
- •Preface
- •Acronyms
- •Introduction
- •Background and objectives
- •Content, format and presentation
- •Radioactive waste management in context
- •Waste sources and classification
- •Introduction
- •Radioactive waste
- •Waste classification
- •Origins of radioactive waste
- •Nuclear fuel cycle
- •Mining
- •Fuel production
- •Reactor operation
- •Reprocessing
- •Reactor decommissioning
- •Medicine, industry and research
- •Medicine
- •Industry
- •Research
- •Military wastes
- •Conditioning of radioactive wastes
- •Treatment
- •Compaction
- •Incineration
- •Conditioning
- •Cementation
- •Bituminisation
- •Resin
- •Vitrification
- •Spent fuel
- •Process qualification/product quality
- •Volumes of waste
- •Inventories
- •Inventory types
- •Types of data recorded
- •Radiological data
- •Chemical data
- •Physical data
- •Secondary data
- •Radionuclides occurring in the nuclear fuel cycle
- •Simplifying the number of waste types
- •Radionuclide inventory priorities
- •Material priorities
- •Inventory evolution
- •Assumptions
- •Errors
- •Uncertainties
- •Conclusions
- •Acknowledgements
- •References
- •Development of geological disposal concepts
- •Introduction
- •Historical evolution of geological disposal concepts
- •Geological disposal
- •Definitions and comparison with near-surface disposal
- •Development of geological disposal concepts
- •Roles of the geosphere in disposal options
- •Physical stability
- •Hydrogeology
- •Geochemistry
- •Overview
- •Alternatives to geological disposal
- •Introduction
- •Politically blocked options: sub-seabed and Antarctic icecap disposal
- •Sea dumping and sub-seabed disposal
- •Antarctic icesheet disposal
- •Technically impractical options; partitioning and transmutation, space disposal and icesheet disposal
- •Partitioning and Transmutation
- •Space disposal
- •Icesheets and permafrost
- •Non-options; long-term surface storage
- •Alternatives to conventional repositories
- •Introduction
- •Alternative geological disposal concepts
- •Utilising existing underground facilities
- •Extended storage options (CARE)
- •Injection into deep aquifers and caverns
- •Deep boreholes
- •Rock melting
- •The international option: technical aspects
- •Alternative concepts: fitting the management option to future boundary conditions
- •Conclusions
- •References
- •Site selection and characterisation
- •Introduction
- •Prescriptive/geologically led
- •Sophisticated/advocacy led
- •Pragmatic/technically led
- •Centralised/geologically led
- •Conclusions to be drawn
- •Lessons to be learned (see Table 4.2)
- •Site characterisation
- •Can we define the natural environment sufficiently thoroughly?
- •Sedimentary environments
- •Hydrogeology
- •The regional hydrogeological model
- •More local hydrogeological model(s)
- •Crystalline rock environments
- •Lithology and structure
- •Hydrogeology
- •Hydrogeochemistry
- •Any geological environment
- •References
- •Repository design
- •Introduction: general framework of the design process
- •Identification of design requirements/constraints
- •Concept development
- •Major components of the disposal system and safety functions
- •A structured approach for concept development
- •Detailed design/specifications of subsystems
- •Near-field processes and design issues
- •Design approach and methodologies
- •Design confirmation and demonstration
- •Interaction with PA/SA
- •Demonstration and QA
- •Repository management
- •Future perspectives
- •References
- •Assessment of the safety and performance of a radioactive waste repository
- •Introduction
- •The role of SA and the safety case in decision-making
- •SA tasks
- •System description
- •Identification of scenarios and cases for analysis
- •Consequence analysis
- •Timescales for evaluation
- •Constructing and presenting a safety case
- •References
- •Repository implementation
- •Legal and regulatory framework; organisational structures
- •Waste management strategies
- •The need for a clear policy and strategy
- •Timetables vary widely
- •Activities in development of a geological repository
- •Concept development
- •Siting
- •Repository design
- •Licensing
- •Construction
- •Operation
- •Monitoring
- •Research and development
- •The staging process
- •Attributes of adaptive staging
- •The decision-making process
- •Status of geological disposal programmes
- •Overview
- •Status of geological disposal projects in selected countries
- •International repositories
- •Costs and financing
- •Cost estimates
- •Financing
- •Conclusions
- •Acknowledgements
- •References
- •Research and development infrastructure
- •Introduction: Management of research and development
- •Drivers for research and development
- •Organisation of R&D
- •R&D in specialised (nuclear) facilities
- •Introduction
- •Inventory
- •Release of radionuclides from waste forms
- •Solubility and sorption
- •Waste form dissolution
- •Colloids
- •Organic degradation products
- •Gas generation
- •Conventional R&D
- •Engineered barriers
- •Corrosion
- •Buffer and backfill materials
- •Container fabrication
- •Natural barriers
- •Geochemistry and groundwater flow
- •Gas transport and two-phase flow
- •Biosphere
- •Radionuclide concentration and dispersion in the biosphere
- •Climate change
- •Landscape change
- •Underground rock laboratories
- •URLs in sediments
- •Nature’s laboratories: studies of the natural environment
- •General
- •Corrosion
- •Cement
- •Clay materials
- •Degradation of organic materials
- •Glass corrosion
- •Radionuclide migration
- •Model and database development
- •Conclusions
- •References
- •Building confidence in the safe disposal of radioactive waste
- •Growing nuclear concerns
- •Communication systems in waste management programmes
- •The Swiss programme
- •The Japanese programme
- •Examples of communication styles in other countries
- •Finland
- •Sweden
- •France
- •United Kingdom
- •Comparisons between communication styles in Finland, France, Sweden and the United Kingdom
- •Lessons for the future
- •What is the way forward?
