
- •Preface
- •Acknowledgments
- •Introduction
- •Cardiac Tissue Engineering
- •Objectives and Scopes
- •Organization of the Monograph
- •Bibliography
- •Introduction
- •The Heart and Cardiac Muscle Structure
- •Myocardial Infarction and Heart Failure
- •Congenital Heart Defects
- •Endogenous Myocardial Regeneration
- •Potential Therapeutic Targets and Strategies to Induce Myocardial Regeneration
- •Bibliography
- •Introduction
- •Human Embryonic Stem Cells
- •Induced Pluripotent Stem Cells
- •Direct Reprogramming of Differentiated Somatic Cells
- •Cardiac Stem/Progenitor Cells
- •Summary and Conclusions
- •Bibliography
- •Introduction
- •Basic Biomaterial Design Criteria
- •Biomaterial Classification
- •Natural Proteins
- •Natural Polysaccharides
- •Synthetic Peptides and Polymers
- •Basic Scaffold Fabrication Forms
- •Hydrogels
- •Macroporous Scaffolds
- •Summary and Conclusions
- •Bibliography
- •Biomaterials as Vehicles for Stem Cell Delivery and Retention in the Infarct
- •Introduction
- •Stem Cell Delivery by Biomaterials
- •Cardiac Stem/Progenitor Cells
- •Clinical Trials
- •Summary and Conclusions
- •Bibliography
- •Introduction
- •Myocardial Tissue Grafts Created in Preformed Implantable Scaffolds
- •Summary and Conclusions
- •Bibliography
- •Introduction
- •Bioreactor Cultivation of Engineered Cardiac Tissue
- •Mass Transfer in 3D Cultures
- •Bioreactor as a Solution for Mass Transfer Challenge
- •Perfusion Bioreactors
- •Inductive Stimulation Patterns in Cardiac Tissue Engineering
- •Mechanotransduction and Physical/Mechanical Stimuli
- •Mechanical Stimulation Induced by Magnetic Field
- •Electrical Stimulation
- •Summary and Conclusions
- •Bibliography
- •Introduction
- •Prevascularization of the Patch by Incorporating Endothelial Cells (ECs)
- •The Body as a Bioreactor for Patch Vascularization
- •Summary and Conclusions
- •Bibliography
- •Introduction
- •Decellularized ECM
- •Injectable Biomaterials
- •Injectable hydrogels based on natural or synthetic polymers
- •Injectable Decellularized ECM Matrices
- •Mechanism of Biomaterial Effects on Cardiac Repair
- •Immunomodulation of the Macrophages by Liposomes for Infarct Repair
- •Inflammation, Apoptosis, and Macrophage Response after MI
- •Summary and Conclusions
- •Bibliography
- •Introduction
- •Evolution of Bioactive Material Approach for Myocardial Regeneration
- •Bioactive Molecules for Myocardial Regeneration and Repair
- •Injectable Systems
- •Sulfation of Alginate Hydrogels and Analysis of Binding
- •Injectable Affinity-Binding Alginate Biomaterial
- •Summary and Conclusions
- •Bibliography

10.4. SCAFFOLDAND HYDROGEL SHEET-BASED MOLECULE DELIVERY 147
10.4SCAFFOLDAND HYDROGEL SHEET-BASED MOLECULE DELIVERY
Various scaffolds or sheet-like structures have been used for growth factor delivery to infarcted myocardium or for the repair of cardiac defects. Ota et al used a decellularized porcine urinary bladder to create a scaffold that was used for the repair of a surgically created defect in the right ventricular wall. The scaffold was loaded with fibronectin collagen-binding domain (CBD)-HGF fusion protein.The presence of CBD significantly improved HGF retention in the scaffold, probably due to its specific interactions with the collagen scaffold. The implantation of this scaffold increased contractility and electrical activity of the heart, and was associated with a homogenous repopulation by host cells and increased angiogenesis in the graft [31].
Growth factor-containing hydrogel sheets, prepared by impregnation of freeze-dried hydrogel sheets in growth factor-containing solution, have been extensively used in cardiovascular tissue engineering [32, 33]. Takehara et al evaluated the effect of controlled delivery of bFGF from gelatin hydrogel sheets in a chronic myocardial infarction model in pigs [34]. At four weeks after implantation, the local sustained delivery of bFGF stimulated myocardial perfusion and increased left ventricular EF. However, these effects were not compared to empty hydrogel controls. Fujiwara and co-workers used the same concept of gelatin hydrogel sheets for erythropoietin (EPO) delivery for infarct repair in rabbits. The patch was placed on the surface risk area immediately after infarct induction. Two months later, the EPO-containing hydrogel sheet improved cardiac function and reduced infarct size, compared to empty sheets or systemic EPO administration [35].
