
Учебники / Head_and_Neck_Cancer_Imaging
.pdf
Neoplasms of the Oral Cavity |
125 |
a |
b |
|
Fig. 6.31a–c. MRI of a 56-year-old man with a local recurrence after resec- |
|
tion of a squamous cell carcinoma of the tongue and respective asymmetric |
|
changes of the anatomy. The T2-weighted image shows a small hyperin- |
|
tense lesion (arrow) deeply between floor of the mouth and tongue (a). |
|
The respective post-contrast axial (b) and coronal (c) T1-weighted images |
c |
demonstrate contrast enhancement of that lesion |
a |
b |
Fig. 6.32a,b. Coronal MRI of a 50-year-old male with a local recurrence at the left tongue. Both the T2-weighted (a) as well as the T1-weighted post-contrast (b) images show a hyperintense lesion

126
References
Al-Ghamdi S, Black MJ, Lafond G (1992) Extracranial head and neck schwannomas. J Otolaryngol 107:186–188
Baker LL, Dillion WP, Hieshima GB et al (1993) Hemangiomas and vascular malformations of the head and neck: MR characterization. AJNR Am J Neuroradiol 14:307–314
Beaman FD, Kransdorf MJ, Menke DM (2004) Schwannoma: radiologic-pathologic correlation. Radiographics 24:1477– 1481
Becker M, Moulin G, Kurt A et al (1998) Non-squamous cell neoplasms of the larynx: radiologic-pathologic correlation. Radiographics 18:1189–1209
Bergman RA, Afifi AK (2002) Nerves of the eyes, nose, and mouth. Atlas of human anatomy. Copyright protected material used with permission of the authors and the University of Iowa’s Virtual Hospital, www.vh.org
Cawson RA (1998) Lucas’s pathology of tumors of the oral tissues, 5th edn. Churchill Livingstone, Harcourt Brace & Co Ltd, Edinburgh
Coit WE, Harnsberger HR, Osborn AG et al (1987) Ranulas and their mimics. Radiology 263:211–216
Douglas PS, Baker AW (1994) Lingual thyroid. Br J Oral Maxillofac Surg 32:123–124
Ferlay J, Bray F, Sankila R et al (1999) EUCAN: cancer incidence, mortality and prevalence in the European Union 1995, version 2.0. IARC CancerBase No. 4., Lyon, IARCPress
Ferner H, Staubesand J (1982) Sobotta – atlas of human anatomy. Urban & Schwarzenberg, Munich
Friedman M, Levin B, Grybauskas V et al (1986) Malignant tumors of the major salivary glands. Otolaryngol Clin North Am 19:625–636
Goodwin WJ (1998) PET and recurrent squamous cell carcinoma of the head and neck: a surgeon’s view. AJNR Am J Neuroradiol 19:1189–1196
Hermans R (2003) Imaging of mandibular osteoradionecrosis. Neuroimaging Clin N Am 13:597–604
Hudgins PA, Burson JG, Gussack GS et al (1994) CT and MR appearance of recurrent malignant head and neck neoplasms after resection and flap reconstruction. AJNR Am J Neuroradiol 15:1698–1694
Johnson JC, Coleman LL (1989) Magnetic resonance imaging of a lingual thyroid gland. Pediatr Radiol 19:461–462
Keberle M, Kenn W, Tschammler A et al (1999) Current value of double-contrast pharyngography and of computed tomography for the detection and for staging of hypopharyngeal, oropharyngeal and supraglottic tumors. Eur Radiol 9:1843–1850
Keberle M, Jenett M, Kessler C et al (2000) New possibilities in the diagnosis and documentation using 3D power Doppler ultrasound with carcinoma of the floor of the mouth as illustration. Ultraschall Med 21:26–31
Keberle M, Tschammler A, Hahn D (2002) Single-bolus technique for spiral CT of laryngopharyngeal squamous cell carcinoma: comparison of different contrast material volumes, flow rates, and start delays. Radiology 22:171–176
Keberle M, Hoppe F, Dotzel S et al (2003) Prognostic value of pretreatment CT regarding local control in oropharyngeal cancer after primary surgical resection. Fortschr Röntgenstr 175:61–66
Keberle M, Ströbel P, Relic A (2005) Simultaneous pleomorphic adenomas of the parotid and the submandibular gland. Fortschr Röntgenstr 177:436–438
M. Keberle
Kennedy TL (1989) Cystic hygroma-lymphangioma: a rare and still unclear entity. Laryngoscope 99:1–10
