
Учебники / Head_and_Neck_Cancer_Imaging
.pdf
166 |
R. Hermans and D. Farina |
a |
b |
Fig. 9.3a,b. Axial gadolinium-enhanced T1-weighted spin-echo images, showing the relationship between the superior constrictor muscle, the pharyngobasilar fascia and a nasopharyngeal cancer. a Axial section at level of soft palate. The superior constrictor muscle (arrowheads) is surrounding the nasopharynx; the tumor (asterisk) is confined to the nasopharyngeal lumen. b. At a higher level, the nasopharyngeal lumen is bordered by the pharyngobasilar fascia (arrowheads). Bilaterally, the tensor veli palatini muscle (black arrow) is visible on the outer side of this fascial layer. On the right, the tumor extends into the PPS through the pharyngobasilar fascia (white arrow); this site likely corresponds to the sinus of Morgagni
mentally outlined on axial scans by using the tensor veli palatini muscle and styloid process as anatomical landmarks. This last structure, easily detected with CT, is seen on MR with a characteristic ‘target’ appearance: hypointense external cortical rim with hyperintense bone marrow in its center.
The lateral fascial border of the PPS is not identified, but its cranial attachment lies just medial to foramen ovale (Fig. 9.1). MR is able to depict the course of the mandibular nerve – from Meckel’s cave, through the foramen ovale – and also the proximal segment of the main terminal branch, the inferior alveolar nerve, as it runs to the entrance of the mandibular canal.
The ICA and IJV, just medial to the styloid process, appear hyperdense on enhanced CT, while they generally show a flow void on T1and T2-weighted MR sequences. High or inhomogeneous signal is often seen in the jugular vein due to turbulent or slow flow and should not be mistaken for a strongly enhancing mass (e.g. glomus jugulare tumor).
9.3
Imaging of Parapharyngeal Space Lesions
The role of imaging in lesions at the level of the PPS can be summarized in two key points:
•To precisely identify the space of origin; this allows to reduce the differential diagnostic list to a limited number of possibilities.
•To identify hypervascular neoplasms in order to avoid biopsy and its possible complications.
9.3.1
Primary Lesions of the Parapharyngeal Space
Primary lesions of the PPS are rare and most are benign. These lesions can be classified based on histology (three main groups: salivary, neurogenic, paragangliomas), or according to the compartment of origin (prestyloid or retrostyloid).
9.3.2.1
Prestyloid Lesions
The overwhelming majority of neoplasms in the prestyloid compartment are salivary gland tumors. Most other lesions in this compartment are related to anomalies of the branchial apparatus.
Pleomorphic adenoma is the most frequent salivary tumor. These tumors may arise from ectopic minor salivary glands, localised in the prestyloid compartment along the embryological growth path of the parotid gland, but much more often they originate from the deep lobe of the parotid gland. Making the differentiation between a primary prestyloid PPS lesion and a neoplasm of the deep lobe of the parotid gland is important, because, in the latter case, the deep lobe of the parotid gland also needs to be resected.

Parapharyngeal Space Neoplasms |
167 |
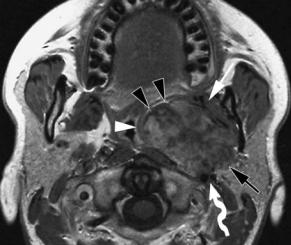
The most reliable sign of a primary PPS tumor is the presence of a fat layer separating the tumor from the deep lobe of the gland. A deep parotid lobe tumor appears as a more or less dumbbell-shaped mass, connected to the parotid gland, potentially widening the stylomandibular tunnel, and displacing anteromedially the PPS fat (Fig. 9.4).
Pleomorphic adenomas usually show the following MR appearance: hyperintense signal intensity on T2-weighted sequences, related to their myxoid component, and often pronounced enhancement with focal areas of hypointensity on T1-weighted sequences. However, various signal intensities due to haemorrhage, calcifications and necrosis may be seen within such a tumor (Fig. 9.5).
Although pleomorphic adenoma is a benign tumor, it may recur locally if the thin capsule is interrupted during surgical resection. Most frequently, these relapses are multifocal and appear very hyperintense on T2-weighted sequences.
Salivary malignancies are infrequent, with mucoepidermoid and adenoid cystic carcinoma being the most common histologic types. CT and MR characteristics do not allow differentiation of a malignant from a benign lesion, only the presence of infiltration of adjacent structures suggests malignancy.
