
|
|
||
|
|
|
|
Figure 10.10: HGF retention at infarct, after injection into rat acute MI model.A. HGF retention profile when injected in various formulations. B. Nonlinear regression of data obtained from HGF retention studies in infarcted myocardium. AUC – area under the curve. Reprinted with permission from [50].
10.6. AFFINITY-BINDING ALGINATE BIOMATERIAL 167
Growth factors Alginate-sulfate
|
SO3 |
SO3 |
|
||
Incubation |
SO3 |
SO3 |
Bioconjugate nanoparticles with various proteins
Release based on
binding constant and factor concentration
Partially cross-linked hydrogel
Presentation
 Hydrogel dissolution
Hydrogel dissolution
In situ gelation
Different factor bioconjugates entrapped within cross-linked alginate chains – a single device
Figure 10.11: The concept of injectable affinity-binding alginate biomaterial, and the proposed modes for protein release and action.
Therapeutic Efficacy of the Combination Platform of Biomaterial and Sustained Factor Delivery
The therapeutic outcome of a single or multiple growth factor delivery by injectable affinity-binding alginate was evaluated in two ischemic disease models: 1) severe hindlimb ischemia in mice; and 2) rat model of acute MI.
Therapeutic angiogenesis in hindlimb ischemia model
Therapeutic angiogenesis is one of the key constituents for successful therapy of ischemic heart disease, peripheral artery disease, and other disorders. Induction of re-vascularization can salvage damaged ischemic tissues and facilitate self-repair [74]. As HGF is a potent angiogenic factor, we tested whether its controlled delivery by an affinity-binding alginate system would prolong and maximize its therapeutic action in a murine disease model of hindlimb ischemia [5, 70]. This strategy resulted in improved tissue perfusion, as judged by laser Doppler analysis nine days after the operation, compared to other treatment groups (Fig. 10.12).
Moreover, the treatment with HGF delivered by the affinity-binding alginate system resulted in a greater density of mature blood vessel network [50]. More recently, improved tissue blood perfusion and marked angiogenesis in the same model were observed using an injectable affinity-

168 10. BIOMATERIAL-BASED CONTROLLED DELIVERY OF BIOACTIVE MOLECULES
$
% |
|
|
& |
|
|
|
|
|
|
|
|
+LQGOLPE SHUIXVLRQ UDWLR LVFKHPLF QRQ LVFKHPLF |
|
|
|
||
|
|
|
|
||
|
|
|
|
||
|
|
|
|||
|
|
EORRG YHVVHOV PP |
|
||
|
|
|
|
||
|
|
|
|||
|
|
|
|
||
|
|
|
|||
|
|
|
|
||
|
|
|
|
|
|
|
GD\ |
GD\ |
|
|
|
|
|
|
|
Figure 10.12: Prolonged delivery of HGF from affinity-binding alginate improves limb perfusion. A. Representative laser Doppler scans, 9 days after ischemia induction. B. Calculated perfusion percentage in different treatment groups at baseline and 9 days after operation. Green bars – saline; blue bars- affinity-binding alginate; red bars – saline-soluble HGF; brown bars – HGF-affinity-binding alginate. P (interaction, two-way repeated measures ANOVA) < 0.0001. − p < 0.05 (Bonferroni’s post-hoc test), n=10/group. C. Quantitative analysis of α-SMA-positive blood vessel density. P (one-way ANOVA) < 0.0001. Pairs indicated have significant differences (p < 0.05) (Bonferroni’s posthoc test). n=10/group. Reprinted with permission from [50].
binding alginate biomaterial loaded with three angiogenic factors, VEGF, TGF-β1, and PDGF-BB (unpublished results).
The affinity-binding alginate was able to translate the known angiogenic effect of HGF into an improved therapeutic outcome, due to creation of a temporary favorable microenvironment for self-repair on one hand, and successful controlled delivery of the protein together with protection from enzymatic degradation and fast elimination in ischemic tissue on the other hand.
Acute MI model
Short (one week) and long (four and eight weeks) - term effects of dual (IGF-1 and HGF) factor delivery from the injectable affinity-binding alginate were tested in a rat model of acute MI. We focused on various parameters and aspects in myocardial tissue regeneration and repair, in order to