King RC, Smith BR, Burk JL (1994) Dermoid cysts in the floor of the mouth. Review of the literature and case reports. Oral Surg Oral Med Oral Pathol 78:567–576
Koeller KK, Alamo L, Adair CF et al (1999) Congenital cystic masses of the head and neck: radiologic-pathologic correlation. Radiographics 19:121–146
Kösling S, Schmidtke M, Vothel F et al (2000) The value of spiral CT in the staging of carcinomas of the oral cavity and of the oroand hypopharynx. Radiologe 40:632–639
Lapela M, Grenman R, Kurki T et al (1995) Head and neck cancer: detection of recurrence with PET and 2-(F- 18)Fluoro-2-deoxy-D-glucose. Radiology 197:205–211
Lee YY, van Tassel P, Nauert C et al (1987) Lymphomas of the head and neck: CT findings at initial presentation. AJNR Am J Neuroradiol 8:665–671
Lell M, Baum U, Koester M et al (1999) The morphological and functional diagnosis of the head-neck area with multiplanar spiral CT. Radiologe 39:932–938
Lell M, Baum U, Greess H et al (2000) Head and neck tumors: imaging recurrent tumor and post-therapeutic changes with CT and MRI. Eur J Radiol 33:239–247
Lenz M, Hermans R (1996) Imaging of the oropharynx and the oral cavity. Part II: pathology. Eur Radiol 6:536–549
Leslie A, Fyfe E, Guest P et al (1999) Staging of squamous cell carcinoma of the oral cavity and oropharynx: a comparison of MRI and CT in T- and N-staging. J Comput Assist Tomogr 23:43–49
Magrin J, Kowalski L (2000) Bilateral radical neck dissection: results in 193 cases. J Surg Oncol 75:232–240
Mashberg A, Meyers H (1976) Anatomical site and size of 222 early asymptomatic oral squamous cell carcinomas. Cancer 37:2149–2157
Mende U, Zöller J, Dietz A et al (1996) The sonography in the primary staging of head and neck tumors. Radiologe 36:207–216
Mukherji SK, Weeks SM, Castillo M et al (1996) Squamous cell carcinomas that arise in the oral cavity and tongue base: can CT help predict perineural or vascular invasion? Radiology 198:157–162
Mukherji SK, Pillsbury HR, Castillo M (1997) Imaging squamous cell carcinomas of the upper aerodigestive tract: what clinicians need to know? Radiology 205:629–646
Mukherji SK, Isaacs DL, Creager A et al (2001) CT detection of mandibular invasion by squamous cell carcinoma of the oral cavity. AJR Am J Roentgenol 177:237–243
Mulliken JB (1988) Vascular malformations of the head and neck. In: Mulliken JB, Young AE (eds) Vascular birthmarks, hemangiomas and malformations. WB Saunders, Philadelphia, pp 301–342
Mulliken JB, Glowacki J (1982) Hemangiomas and vascular malformations in infants and children: a classification based on endothelial characteristics. Plast Reconstr Surg 69:412–420
Okahara M, Kiyosue H, Hori Y et al (2003) Parotid tumors: MR imaging with pathological correlation. Eur Radiol 13[Suppl 4]:L25–33
Parker GD, Harnsberger HR (1991) Clinical-radiologic issues in perineural tumor spread of malignant diseases of the extracranial head and neck. Radiographics 11:383–399
Peters E, Cohen M, Altini M et al (1989) Rhabdomyosarcoma of the oral and paraoral region. Cancer 63:963–966

Neoplasms of the Oral Cavity
Sigal R, Monnet O, de Baere T et al (1992) Adenoid cystic carcinoma of the head and neck. Radiology 184:95–101
Sigal R, Zagdanski A, Schwaab G et al (1996) CT and MR imaging of squamous cell carcinoma of the tongue and floor of the mouth. Radiographics 16:787–810
Smoker WRK (2003) The oral cavity. In: Som PM, Curtin HD (eds) Head and neck imaging, 4th edn.. Mosby, St. Louis, pp 1377–1464
Som PM, Brandwein MS (2003) Salivary glands: anatomy and pathology. In: Som PM, Curtin HD (eds) Head and neck imaging, 4th edn. Mosby, St. Louis, pp 2005–2133
UICC Union international contre le cancer (2002) TNM atlas. Springer, Berlin Heidelberg New York
van den Brekel MWM, Runne RW, Smeele LE et al (1998) Assessment of tumour invasion into the mandible: the
127
value of different imaging techniques. Eur Radiol 8:1552– 1557