Much rarer neoplasms in the prestyloid compartment are lipomas (Kakani et al. 1992) and muscular
neoplasms (rhabdomyomas and rhabdomyosarcomas).
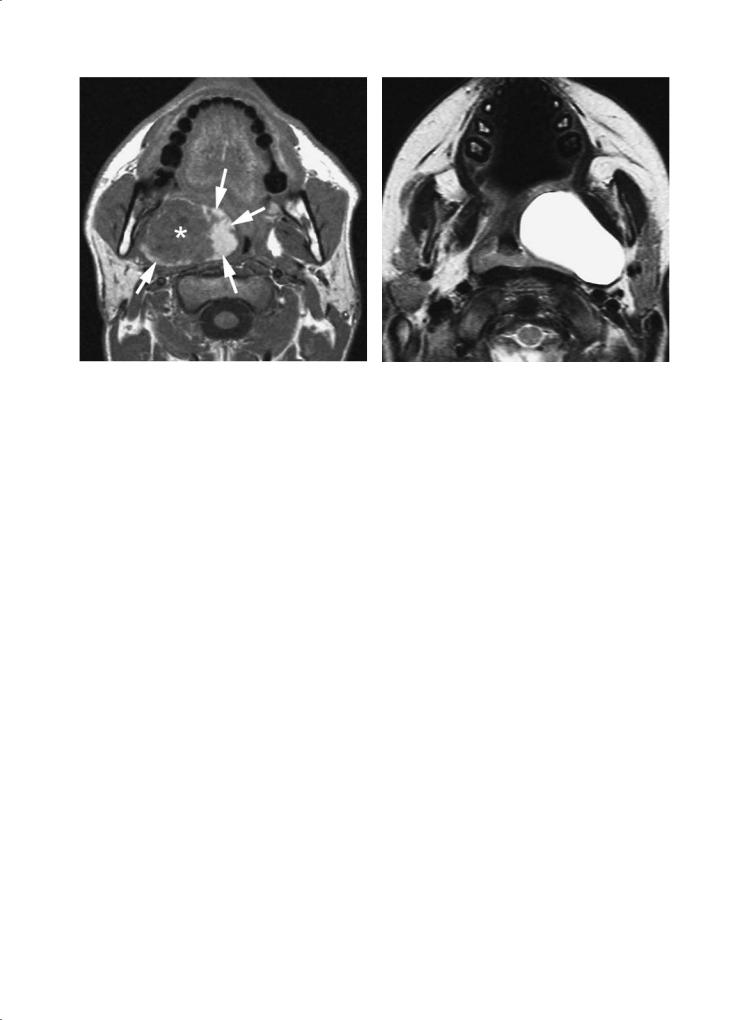
Anomalies of the second branchial apparatus represent a spectrum of manifestations, ranging from a fistula to an isolated cyst. One end of the spectrum is a fistula between the anterior side of the sternocleidomastoid muscle and the pharyngeal wall at the level of the palatine tonsil. The other end of the spectrum is a cyst, potentially localised anywhere along this tract. The most typical localisation of such a cyst is just below the mandibular angle, posterior to the submandibular salivary gland, lateral to the vessels and anterior to the sternocleidomastoid muscle. As the trajectory of such a branchial apparatus anomaly runs through the PPS, such cysts may also occur within the PPS (Fig. 9.6). Thickened, irregular walls may be seen in branchiogenic cysts that are, or have been infected. In the absence of a history of inflammation, an atypical cystic lesion should also raise the possibility of a cystic (retropharyngeal) adenopathy, as can be seen with papillary thyroid carcinoma (Fig. 9.7) (Lombardi et al. 2004).
A possible imaging pitfall in the prestyloid compartment is an hypertrophic pterygoid venous plexus mimicking a vascular lesion; in these cases imaging techniques will demonstrate the presence of multiple serpiginous flow voids (on MR) or enhancing vessels (on CT) just medial to the medial pterygoid muscle.