10.6. AFFINITY-BINDING ALGINATE BIOMATERIAL 169
examine the efficacy of the delivery system to translate known effects of the growth factors into a significant therapeutic outcome.
The sequential delivery of IGF-1 and HGF from this system reduced scar fibrosis, increased scar thickness and prevented infarct expansion, one and four weeks after injection. It also induced angiogenesis and mature blood vessel network formation, and reduced cell apoptosis at the infarct. These therapeutic effects were preserved for eight weeks after injection (unpublished data) (Fig. 10.13A).
We then evaluated the contribution of endogenous regeneration to increased tissue salvage.We focused on two processes that can be responsible for endogenous regeneration of cardiac muscle: adult cardiomyocyte proliferation (shown by staining for mitotic marker Ki-67) and the existence of cardiac stem/progenitor cells (identified by staining for transcription factor GATA-4, generally associated with cardiomyogenic differentiation) (Fig. 10.13B-D). Of note, these results were deduced based on simple morphological discrimination and single marker staining. Thus, more extensive analyzes (e.g., genetic mapping, simultaneous staining) are required to confirm the effects of sequential IGF- 1/HGF delivery on endogenous myocardial regeneration.
Based on staining pattern and morphological discrimination, we identified Ki-67-positive cardiomyocytes at the infarct border of all animal groups, one week after treatment. Due to the high ultrastructural fiber organization of Ki-67-positve cells, the possible explanation for this phenomenon is cell cycle re-entry that can occur at higher incidence after MI [75]. Strikingly, the sequential delivery of IGF-1/HGF from the affinity-binding alginate biomaterial in the infarct increased the incidence of Ki-67-positive cardiomyocytes (Fig. 10.13B, D) [51]. Cell cycle re-entry could point to actual cell proliferation, an important constituent of myocardial regeneration, especially in light of recent data suggesting that endogenous myocardial regeneration could be driven by the induction of cell cycle re-entry and proliferation of existing cardiomyocytes rather than by stem or progenitor cells [76].
As an additional aspect of possible myocardial regeneration, we examined GATA-4 expression in the infarcts (Fig. 10.13C, D). Recent data show that along with its established critical role in early and late heart development and morphogenesis, GATA-4 also acts as a pro-angiogenic, antiapoptotic, and stem cell-recruiting factor post-MI [77, 78]. The GATA-4-positive cell clusters were found only in the peri-infarct areas of animals treated with the factors, with the highest incidence detected four weeks post-MI in the animals treated with the sequentially delivered factors (Fig. 10.13D) [51]. From the cell cluster organization, we propose that these cells could be stem or progenitor cells of unknown origin. IGF-1 and HGF were previously shown to activate distinct subsets of cardiac stem cells [79]. The long-term delivery of both factors could induce the migration of these cells to the infarct region and their subsequent activation, which in turn, can partially contribute to myocardial tissue salvage. Increased GATA-4 expression could by itself contribute to pro-angiogenic and anti-apoptotic properties of the delivered proteins [77].
The extent of possible endogenous regeneration (based on Ki-67 and GATA-4 staining) after the treatment with sequentially delivered proteins in affinity-binding alginate hydrogel was

170 10. BIOMATERIAL-BASED CONTROLLED DELIVERY OF BIOACTIVE MOLECULES
$ |
,QIDUFW H[SDQVLRQ LQGH[ |
|
)LEURWLF DUHD VFDU |
|
|
|
|
||||
|
|
|
|
|
|
%ORRG YHVVHO GHQVLW\ Į 60$ $SRSWRVLV DFWLYH FDVSDVH
Figure 10.13: Therapeutic efficacy and cardiac tissue regeneration induced by the dual factor delivery from the injectable affinity-binding alginate system in acute MI model. A. The effects of a sequential delivery of IGF-1/HGF by injectable affinity-binding alginate biomaterial on various aspects of tissue regeneration. Infarct expansion index was evaluated by Masson’s trichrome staining. Blood vessels were identified by α-SMA staining. Apoptotic cells were identified by active caspase-3 staining. − p < 0.05. Reprinted with permission from [51] and unpublished data.
significantly reduced after a longer period of time (four weeks) [51]. This can be explained by the suboptimal concentration of the growth factors due to cessation of growth factor release and action, and also by the lack of additional endogenous signals, that could act synergistically with the delivered proteins, and are present only for a limited period of time after initial infarct. This can be overcome in future studies by administering several injections of the growth factor/biomaterial combinations.