Vogl TJ, Steger W, Ihrer S et al (1993) Cystic masses in the floor of the mouth: value of MR imaging in planning surgery. AJR Am J Roentgenol 161:183–186
Weber AL, Kaneda T, Scrivani SJ et al (2003) Jaw: cysts, tumors, and nontumorous lesions. In: Som PM, Curtin HD (eds) Head and neck imaging, 4th edn. Mosby, St. Louis, pp 930– 994
Yasumoto M, Shibuya H, Takeda M et al (1995) Squamous cell carcinoma of the oral cavity: MR findings and value of T1versus T2-weighted fast spin-echo images. AJR Am J Roentgenol 164:981–987
Yousem DM, Kraut MA, Chalian AA (2000) Major salivary gland imaging. Radiology 216:19–29

Neoplasms of the Oropharynx |
129 |
7Neoplasms of the Oropharynx
Robert Hermans
CONTENTS
7.1Introduction 129
7.2Normal Anatomy 129
7.3 |
Squamous Cell Carcinoma 132 |
7.3.1Tonsillar Cancer 132
7.3.2 |
Tongue Base Cancer |
133 |
7.3.3 |
Soft Palate Cancer |
135 |
7.3.4 |
Posterior Oropharyngeal Wall Cancer 136 |
|
7.3.5Lymphatic Spread 137
7.4Treatment 137
7.5Post-treatment Imaging 138
7.6. Other Neoplastic Disease 139
7.6.1Non-Hodgkin Lymphoma 139
7.6.2 Salivary Gland Tumors 140
7.6.3Other 140 References 142
7.1 Introduction
Head and neck cancer commonly originates from the oropharynx. As in most head and neck sites, squamous cell cancer is the most frequently encountered malignant disease. Cigarette smoking and excessive alcohol consumption are well-known risk factors. The accuracy of pretherapeutic staging is an important factor in the treatment planning of oropharyngeal neoplasms; clinical examination and imaging studies are complementary in precisely evaluating tumor extent. As an adjunct to clinical surveillance, imaging can be used to monitor tumor response and to detect recurrent or persistent disease as early as possible.
R. Hermans, MD, PhD
Professor, Department of Radiology, University Hospitals
Leuven, Herestraat 49, 3000 Leuven, Belgium
7.2
Normal Anatomy
The pharynx is divided in three sections: the nasopharynx lies behind the nasal cavity, the oropharynx behind the oral cavity, and the hypopharynx merging with the proximal oesophagus at the lower level of the cricoid bone, behind the larynx.
The pharyngeal constrictor muscles compose the posterior and lateral wall of the pharynx. They all insert on a midline localised fibrous raphe. This posterolateral wall of the pharynx is a continuous structure, without markings allowing separation in a naso-, oroor hypopharyngeal level.
The soft palate separates the nasopharynx from the oropharynx. On imaging studies, often one uses a line extending the level of the hard palate as demarcation line on the lateral and posterior wall; an alternative is to use a horizontal line through the C1–C2 articulation (Chong et al. 1999). Direct sagittal MR images, or sagittally reformatted CT images are well suited for defining the border between the nasoand oropharynx.
The oropharynx is separated from the hypopharynx by the pharyngoepiglottic folds. The border between the oropharynx and oral cavity is more complex, being ring-like and composed by several structures. The upper part of this ring is formed by the junction between the hard and soft palate; therefore, the hard palate is a structure belonging to the oral cavity, while the soft palate is an oropharyngeal structure (actually only its lower surface – the upper surface belongs to the nasopharynx). A mucosal fold, known as the anterior tonsillar pillar, forms the lateral part of the ring. This mucosal fold marks the anterior border of the tonsillar fossa. The lower part of the ring is formed by a row of small structures on the back of the tongue, the circumvallate papillae. These papillae are on a V-shaped line, with the tip of the V pointing posteriorly. By definition, the posterior third of the tongue (called tongue base) is part of the oropharynx, and not of the oral cavity (Hermans and Lenz 1996).