Zhang and co-workers created an SDF-1α-containing PEGylated fibrin patch by incubation of SDF-1 with PEGylated fibrinogen, followed by gelation with thrombin, and tested the effect of this delivery system in murine MI model. The patch increased c-kit+ stem cell homing to myocardium and improved cardiac function [36].
10.5 INJECTABLE SYSTEMS
Injectable systems composed of biomaterial-bioactive molecule combinations offer an additional advantage to above mentioned aspects of this strategy, i.e., greater applicability in clinical settings due to the less invasive route of delivery.
Gelatin hydrogel microspheres are widely used as a carrier for growth factor delivery in various settings, including into the infarcted heart. For instance, Iwakura et al used gelatin hydrogel microspheres for controlled delivery of bFGF into infarcted myocardium of rats.The treatment resulted in increased angiogenesis and improved systolic and diastolic function [37, 38]. In a similar approach, Deng and coworkers injected bFGF-containing gelatin hydrogel microspheres into infarcted hearts of dogs. MRI showed improved LV function and angiogenesis [39].
Injectable self-assembling peptide nanofibers (NF) were developed by Richard Lee and colleagues and were investigated as a supporting matrix and for protein delivery into an infarcted heart [40].
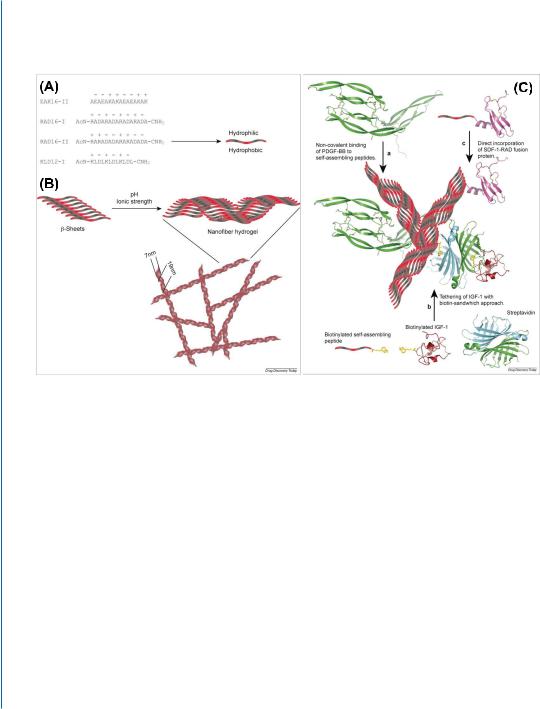
148 10. BIOMATERIAL-BASED CONTROLLED DELIVERY OF BIOACTIVE MOLECULES
Figure 10.2: Self-assembling peptide nanofiber system for growth factor delivery. A. Examples of different sequences of self-assembling peptides. Hydrophobic amino acids (alanine (A) or leucine (L)) alternate with positively (lysine (K) and arginine (R)) and negatively charged amino acids (glutamate (E) and aspartate (D)). Hydrophobic amino acids are directed to one side and hydrophilic (positively and negative charged) amino acids to the other side of peptides. B. Self-assembling peptides are arranged in stable β-sheets at low pH and low ionic strength. Upon exposure to physiological pH and ionic strength, they form stable nanofibers with a diameter of 10 and 50–200 nm pores. C. Self-assembling peptides allow delivery and tethering of proteins in different manners. (a) Self-assembling peptides allow long-term delivery in vivo of PDGF-BB. PDGF-BB binds to self-assembling peptides non-covalently by electrostatic interactions. (b) Biotinylated variants of self-assembling peptides have been synthesized. They allow binding of every protein that can be biotinylated using streptavidin as a linker. (c) The sequence of self-assembling peptides can be incorporated at the N- or C-terminus of recombinant proteins, allowing incorporation of proteins in the hydrogel. Reprinted with permission from [40, 41].