- •Acknowledgements
- •References
- •A look to the future
- •Introduction
- •Current trends in repository programmes
- •Priorities for future efforts
- •Waste characterisation
- •Operational safety
- •Emplacement technologies
- •Knowledge management
- •Alternative designs and optimisation processes
- •Materials technology
- •Novel construction/immobilisation materials: the example of low pH cement
- •Future SA code development
- •Implications for environmental protection: disposal of other wastes
- •Conclusions
- •References
- •Index
32 |
D.F. McGinnes |
activity by at least an order of magnitude as against a simplistic non-decayed radionuclide inventory.
Surface to mass ratios for metallic materials To determine the amount of gas a metallic component may produce, it is necessary to know the available surface area in relation to its total mass, i.e., the rate of gas production is much higher from sheets of aluminium foil in comparison to that from aluminium laboratory apparatus support stands (rods).
2.7.3. Secondary data
To have an inventory that can be used to respond to questions, e.g., requests from safety assessors, regulators, etc., in a timely fashion, it is recommended that processing functions are built into any inventory database. The types of secondary data that are required during a repository project are:
Summation over any combination of individual waste types (volume, activities, masses, etc.)
Decay and arisings calculations
Radiotoxicity calculations
Classification of material into chemical groups (e.g., inorganic, low-or high-molecular weight organic, etc.)
Elemental composition
For examples of the various inventories that have been derived in Europe and the US, see Alder and McGinnes (1994), Carlsson (1999), ENEA (2000), Riggar and Johansson (2001), USDOE (2001), Nirex (2002) and McGinnes (2002).
2.7.4. Radionuclides occurring in the nuclear fuel cycle
Within the nuclear fuel cycle, radionuclides arise in three groups: activation products, fission products and actinides.
Activation products: In a reactor, a certain amount of the neutrons produced are absorbed by fuel impurities or by fuel and reactor structural materials. The most common
reaction is where a stable isotope absorbs a neutron and emits a -ray, e.g., 59Co (n, ) 60C or 62Ni (n, ) 63Ni.
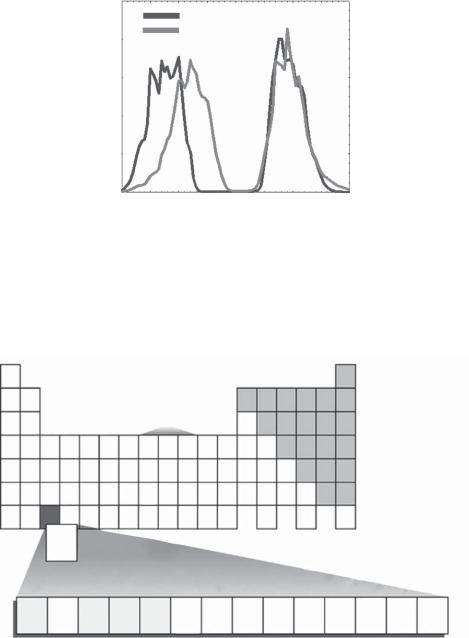
Fission products: When a nuclide undergoes fission, the resulting nuclei are termed ‘‘fission products’’. Depending on the nuclide that undergoes fission, a distinct distribution of fission products occurs. In Fig. 2.16, for 235U fission (235U is the initial fissile
component of uranium oxide fuels), it can be seen that certain mass numbers dominate, e.g., A =90(90Sr), A =99(99Tc), A =137(137Cs).