130
The soft palate is a complex structure, composed of muscles, some fat and lymphoid tissue. Two muscles, arising from the skull base, take part in the formation of the soft palate. The more medial one is known as the levator veli palatini, the more lateral one as the tensor veli palatini. These muscles have an important role during deglutition and phonation. They also form a functional unit with the Eustachian tube; their action opens the Eustachian tube fissure during swallowing and yawning. Dysfunction of the Eustachian tube causes serous otitis, a common finding in nasopharyngeal cancer.
The anterior and posterior tonsillar pillar define the triangular tonsillar fossa. Both pillars are mucosal folds produced by underlying muscular structures. The anterior muscle is the palatoglossal muscle, connecting the soft palate with the tongue base. The posterior muscle is the palatopharyngeal muscle, which takes part in the formation of the muscular pharyngeal wall. Oropharyngeal cancers commonly arise on the anterior tonsillar pillar, using this muscle and the overlying mucosa as a pathway to spread into the soft palate and tongue base. The tonsillar fossa harbours the palatine tonsil, consisting of encapsulated lymphoid tissue. The palatine tonsil is one of the major tonsils in the lymphoid ring of Waldeyer; the other major tonsils are the lingual tonsil in the tongue base, and the pharyngeal tonsil in the roof of the nasopharynx.
The pit between the tongue base and the free edge of the epiglottis is divided by a median mucosal fold running from the base of the tongue to the epiglottis; this fold is known as the glossoepiglottic fold, and separates both valleculae. The valleculae are part of the oropharynx. Anatomically, the lingual side of the free epiglottic margin is the posterior border of the valleculae. For staging purposes, it is important to know that the entire epiglottis is considered as part of the larynx.
Underneath the vallecular floor a laryngeal fat plane is present, known as the preepiglottic fat plane; this fatty tissue is sometimes used by oropharyngeal tumours to dive into the larynx, an extension that is occult to the examining clinician.
The different subsites of the oropharynx,important for staging purposes, are summarized in Table 7.1.
The largest part of the tongue base is composed of muscles, both intrinsic and extrinsic muscles. On axial imaging, these intrinsic muscular fibres may produce a relative high density in the tongue base on CT images, which should not be confused with an infiltrating mass lesion; the regular and symmetric appearance of these normal muscles allows avoidance of this pitfall.
R. Hermans
Table 7.1. Subsites within the oropharynx (UICC 2002)
Anterior wall
–Base of tongue (posterior to circumvallate papillae or posterior third)
–Vallecula
Lateral wall
–Tonsil
–Tonsillar fossa and tonsillar (faucial) pillars
–Glossotonsillar sulci
Posterior wall
Superior wall
–Inferior surface of soft palate
–Uvula
The lingual artery is the main arterial supply to the tongue. It is a branch of the external carotid artery, arising just distal from the superior thyroid artery and proximal to the facial artery. Three segments can be distinguished (Portugalli et al. 2003):
•A proximal segment, running anterosuperior from the external carotid artery, entering the tongue base just above the hyoid bone, medial to the hyoglossal muscle. The hypoglossal nerve crosses the origin of the lingual artery, but runs lateral to the hyoglossal muscle.
•A middle segment, running along the medial side of the hyoglossal muscle.
•A branching distal segment, dividing into the deep lingual artery and sublingual artery.
The lingual nerve, a branch of the third portion of the trigeminal nerve, enters the tongue at a higher level than the lingual artery; this nerve enters the tongue from the parapharyngeal space, lateral to the stylohyoid and hyoglossal muscle. The lingual nerve contains sensitive and sensory (via the chorda tympani, branch of the facial nerve) fibres for the anterior two-thirds of the tongue. The sensory and sensitive innervation of the tongue base is via the glossopharyngeal nerve.
The lymphoid tissue within the ring of Waldeyer can appear prominent in younger subjects, prolapsing into the pharyngeal lumen. The amount of the lymphoid tissue decreases with age. Patients older than 40 years are not expected to have a significant amount of residual lymphatic tissue, but small tags of tissue may persist. The volume of the tonsils may increase due to an upper respiratory tract infection, but also due to an extranodal lymphoma localisation. A persistent or asymmetric appearing lingual tonsil can cause problems, as differentiation with a malignant tumour is not always possible. Clues, which may help to differentiate,

Neoplasms of the Oropharynx |
131 |
are air-filled crypts and the presence of small calcifications within the lymphatic tissue. Lymphatic tissue does not invade the deeper structures (Fig. 7.1; see also Fig. 7.9). One should however always stay prudent, and in the case of doubt, biopsies are warranted.