a 
 b
b
Fig. 9.4a,b. Axial T2-weighted (a) and gadolinium-enhanced T1-weighted spin-echo image (b) in a patient with a coincidentally discovered PPS tumor. The lesion can not be separated from the deep lobe of the parotid gland (black arrow), and largely fills the prestyloid compartment; a thin layer of fat is still visible at the anteromedial margin of the tumor (black arrowheads, b). The pharyngeal wall (white arrowheads) and medial pterygoid muscle (white arrow) are displaced. The large vessels (curved arrow) are displaced posteriorly. The styloid musculature produces an indentation in the posterior tumor margin (black arrowhead, a). The tumor was removed via a cervical approach, with resection of the deep parotid lobe. Pathologic examination showed pleomorphic adenoma
168
Fig. 9.5. Axial plain T1-weighted spin-echo image in a patient with a peritonsillar swelling on the right, rapidly increasing over a few hours time. A soft tissue mass (asterisk) is seen in the prestyloid compartment of the PPS; the mass periphery, mainly on the tonsillar side, shows spontaneous hyperintensity, compatible with recent hemorrhage (arrows). The mass was resected; pathologic examination showed pleomorphic adenoma
R. Hermans and D. Farina
Fig. 9.6. A 3-year-old patient presenting with peritonsillar swelling. Axial T2-weighted spin-echo image shows a cystic mass in the prestyloid compartment of the PPS, displacing the pharyngeal wall. The cyst was resected and confirmed to be a branchiogenic cyst
a 
 b
b
Fig. 9.7a,b. An asymptomatic, 42-year-old female patient, with coincidentally discovered peritonsillar swelling on right side. Axial T2-weighted (a) and gadolinium-enhanced T1-weighted (b) spin-echo images show largely cystic lesion, filling the prestyloid compartment of the PPS. The mass is centered between the prevertebral muscle (black arrowhead) and internal carotid artery (white arrowhead), a typical localisation for retropharyngeal adenopathy. The internal carotid artery, as well as the internal jugular vein (white arrow) are displaced posterolaterally. The cystic mass contains a solid component (black arrow), showing enhancement. A lesion showing such appearance, especially in a young female patient, should raise the possibility of metastatic papillary thyroid cancer. Cytologic examination of the cyst content was compatible with (although not conclusive for) metastatic papillary thyroid cancer. Resection of the thyroid gland eventually confirmed papillary cancer in the right lobe; after subsequent resection of the retropharyngeal mass, metastatic thyroid cancer was histologically proven also at this level

Parapharyngeal Space Neoplasms |
169 |
9.3.2.2
Retrostyloid Lesions
A retrostyloid tumor can easily be identified when an anterior displacement of the ICA, and possibly the styloid process, is present. These lesions also displace the prestyloid PPS fat anteriorly. The most common primary lesions in this compartment are neurogenic tumors (17%–25% of all PPS neoplasms) and paragangliomas (10%–15%).
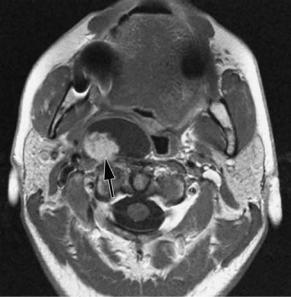
Schwannomas more frequently arise from the vagal nerve or from the cervical sympathetic chain. Malignant degeneration has seldom been reported (Al Otieshan et al. 1998). The typical MR appearance of a schwannoma is that of an ovoid mass with a slightly hyperintense signal on T2-weighted images; the lesion can be inhomogeneous because of areas of hemorrhage or cystic degeneration (Fig. 9.8). After the administration of a contrast agent, marked enhancement may lead to a misdiagnosis of a hypervascular tumor.Actually, a schwannoma is a relatively hypovascular lesion and the enhancement is due to extravascular leakage through abnormally permeable vessels with poor venous drainage (Fig. 9.9).
Less frequent are neurofibromas,in 10% of cases associated to von Recklinghausen syndrome (Tandon et al. 1992). These lesions may undergo marked fatty replacement.
Paragangliomas arise from chemoreceptor cells, basically present at three different anatomical sites: at the level of the nodose ganglion of vagal nerve, just below the skull base (vagal paraganglioma), at the ca-
rotid bifurcation (carotid paraganglioma) and at the level of the jugular foramen (jugular paraganglioma). A marked enhancement and, at MR, a‘salt and pepper’ pattern are quite typical: this is due to the presence of tortuous large caliber vessels (detected as flow voids) within the mass (Fig. 9.10). Nevertheless, this pattern can be difficult to see or even absent, particularly in small lesions with predominantly small caliber feeding vessels (Fig. 9.11) (Som and Curtin 1995). Carotid body tumors typically splay the internal and external carotid artery and may extend superiorly in the PPS. A vagal glomus tumor has a prevalent extracranial growth and only rarely reaches the carotid bifurcation. A jugular paraganglioma is centered in the jugular foramen, often eroding the surrounding bone and extending into the middle ear (Swartz et al. 1998). CT and MR allow an accurate diagnosis of glomus tumors in most cases; the role of angiography is restricted to accurate assessment of the arterial feeders and to preoperative embolization.