Transuranic (TRU) nuclides are those essentially man-made nuclides occurring after uranium in the actinide element series (see highlighted area in Fig. 2.17).
Table 2.3 identifies the most important sources of radionuclides in light water reactors. For each of these nuclides, it also gives the dominant emission ( , , , etc.), its half-life and, in the case of activation products, which reactor material (steel or concrete).
To put radwaste inventories in perspective, the time for vitrified HLW reprocessing wastes to reach the same activity level as in the initial uranium ore from which the fuel is
Waste sources and classification |
33 |
Independent mass yield [%]
10
U-235
Cm-245
8 |
|
|
|
|
6 |
|
|
|
|
4 |
|
|
|
|
2 |
|
|
|
|
0 |
100 |
120 |
140 |
160 |
80 |
Mass number A
Actinides: The actinides consist of the elements from actinium (Z = 89) to lawrencium (Z =103)
Fig. 2.16. Fission products of 235U fission.
IA |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
0 |
|
|
1 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
2 |
The elements from |
|
H |
IIA |
|
|
|
|
|
|
|
|
|
|
IIIA |
IVA |
VA |
VIA |
VIIA |
He |
actinium (element 89) |
|
3 |
4 |
|
|
|
|
|
|
|
|
|
|
5 |
6 |
7 |
8 |
9 |
10 |
to lawrencium |
|
Li |
Bc |
|
|
|
|
|
|
|
|
|
|
B |
C |
N |
O |
F |
Ne |
(element 103) form |
|
11 |
12 |
|
|
|
|
|
|
|
|
|
|
13 |
14 |
15 |
16 |
17 |
18 |
a distinct group– |
|
|
|
|
|
|
|
VIIIB |
|
|
|
the actinides–within |
|||||||||
Na |
Mg |
|
|
|
|
|
|
|
|
|
Al |
Sl |
P |
S |
Cl |
Ar |
|||
|
|
IIIB |
IVB |
VB |
VIB |
VIIB |
|
|
|
IB |
IIB |
|
|
|
|
|
|
the periodic table. |
|
19 |
20 |
21 |
22 |
23 |
24 |
25 |
26 |
27 |
28 |
29 |
30 |
31 |
32 |
33 |
34 |
35 |
36 |
||
|
|||||||||||||||||||
K |
Ca |
Sc |
Ti |
V |
Cr |
Mn |
Fe |
Co |
Ni |
Cu |
Zn |
Ga |
Ge |
As |
Se |
Br |
Kr |
|
|
37 |
38 |
39 |
40 |
41 |
42 |
43 |
44 |
45 |
46 |
47 |
48 |
49 |
50 |
51 |
52 |
53 |
54 |
|
|
Rb |
Sr |
Y |
Zr |
Nb |
Mo |
|
Ru |
Rh |
Pd |
Ag |
Cd |
In |
Sn |
Sb |
Te |
I |
Xe |
|
|
55 |
56 |
57 |
72 |
73 |
74 |
75 |
76 |
77 |
78 |
79 |
80 |
81 |
82 |
83 |
84 |
85 |
86 |
|
|
Cs |
Ba |
La |
Hi |
Ta |
W |
Re |
Os |
Ir |
Pt |
Au |
Hg |
Tl |
Pb |
Bi |
Po |
At |
Rn |
|
|
|
|
|
178.49 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
87 |
88 |
|
104 |
105 |
106 |
107 |
108 |
109 |
110 |
111 |
112 |
|
114 |
|
116 |
|
118 |
|
|
Fr |
Ra |
|
(Rf) |
Db |
Sg |
Bh |
Hs |
Mt |
|
|
|
|
|
|
|
|
|
|
89
Ac Actinides
(227)
Thorium ProtactiniumUranium
NeptuniumPlutonium
um |
|
Americi |
Curium |
Berk |
Califor |
EinsteiniumFem |
vium |
Lawrencium |
|
Mendele Nobelium |
|||||
elium |
nium |
nium |
|
|
|
90 |
91 |
92 |
93 |
94 |
95 |
96 |
97 |
98 |
99 |
100 |
101 |
102 |
103 |
Th |
Pa |
U |
Np |
Pu |
Am |
Cm |
Bk |
Cf |
Es |
Fm |
Md |
No |
Lr |
(232) |
(231) |
(238) |
(237) |
(244) |
(243) |
(247) |
(247) |
(251) |
(252) |
(257) |
(258) |
(259) |
(260) |
Fig. 2.17. The periodic table with the actinide elements highlighted.