The parapharyngeal space runs from the skull base to the submandibular salivary glands. It essentially consists of fat and is bordered medially by the pharyngeal walls and laterally by the infratemporal space (containing the masticatory muscles). Contrary to the superficial tissues of the oropharynx, which may appear somewhat asymmetric, this deeper lying parapharyngeal space should always appear symmetric; any
asymmetry should be treated with extreme caution (Farina et al. 1999). The parapharyngeal space has a small anterior extension between the oropharyngeal wall and the medial pterygoid muscle; this narrow space is known as the pterygomandibular space, reaching the mandible; it contains the lingual nerve. This small pterygomandibular space is actually a crossroads of several structures belonging to different spaces. The anterior tonsillar pillar, connecting the soft palate to the tongue base, and using the pterygomandibular space as access to the parapharyngeal space, is very close, as well as the retromolar trigonum of the mandible, a small triangular bony space, localised just
a |
b |
c 
 d
d
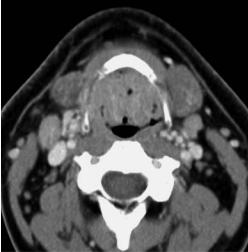
Fig. 7.1.a–d. Contrast-enhanced CT images in a 63-year-old patient (a,b). The study was performed because of a parotid tumor. The lingual tonsil appear prominent. The symmetric appearance, lack of invasion in the tongue base tissues (arrows, a), and the presence of air-filled crypts, as visible at the vallecular level (b), indicate benign enlargement of the tonsillar lymphoid tissue. Epiglottic rim, (arrowheads) (b). No biopsies were obtained. c,d Contrast-enhanced CT images in a 40-year-old patient complaining of globus sensation. Large volume of the lingual tonsil, narrowing the oropharyngeal airway, both at the level of the tongue base (a) and valleculae (b). The tissue appears symmetrical, does not invade the tongue base (arrows) and contains air-filled crypts, suggesting benign enlargement of the tonsillar lymphoid tissue. Because of the large size of this tonsil, and the associated symptoms, biopsies were obtained on three occasions over a period of 6 months: histological examination always showed follicular hyperplasia, without evidence of tumor

132 |
R. Hermans |
behind the third lower molar. The mucosal surface at the level of the retromolar trigone also covers the fibrous pterygomandibular raphe. On the posterior side of this raphe, part of the pharyngeal constrictor muscle originates, while on the anterior side, the buccinator muscle takes its origin, eventually forming the muscular substrate of the cheek.
The parapharyngeal space and its neoplastic pathology is discussed in detail in Chap. 9.
7.3
Squamous Cell Carcinoma
Squamous cell carcinoma is the most frequent malignant tumour of the oropharynx (90%). Most patients complain of sore throat, otalgia or dysphagia; more advanced, invasive tumours may cause severe pain and trismus.
The T staging is based on tumour size, and involvement of adjacent structures (Table 7.2). The most common site of origin of oropharyngeal cancer is the anterior tonsillar pillar.
7.3.1
Tonsillar Cancer
Nearly all tonsillar cancers originate from the anterior tonsillar pillar. These cancers commonly spread
anteroinferiorly to the tongue base, and superomedially to the soft palate, both along the palatoglossal muscle. Anterolateral spread, along the pharyngeal constrictor muscle to the pterygomandibular raphe and retromolar trigone, is also commonly seen (Fig. 7.2). Advanced lesions may invade the mandible, spread along the pharyngeal wall to the hypoand/or nasopharynx, or invade the parapharyngeal space through the pharyngeal wall (Figs. 7.3 and 7.4). Spread to the infratemporal space, with involvement of the muscles of mastication and neurovascular structures in this space may be seen in advanced cases (Fig. 7.5).
Lesions originating from the posterior tonsillar pillar are rare; these may spread inferiorly along the palatopharyngeal muscle.