Several vascular anomalies may be encountered in the retrostyloid compartment. Sometimes the ICA shows a tortuous course,running behind the posterior pharyngeal wall: correct assessment avoids a disastrous biopsy of a misinterpreted submucosal pharyngeal lesion. An occlusion of ICA, or a IJV thrombosis, are uncommonly encountered: MR shows a complex pattern of signal intensities of the intraluminal clot reflecting the different phases of hemoglobin degradation. MR is an accurate technique to diagnose dissection of the ICA. Aneurysms of the extracranial part of the ICA occur; rarely these aneurysms cause
a 
 b
b
Fig. 9.8a,b. T2-weighted (a) and gadolinium-enhanced T1-weighted (b) spin-echo images, showing a inhomogeneous mass in the retrostyloid compartment of the PPS, displacing the internal carotid artery (arrowhead) anteromedially, and the internal jugular vein (arrow) posterolaterally. Vagal schwannoma

170 |
R. Hermans and D. Farina |
a |
b |
d
c
Fig. 9.9a–d. Axial T2-weighted (a), axial plain T1-weighted spin-echo image (b), gadolinium-enhanced 3D-gradient echo T1weighted sequence, reformatted in oblique axial (c) and sagittal (d) plane. Solid mass lesion in the right PPS: anterior displacement of the internal carotid artery (black arrows) and stylopharyngeal muscle (arrowheads) indicates the retrostyloid compartment as the site of origin. T2 hyperintensity and bright enhancement (in the absence of vascular flow voids) are consistent with neurogenic tumor. Tumor extension within the condylar canal (white arrow) along with denervation atrophy of the right hemitongue (asterisk) suggests schwannoma of the XIIth cranial nerve (hypoglossal nerve). The diagnosis was confirmed at surgery
cranial nerve palsy. The diagnosis of such an aneurysm is usually straightforward (Fig. 9.12).
9.3.2
Secondary Lesions of the Parapharyngeal Space
The key to correctly identify the site of origin of a lesion arising from a space neighbouring the PPS, is
to carefully assess its relationships with the PPS fat and large vessels. A neoplasm of the masticator space displaces this fat plane posteromedially (Fig. 9.13), while a tumor of the pharyngeal mucosal space will usually infiltrate the retrostyloid compartment (Fig. 9.14), displacing the fat tissue laterally. A retropharyngeal lesion (most often a retropharyngeal adenopathy) displaces the fat of the PPS anterolaterally (Fig. 9.15).

Parapharyngeal Space Neoplasms
Fig. 9.10. Axial gadolinium-enhanced T1-weighted spin-echo image. A strongly enhancing mass, showing intratumoral signal voids, is present in the retrostyloid compartment of the PPS. The internal carotid artery is displaced anteromedially (arrowhead); the internal jugular vein is compressed and displaced posteromedially, not clearly identifiable on this image. (Image courtesy of Bert De Foer, MD, Antwerp, Belgium)
171
Fig. 9.12. Axial contrast-enhanced CT image shows nodular lesion in the retrostyloid compartment of the PPS. At first sight one may think about retropharyngeal adenopathy. However, the narrowed lumen of the internal carotid artery (white arrowhead) is within the peripheral part of the mass, which also shows some peripheral calcifications (black arrowhead): these findings are compatible with an internal carotid artery aneurysm
a |
b |
Fig. 9.11a–c. Gadolinium-enhanced |
3D gradient-echo T1- |
weighted sequence in axial plane. Bilateral retrostyloid PPS |
|
mass (a,b): on the right side the internal carotid artery is |
|
anteromedially displaced (white arrows), and the stylopha- |
|
ryngeal muscle is anteriorly displaced (white arrowhead); on |
|
the left, anterior displacement of internal carotid artery is |
|
more evident (black arrow). Serpiginous flow voids are more |
|
clearly demonstrated at the periphery of the left lesion, prob- |
|
ably due to its larger size. More caudally (c), a third lesion |
|
is demonstrated just medial to internal (i) and external (e) |
|
carotid artery, few millimetres above carotid bifurcation. MR |
|
findings are consistent with bilateral vagal paraganglioma |
|
and right carotid body tumor; this diagnosis is also strongly |
|
supported by a familial history for paraganglioma |
c |

172 |
R. Hermans and D. Farina |
a 
 b
b
Fig. 9.13a,b. Axial T2-weighted (a) and plain T1-weighted spin-echo image (b) in a 22-year old patient presenting with hearing loss on left side and increasing difficulties with mastication. A soft tissue mass (black arrows) is seen on the deep side of the mandible, extending into the PPS; a thin layer of fat can be recognized on the posteromedial side of the mass (white arrow). The lesion involves the deep lobe of the parotid gland. Contrary to the patient shown in Fig. 9.4, the pterygoid musculature can not be recognized anymore, suggesting that the point of origin is within the masticator space. Fluid is present in the mastoid, secondary to dysfunction of the eustachian tube. Biopsy revealed rhabdomyosarcoma