|
|
10.6. AFFINITY-BINDING ALGINATE BIOMATERIAL 171 |
% |
|
|
|
|
$IILQLW\ ELQGLQJ DOJLQDWH |
,*) +*) |
|
&0 |
&0 |
&0 |
.L |
|
.L |
&
|
|
$IILQLW\ ELQGLQJ DOJLQDWH |
,*) +*) |
|
*$7$ |
|
*$7$ |
Figure 10.13: Therapeutic efficacy and cardiac tissue regeneration induced by the dual factor delivery from the injectable affinity-binding alginate system in acute MI model. B. Mitotic marker Ki-67 staining (brown) in healthy/infarct border region, one week after injection. Arrows show nuclear localization or unstained nuclei. Bar = 50 μm. CM-cardiomyocytes. Reprinted with permission from [51] and unpublished data. C. Cardiac progenitor cell marker GATA-4 staining (brown) in infarcted hearts (periinfarct region), four weeks after injection. Arrows show nuclear localization. Bar = 50 μm. Reprinted with permission from [51] and unpublished data.

172 10. BIOMATERIAL-BASED CONTROLLED DELIVERY OF BIOACTIVE MOLECULES
' |
|
|
|
|
|
D |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
.L SRVWLYH FDUGLRP\RF\WHV ILHOG |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
$IILQLW\ ELQGLQJ |
6DOLQH |
||
|
|
DOJLQDWH |
|||
|
|
|
|
||
,*) +*) |
|
|
|
|
|
E
*$7$ LQFLGHQFH
|
$IILQLW\ ELQGLQJ DOJ |
$IILQLW\ ELQGLQJ |
6DOLQH +*) ,*) |
6DOLQH |
|
+*) ,*) |
DOJ |
|
|
|
|
|
|
|
ZHHN |
|
|
|
|
|
||||
|
|
|
|
|
ZHHNV |
|
|
|
|
|
||||
|
|
|
|
|
Figure 10.13: Therapeutic efficacy and cardiac tissue regeneration induced by the dual factor delivery from the injectable affinity-binding alginate system in acute MI model. D-a. Quantitative analysis of Ki-67-positive cardiomyocytes (based on morphological discrimination) in short-term (one week) experiment. − < 0.001. D-b. Incidence of GATA-4-positive cell clusters in different treatment groups. Incidence = (number of animals with positive staining/total number of animals in each group) × 100. Reprinted with permission from [51] and unpublished data.
The beneficial effects of the sequential growth factor delivery by affinity-binding alginate biomaterial were shown to be specifically mediated by the active components of the system, i.e., IGF-1 and HGF.The effect of the biomaterial, if found, was limited to only short-term effects which were not seen after four weeks. This initial effect is possibly due to the sequestering and increased local retention of various endogenous cardioprotective and angiogenic factors by the affinity-binding system.
The results observed after the treatment with the sequentially delivered proteins from the affinity-binding alginate system collectively suggest that marked salvage of the functional tissue occurred due to the enhanced retention of the bioactive factors at the infarct and their sequential release pattern, mediated by the affinity-binding mechanism to alginate-sulfate and in situ gelation of the system (Fig. 10.11). Increased retention and prolonged release of the growth factors facilitate a more favorable course of repair, by supplying the required protective signals for longer periods of time. As a result, prolonged growth factor activity leads to more favorable remodeling at later stages (four and eight weeks after MI). These effects could also be accompanied by passive tissue support and mechanical stabilization of the infarct conferred by the alginate hydrogel itself [80, 81].