Table 7.2. T staging of oropharyngeal carcinoma (UICC 2002)
Tis Carcinoma in situ
T1 Tumour ≤ 2 cm in greatest dimension
T2 Tumour > 2 cm but ≤ 4 cm in greatest dimension T3 Tumour measures > 4 cm in greatest dimension T4a Tumour invades any of the following : larynx,
deep/extrinsic muscle of the tongue (genioglossus, hyoglossus, palatoglossus, and styloglossus), medial pterygoid, hard palate, and mandible
T4b Tumour invades any of the following: lateral pterygoid muscle, pterygoid plates, lateral nasopharynx, skull base, or encases the carotid artery
a 
 b
b
Fig. 7.2a,b. Axial contrast-enhanced CT images in a patient with right-sided tonsillar cancer. a Soft tissue thickening and increased enhancement is seen in the right anterior tonsillar pillar (white arrowheads), extending into pterygomandibular raphe (black arrowhead). b The enhancing soft tissue mass extends along the glossotonsillar sulcus (arrow) into the tongue base (arrowheads)

Neoplasms of the Oropharynx |
133 |
a 
 b
b
Fig. 7.3a,b. Axial T2-weighted (a) and plain T1-weighted spin echo image (b) in a patient with a left-sided tonsillar cancer. The soft tissue mass involves the palatine tonsil, grows through the pharyngeal constrictor muscle into the parapharyngeal space (arrows, compare to opposite side). The mass lesion reaches the retromolar trigone, and slightly compresses the tongue base. Retropharyngeal adenopathy (asterisk). Normal pharyngeal constrictor muscle on right side (arrowheads)
a |
b |
Fig. 7.4a,b. Patient presenting with left-sided otalgia. Clinical examination show left-sided tonsillar tumor. Axial contrast-en- hanced CT images. a Level of oropharynx: enhancing mass lesion in left oropharyngeal tonsil (white arrowhead), extending into the retrostyloid compartment of the parapharyngeal space (arrow), encasing the internal carotid artery (black arrowhead). b Level of nasopharynx. Submucosal perivascular tumor extension (black arrowhead) along the internal carotid artery (white arrowhead)
7.3.2
Tongue Base Cancer
Cancer in the tongue base tends to grow silently and deeply, and is often larger than suspected at clinical examination. Tumours may spread along the palatoglossal muscle, cornering the glossotonsillar sulcus, to involve the anterior tonsillar pillar.
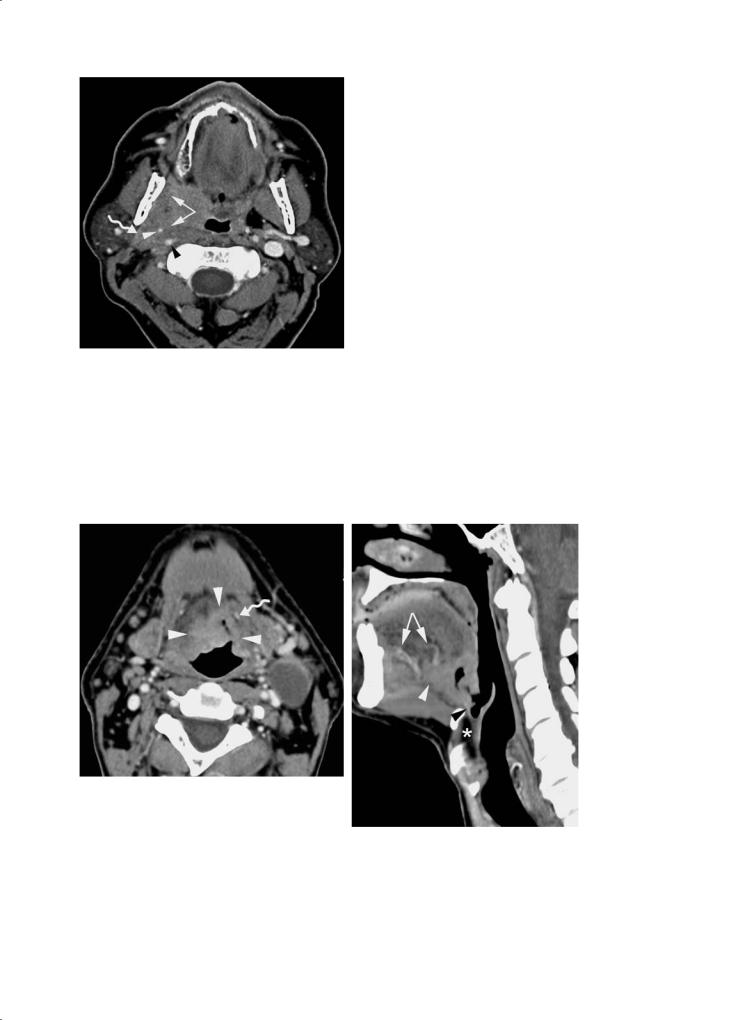
Anterior spread into the floor of the mouth and/ or tongue body may occur, along the myloand/or hyoglossal muscle, and/or along the lingual neurovascular bundle (Fig. 7.6). Tongue base cancer may also grow in a retrograde fashion along the lingual vessels towards the external carotid artery (Dubin et al. 2002).Vascular and perineural tumor spread is associated with reduced local and regional tumor
134 |
R. Hermans |
Fig. 7.5 Contrast-enhanced CT image in a patient with a right sided tonsillar cancer. The mass extends into the infratemporal space, parapharyngeal and carotid space (arrows). Encasement of the external carotid artery (white arrowhead), and partial encasement of the internal carotid artery (black arrowhead). Laterally, the mass is bordered by the posterior belly and the digastric muscle (curved arrow) and parotid gland
control and reduced patient survival. A tumor mass with a overall diameter of more than 2 cm on imaging predicts vascular and perineural tumor spread (Mukherji et al. 1996). Infiltration of the normal fatty tissue planes in the base of the tongue, of the fat in the sublingual space, as well as irregular tumor margins are also associated with an increased risk of vascular and perineural tumor spread. Such findings are related to overall tumor bulk.