Fig. 9.14. Patient presenting with increasing left-sided neck and facial pain. Axial gadolinium-enhanced T1-weighted spinecho image shows poorly defined, partially liquefied mass in the left PPS, mainly within the retrostyloid compartment. The adjacent pharyngeal wall (arrow) appears inhomogeneous, with disruption of the constrictor muscle. Anterolateral displacement of the internal carotid artery (arrowhead), as well as of the prestyloid fat plane. Involvement of the prevertebral and paraspinal compartment is seen. Pharyngeal biopsy showed squamous cell cancer
Fig. 9.15. Axial contrast-enhanced CT image at level of nasopharynx. Centrally necrotic retropharyngeal adenopathy (lymphatic metastasis of recurrent laryngeal cancer). A retropharyngeal adenopathy is typically situated between the prevertebral muscle (arrowhead) and the internal carotid artery (arrow); large adenopathies, such as in this case, displace the prestyloid fat anterolaterally

Parapharyngeal Space Neoplasms |
173 |
Large and aggressive neoplasms, such as sometimes seen with sarcoma, nasopharyngeal cancer or lymphoma, may show a transspatial growth pattern; in such circumstances, a precise diagnosis on imaging studies is not possible.
Secondary involvement of the parapharyngeal space may also be observed in benign multicompartmental lesions, i.e. lesions characterized by the aptitude to grow across the boundaries that separate the deep spaces of the head and neck, thus invading simultaneously more than two compartments. Among these, hemangioma and lymphangioma exhibit a rather peculiar imaging pattern consisting of hypodensity on plain CT and hyperintensity
on T2-weighted MR sequences. Dotted calcifications – better demonstrated on CT – may be seen in hemangiomas; MR may reveal serpiginous flow voids representing dilated vascular channels. On the other hand, lymphangioma may exhibit spontaneous T1 hyperintensity, due to highly hyperproteinaceous or fatty content. Contrast agent application better differentiates the two entities: hemangioma shows bright (though frequently non-homoge- neous) enhancement; lymphangioma, on the other hand, shows no contrast uptake, though enhancement of the multiple subtle septa crossing the lesion is occasionally observed (Figs. 9.16 and 9.17) (Sigal 1998).
a |
b |
Fig. 9.16a–c. T2-weighted spin-echo (a), plain (b) and gadolin- ium-enhanced T1-weighted spin-echo (c) images in the axial
|
plane. Recurrent lymphangioma. The patient had received sur- |
|
gery 1 year before, as demonstrated by skin retraction (white |
|
arrowhead) and deep scar tissue in the proximity of external |
|
and internal carotid artery (black arrow). Residual lymphan- |
|
gioma is demonstrated in the PPS medially extending to the |
|
pharyngeal mucosal space (tonsillar fossa, base of tongue) |
|
(white arrows). The lesion shows bright T2 signal, no signifi- |
|
cant enhancement is observed after contrast application. The |
|
diagnosis was confirmed with fine needle aspiration under |
c |
ultrasound guidance |

174 |
R. Hermans and D. Farina |
a |
b |
Fig. 9.17a,b. A T2-weighted spin-echo (a) and gadolinium-enhanced T1-weighted spin-echo image (b), axial plane. Multicompartmental lesion invading the left parotid (pa), parapharyngeal (pps) and pharyngeal mucosal (pm) space. The lesion exhibits T2 hyperintense signal, the slightly more caudal T1 section demonstrates enhancement of the parapharyngeal component. Findings are consistent with hemangioma
Parapharyngeal abscess is a rare event in the era of broad spectrum antibiotics, generally secondary to head and neck infections (odontogenic, pharyngeal, tonsillar, otomastoideal, or of salivary origin). On CT and MR, the detection of a fluid filled cavity lined by an enhancing peripheral rim rather safely indicates the presence of an abscess, whereas a soft tissue swelling associated with non-homogeneity and effacement of fat planes suggests cellulitis. Actually, the discrimination between the two entities, potentially critical for treatment planning, may be hampered by a significant number of false positives, particularly when cellulitis must be differentiated from an early abscess (Elden et al. 2001). Imaging also plays a key role in detecting major complications, more commonly occurring when the infectious process involves the retrostyloid compartment, such as IJV thrombosis, skull base osteomyelitis or extension in the retropharyngeal space (Nicolai et al. 2005).