10.5. INJECTABLE SYSTEMS 149
(
'
)
Figure 10.2: Self-assembling peptide nanofiber system for growth factor delivery. D-F.Myocardial repair and regeneration by combined cardiac progenitor cells (CPCs) and NF-IGF-1 therapy. D. Regions of regenerated myocardium (dashed line) in infarcts treated with CPCs (top panel), NF-IGF-1 (middle panel), and CPCs-NF-IGF-1 (bottom panel). In the insert, new myocytes (α-sarcomeric actin [α-SA], red) express EGFP (top and bottom panels, green) or are labeled by BrdU (middle panel, white). E. Average volume, number, and aggregate mass of regenerated myocytes. The latter was employed to compute infarct size. *P <0.05 vs. infarcts treated with CPCs only; **P <0.05 vs. infarcts treated with NF-IGF-1 only. F. Combination therapy (CPC-NF-IGF-1) improved cardiac performance. *P <0.05 vs. sham-operated (SO); **P <0.05 vs. untreated infarcts (UN); †P <0.05 vs. infarcts treated with CPCs;
‡P <0.05 vs. infarcts treated with NF-IGF-1. EF, ejection fraction; MI, myocardial infarction; SO, shamoperated; and UN, untreated infarcts. Reprinted with permission from [40, 41].

150 10. BIOMATERIAL-BASED CONTROLLED DELIVERY OF BIOACTIVE MOLECULES
Self-assembling peptides are typically 8–16 amino acids long and are composed of alternating hydrophilic and hydrophobic residues. They form stable β-sheets in water, and upon exposure to physiological salt concentration or pH, they form a stable hydrogel of flexible NFs (7–20 nm in diameter) consisting of more than 99% water (Fig. 10.2) [40]. Slow release of the proteins (e.g., PDGF-BB) from such a system has been achieved by the physical entrapment of the protein in the hydrogel and possibly by its adsorption on the self-assembling peptides by non-covalent interactions (Fig. 10.2) [42]. To improve protein retention in the hydrogel, biotinylation of the self-assembling peptides can be performed. By this method, for example, IGF-1 was tethered to the biotinylated selfassembling peptides using the biotin sandwich method, where biotinylated IGF-1 and streptavidin were mixed in 1:1 molar ratio, allowing other biotin-binding sites on most tetravalent streptavidins to remain available for subsequent binding to the biotinylated self-assembled NFs (Fig. 10.2) [43]. More recently, Padin-Iruegas et al tested whether the local injection of clonogenic cardiac progenitor cells (CPCs) in NFs with tethered IGF-1 potentiates the activation and differentiation of delivered and resident CPCs enhancing cardiac repair after infarction (Fig. 10.2) [41]. Compared to infarcts treated with either CPCs or NF-IGF-1 alone, the combination therapy resulted in a greater increase in the ratio of left ventricular mass to chamber volume and a better preservation of +dP/dt, −dP/dt, EF, and diastolic wall stress. Of note, the effects of NF-IGF-1 therapy in terms of ventricular function were comparable to CPC transplantation. Myocardial regeneration was detected in all treated infarcts, but the number of newly formed myocytes with the combination therapy was 32% and 230% higher than with CPCs and NF-IGF-1, respectively. Corresponding differences in the volume of regenerated myocytes were 48% and 115%. Importantly, activation of resident CPCs by paracrine effects contributed to cardiomyogenesis and vasculogenesis. Collectively, CPCs and NF-IGF-1 therapy reduced infarct size more than CPCs and NF-IGF-1 alone [41]. In another report, to improve protein stability and confer protection from proteolysis, Segers et al genetically engineered a protease-resistant form of SDF-1 that was subsequently delivered by self-assembling NFs into the infarcted heart by intramyocardial injection [44].
Cardiac regeneration, most likely, would require the presentation and participation of multiple growth factors. Multiple and/or sequential factor delivery has been described by several groups, applying different delivery systems or different combination of polymers [45, 46]. Hao et al used a combination of partially oxidized alginates with low and high molecular weights to produce a hydrogel system that can sequentially deliver VEGF and PDGF-BB into the infarcted myocardium [47]. The factors were adsorbed to the hydrogel via electrostatic interaction and the sequential factor delivery was achieved due to the different degradation rates of the partially oxidized alginates constituting the hydrogel. One week after MI was induced in rats, the modified alginate hydrogels with loaded VEGF and PDGF-BB, were injected intramyocardially along the border of the MI. The sequential growth factor release led to a higher density of α-SMA-positive (mature) vessels compared to the delivery of single factors, and improved cardiac function.