Spread to the valleculae and piriform sinuses, and into the preepiglottic space may be seen. Retrograde tumor growth sometimes occurs along the styloid musculature (Fig. 7.7).
Extension of a tongue base cancer across the midline usually precludes surgical cure, as one lingual neurovascular pedicle needs to be conserved for sufficient functional recovery to allow safe swallowing (Fig. 7.8).
Differentiation of tongue base cancer from normal lymphoid tissue on the surface of the tongue base may be difficult on imaging studies; the only reliable criterion to diagnose cancer is infiltration of the deeper soft tissue structures (see above) (Fig. 7.9).
a
b
Fig. 7.6a,b. Contrast-enhanced CT images in a patient with tongue base cancer. a Axial image. An ulcerated, contrast-enhancing soft tissue mass is seen in the base of the tongue (arrowheads). Irregular tumor margins are present. The lesion crosses the midline, and approaches the left lingual artery (curved arrow). Large, necrotic adenopathy on left side. b Sagittal reformatted image (paramedian section on left side). The anterior spread in the floor of the mouth can be well appreciated (white arrowhead); again, close relationship to the proximal part of the lingual artery is seen (distal branches indicated by arrows). The lesion extends into the vallecula (black arrowhead); the preepiglottic space (asterisk) is not involved

Neoplasms of the Oropharynx |
135 |
a 
 b
b
Fig. 7.7a,b. Patient treated by irradiation for right-sided tonsillar cancer 5 years earlier; clinically an area suspicious for recurrent cancer is seen on the right tonsil. a Axial contrast-enhanced CT image. Area of soft tissue thickening and increased enhancement in right tonsillar area, extending into tongue base (arrowheads): recurrent cancer. Some anterior extension along the hyoglossus muscle is suspected (arrow). The lesion abuts the styloglossal muscle, which has a slightly irregular contour (curved arrow). The fat planes surrounding the external and internal carotid artery are obliterated, but do not show increased enhancement: probably post-radiotherapy. b Coronal reformatted image. The cancer is extending into the soft palate (upper arrowhead) and down to the pharyngoepiglottic fold (lower arrowhead). Obliteration of fat planes along the styloglossal muscle, showing increased enhancement: perimuscular tumor extension
Fig. 7.8. Patient with a left-sided tongue base cancer. Axial plain T1-weighted spin echo image shows soft tissue mass growing over the midline (arrowheads), involving the left sublingual space (asterisk) and genioglossus/geniohyoid muscles (arrow)
Fig. 7.9. Axial contrast-enhanced CT image in a patient with a right-sided tongue base cancer (asterisk). The lesion shows the same density as the lingual tonsil (arrowheads), but can be identified because it infiltrates the deeper tissues of the tongue base (arrows, compare to opposite side)
7.3.3
Soft Palate Cancer
Soft palate cancer may spread laterally and inferiorly along the tonsillar pillars. Superior spread
to the nasopharynx occurs in advanced disease (Fig. 7.10). Carcinoma of the soft palate may occasionally spread perineurally along palatine branches of the maxillary nerve (Ginsberg and
DeMonte 1998).