References
Al Otieschan AA, Saleem M, Manohar MB, Larson S, Atallah A (1998) Malignant schwannoma of the parapharyngeal space. J Laryngol Otol, 112:883–887
Elden LM, Grundfast KM, Vezina G (2001) Accuracy and usefulness of radiographic assessment of cervical neck infections in children. J Otolaryngol 30:82–89
Farina D, Hermans R, Lemmerling M, Op de beeck K (1999) Imaging of the parapharyngeal space. J Belge Radiol 82:234–240
Grégoire V, Levendag P, Ang KK, et al (2003) CT-based delineation of lymph node levels and related CTVs in the nodenegative neck: DAHANCA, EORTC, GORTEC, NCIC,RTOG consensus guidelines. Radiother Oncol 69:227–236
Kakani RS, Bahadur S, Kumar S, Tandon DA (1992) Parapharyngeal lipoma. J Laryngol Otol 106:279–281
Lombardi D, Nicolai P, Antonelli AR, Maroldi R, Farina D, Shaha AR (2004) Parapharyngeal lymph node metastasis: an unusual presentation of papillary thyroid carcinoma. Head Neck 26:190–196
Luna-Ortiz K, Navarrete-Alemán JE, Granados-García M, Herrera-Gómez A (2005) Primary parapharyngeal space tumors in a Mexican cancer center. Otolaryngol Head Neck Surg 132:587–591
Maroldi R, Battaglia G, Maculotti P, Bondioni MP, Gheza G, Chiesa A (1994) Diagnostica per immagini delle lesioni dello spazio parafaringeo. In: Mosciaro O (ed) I Tumori parafaringei. Printed by Studio Forma, Verona, pp 69– 103
Miller FR, Wanamaker J., Lavertu P, Wood BG (1996) Magnetic resonance imaging and the management of parapharyngeal space tumors. Head Neck 18:67–77
Mukherji SK, Castillo M (1998) A simplified approach to the spaces of the suprahyoid neck. Radiol Clin North Am 36:761–780
Nasser JG, Attia EL (1990) A conceptual approach to learning

Parapharyngeal Space Neoplasms
and organizing the surgical anatomy of the skull base. J Otolaryngol 19:114–121
Nicolai P, Lombardi D, Berlucchi M, Farina D, Zanetti D (2005) Drainage of retro-parapharyngeal abscess: an additional indication for endoscopic sinus surgery. Eur Arch Otorhinolaryngol Jan 25; [Epub ahead of print]
Olsen KD (1994) Tumors and surgery of the parapharyngeal space. Laryngoscope 104:1–28
Pang KP, Goh CH, Tan HM (2002) Parapharyngeal space tumours: an 18 year review. J Laryngol Otol 116:170–175 Sigal R (1998) Infrahyoid neck. Radiol Clin North Am 36:781–
799
175
Som PM, Curtin HD (1995) Lesions of the parapharyngeal space. Role of MR imaging. Otolaryngol Clin North Am 28:515–542
Stell PM, Mansfield AO, Stoney PJ (1985) Surgical approaches to tumors of the parapharyngeal space. Am J Otolaryngol 6:92–97
Swartz JD, Harnsberger HR, Mukherji SK (1998) The temporal bone: contemporary diagnostic dilemmas. Radiol Clin North Am 36:819–854
Tandon DA, Bahadur S, Misra NK, Deka RC, Kapila K (1992) Parapharyngeal neurofibromas. J Laryngol Otol 106:243– 246
