
- •Preface
- •Acknowledgments
- •Introduction
- •Cardiac Tissue Engineering
- •Objectives and Scopes
- •Organization of the Monograph
- •Bibliography
- •Introduction
- •The Heart and Cardiac Muscle Structure
- •Myocardial Infarction and Heart Failure
- •Congenital Heart Defects
- •Endogenous Myocardial Regeneration
- •Potential Therapeutic Targets and Strategies to Induce Myocardial Regeneration
- •Bibliography
- •Introduction
- •Human Embryonic Stem Cells
- •Induced Pluripotent Stem Cells
- •Direct Reprogramming of Differentiated Somatic Cells
- •Cardiac Stem/Progenitor Cells
- •Summary and Conclusions
- •Bibliography
- •Introduction
- •Basic Biomaterial Design Criteria
- •Biomaterial Classification
- •Natural Proteins
- •Natural Polysaccharides
- •Synthetic Peptides and Polymers
- •Basic Scaffold Fabrication Forms
- •Hydrogels
- •Macroporous Scaffolds
- •Summary and Conclusions
- •Bibliography
- •Biomaterials as Vehicles for Stem Cell Delivery and Retention in the Infarct
- •Introduction
- •Stem Cell Delivery by Biomaterials
- •Cardiac Stem/Progenitor Cells
- •Clinical Trials
- •Summary and Conclusions
- •Bibliography
- •Introduction
- •Myocardial Tissue Grafts Created in Preformed Implantable Scaffolds
- •Summary and Conclusions
- •Bibliography
- •Introduction
- •Bioreactor Cultivation of Engineered Cardiac Tissue
- •Mass Transfer in 3D Cultures
- •Bioreactor as a Solution for Mass Transfer Challenge
- •Perfusion Bioreactors
- •Inductive Stimulation Patterns in Cardiac Tissue Engineering
- •Mechanotransduction and Physical/Mechanical Stimuli
- •Mechanical Stimulation Induced by Magnetic Field
- •Electrical Stimulation
- •Summary and Conclusions
- •Bibliography
- •Introduction
- •Prevascularization of the Patch by Incorporating Endothelial Cells (ECs)
- •The Body as a Bioreactor for Patch Vascularization
- •Summary and Conclusions
- •Bibliography
- •Introduction
- •Decellularized ECM
- •Injectable Biomaterials
- •Injectable hydrogels based on natural or synthetic polymers
- •Injectable Decellularized ECM Matrices
- •Mechanism of Biomaterial Effects on Cardiac Repair
- •Immunomodulation of the Macrophages by Liposomes for Infarct Repair
- •Inflammation, Apoptosis, and Macrophage Response after MI
- •Summary and Conclusions
- •Bibliography
- •Introduction
- •Evolution of Bioactive Material Approach for Myocardial Regeneration
- •Bioactive Molecules for Myocardial Regeneration and Repair
- •Injectable Systems
- •Sulfation of Alginate Hydrogels and Analysis of Binding
- •Injectable Affinity-Binding Alginate Biomaterial
- •Summary and Conclusions
- •Bibliography

63
C H A P T E R 6
Bioengineering of Cardiac
Patches, In Vitro
CHAPTER SUMMARY
In vitro-generated cardiac tissue grafts represent an ideal solution when the replacement of significant portions of the myocardium (damaged by myocardial infarction or bearing a structural defect) is required.This chapter introduces the principles of in vitro cardiac tissue engineering and presents three main strategies developed to attain cardiac patches, in vitro: cell entrapment in hydrogels, cell sheet technology, and cell seeding in preformed macroporous scaffolds. It describes the various materials and scaffolds in use, the evolution of new synthetic scaffolds by integration of cell-matrix interactions bio-inspired by ECM to improve tissue organization and introduces recent advancements in microand nano-fabrication techniques applied for the fabrication of scaffolds bio-mimicking the physical structure and architecture of cardiac ECM. In Chapter 7, we will present the dynamic environment created by perfusion bioreactors and stimulation patterns, which is necessary for attaining a functional cardiac patch.
6.1INTRODUCTION
The replacement of a large scar tissue after MI and the corrections of congenital heart malformations, such as septal defects, require the transplantation of a fully developed cardiac tissue graft prepared in vitro. The engineered cardiac patch should be thick and has to display the functional and morphological properties of the native cardiac muscle. Once integrated into the heart, the cardiac patch must develop systolic force, withstand diastolic load with appropriate compliance, and form an electrical and functional syncytium with the host myocardium [1].
Three main strategies for in vitro cardiac patch reconstruction have been developed over the last decade or so and they are based on seeding the cardiac cells in scaffolds in the form of hydrogels, polymer layers, and pre-formed macro-porous scaffolds (Fig. 6.1).
The scaffolds are an important component in the in vitro construction of the cardiac patch as they provide the physical support and biological cues and instruct tissue formation. Early studies in cardiac tissue engineering investigated scaffolds fabricated from natural polymers, such as alginate, chitosan, collagen, and gelatin, or from synthetic polyesters and polyethers. To improve survival and cell organization in these scaffolds, the cardiac cells were frequently seeded mixed with Matrigel to enable cell adhesion. However, Matrigel, being a gelatinous protein mixture secreted by mouse tumor

64 6. BIOENGINEERING OF CARDIAC PATCHES, IN VITRO
,Q YLWURWLVVXH HQJLQHHULQJ
&HOO |
&HOO VKHHW |
' |
|
HQWUDSPHQW |
|||
WHFKQRORJ\ |
VFDIIROGV |
||
(+7 |
|||
|
|
0DWXUDWLRQ YDVFXODUL]DWLRQ
VWLPXODWLRQ VWHSV
,Q YLYRLPSODQWDWLRQ
Figure 6.1: Strategies for reconstructing the cardiac patch. Three major strategies are used for preparation of cardiac patches: cell entrapment in hydrogels (EHT), cell sheet engineering, and cell seeding in preformed macroporous 3D scaffolds. After initial culture, various stimulation strategies could be applied (i.e., perfusion bioreactors, electrical stimulation) in order to improve cell organization and tissue maturation. Finally, the constructs can be implanted into infarcted heart. See text for more details.
cells, contains growth factors and unknown proteins that limit its desirability for experiments, as they require precise conditions or implantation into human beings. Consequently, scaffold research in recent years has advanced to designing, synthesizing, and modifying materials to selectively and spatially interact with cells through defined bio-molecular recognition events. More recently, with the advancements in nanoand micro-fabrication techniques, these tools have been employed in the fabrication of scaffolds bio-mimicking the physical structure and architecture of cardiac ECM. In particular, these technologies have been adapted for enabling and promoting of patch vascularization.
As a tribute to the pioneers in this exciting field, we first introduce their works which lay the foundation for cardiac tissue engineering.

6.2. CARDIAC CELL ENTRAPMENT IN HYDROGELS—ENGINEERED HEARTTISSUE (EHT) 65
6.2CARDIAC CELL ENTRAPMENT IN HYDROGELS—ENGINEERED HEART TISSUE (EHT)
In this strategy, cardiac cells are mixed with a liquid form of the biomaterial, followed by its solidification to create a mixed 3D cell hydrogel. This strategy has been developed by Eschenhagen and collaborators, who cultured embryonic cardiac myocytes in a collagen type I matrix to produce a coherently contracting 3D Engineered Heart Tissue (EHT) (Fig. 6.2) [2].
D E
F |
|
G |
|
|
|
H |
I |
Figure 6.2: An engineered heart tissue (EHT) constructed by cell entrapment method. (a) Multiloop EHT ready for in vivo engraftment. (b) EHTs fixed on the recipient heart. (c) Four weeks after EHT engraftment, the construct formed compact and oriented heart muscle on the top of the infarct scar.
(d) Laser confocal microscopy showing the highly differentiated sarcomeric organization of engrafted cardiomyocytes (actin, green; nuclei, blue). (e-f ) Representative plots of epicardial activation times in sham-operated (e) and EHT-engrafted hearts (f ), showing undelayed coupling of EHT to the host myocardium. Reprinted with permission from [3].
Later, the same group improved the cardiac patch by suspending cardiomyocytes from neonatal rats in a mixture of collagen type I and Matrigel and casting the solidified matrix into circular
66 6. BIOENGINEERING OF CARDIAC PATCHES, IN VITRO
molds [4]. The construct was then subjected to a mechanical stretch, stimulating the tissue to differentiate and mature. The result was a ring-shape EHT that displayed important hallmarks of differentiated myocardium, such as a striated shape and an electrical response to a β-agonist [4]. Implantation of the cardiac patch into infarcted hearts showed undelayed electrical coupling to the native myocardium without arrhythmias, prevented LV dilation, induced systolic wall thickening, and improved cardiac function [3].The EHT construct has also been found to be a valuable tool for preclinical toxicology assay [5]. Although the cardiac cell entrapment in hydrogels has led to the formation of beating cardiac tissue, the strategy in its present form could not promote thick tissue assembly (i.e., over 100 μm in thickness), mainly due to mass transfer limitations to the center of the construct.
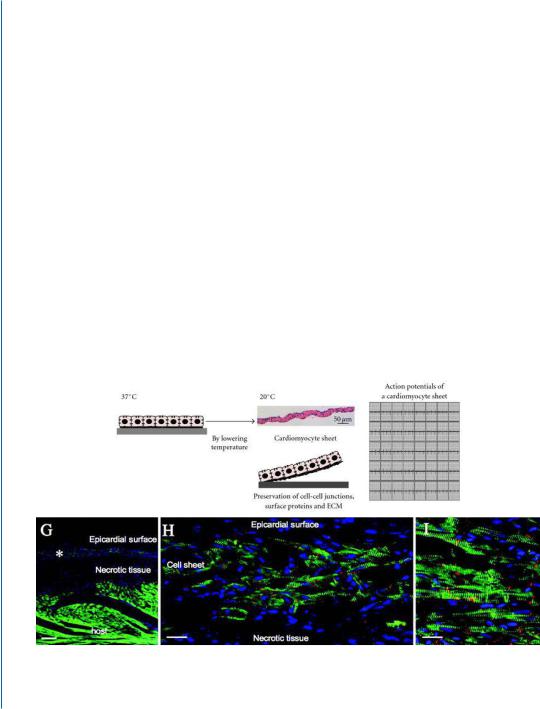
6.3CELL SHEET-BASED CARDIAC TISSUE ENGINEERING
Shimizu and co-workers developed a novel approach in which cardiomyocyte monolayers are assembled to form multi-layered heart muscle constructs [6, 7]. The cardiomyocyte layers were initially formed by cultivating isolated cardiac cells on cell culture surfaces, grafted with the temperatureresponsive polymer, poly (N −isopropylacrylamide) (PIPAAm). Confluent cell monolayers could be detached from the surface as a cell sheet simply by reducing the temperature, without any enzymatic treatments. The released cell layers (2D) were overlaid one on top of the other, forming a multi-layer 3D tissue that began to pulsate simultaneously (Fig. 6.3) [6].
D
E |
|
F |
|
G |
Figure 6.3: Reprinted with permission from [7, 8]. Caption on the next page.
