
- •Modular structure of proteins
- •Structural domains
- •The evolution and shuffling of domains
- •Sequence homology and the acquisition of function
- •Domain function
- •Catalytic domains
- •Protein interaction domains
- •The inventory of domains
- •Detection
- •Classification
- •Examples of domains with roles in signalling
- •Domains that bind oligopeptide motifs
- •SH2 domains
- •PTB domains
- •SH3 domains
- •Phosphoinositide-binding domains
- •PH domains
- •Other phosphoinositide-binding domains
- •Polypeptide modules that bind Ca2
- •Calcium-binding motifs and domains
- •The EF-hand motif
- •C2 domains
- •Zinc finger domains
- •Protein kinase domains
- •Protein kinases share a common domain
- •Structural elements that regulate kinase activity
- •References

Signal Transduction
The SH3 domain binding surface is hydrophobic16 with conserved aromatic side chains forming ridges that interact with the grooves of the polyproline helix. About three turns are involved in binding (see Figure 24.3). SH3 domains fall into two main classes that recognize different target sequences. One contains the motif [R/K]xxPxxP (class I), the other PxxPx[R/K] (class II). Proteins with class I motifs will align in the opposite direction to those with class II. Either way, the binding is weak and promiscuous, unless there are additional contributions from flanking residues. This may be the reason why adaptor proteins, such as Grb2 and Nck, possess two or more SH3 domains (see
Figure 12.6, page 326). In the case of Grb2, which has the architecture SH3- SH2-SH3, the SH3 domains bind to two neighbouring proline-rich regions on the target, thereby increasing the affinity and specificity of binding. The tandem arrangement of SH2 and SH3 domains found in several signalling
proteins provides a conformational mechanism for regulating SH3-dependent interactions through tyrosine phosphorylation.17
Note that although the architecture of the other proline recognition domains (EVH1, WW, etc.) is quite distinct from that of SH3 domains, the binding mechanism is very similar.
Phosphoinositide-binding domains
A common feature of signal transduction following receptor activation is the formation of complexes through the recruitment of cytosolic proteins to membrane sites. This may occur through the recognition of membrane phospholipid headgroups by phosphoinositide-binding domains.18 There are seven main phosphoinositides (see Figure 18.1, page 546) and although they are minority components of cell membranes, they are selectively enriched in different subcellular compartments, such as in the membranes of organelles or in the plasma membrane. For example PI(4,5)P2 is most
abundant at particular sites on the inner leaflet of the plasma membrane and on the surface of the Golgi apparatus, while PI(3)P is enriched on endosomal membranes (see below).
PI(4,5)P2 is a substrate for phospholipase C and PI 3-kinase. Whereas PI(4,5)P2 is present in resting cells, the levels of PI(3,4)P2 and PI(3,4,5)P3 are negligible, but rise acutely at the plasma membrane when PI 3-kinase is recruited from the cytosol, as in signalling through the insulin receptor (see page 554).
Proteins recruited to the membrane by PI(3,4)P2 and PI(3,4,5)P3 have domains that bind to these 3-phosphoinositides selectively and with high affinity, in contrast to those that bind PI(4,5)P2, which tend to exhibit less preference and much lower affinity. This looser binding allows recruited proteins to explore different areas of membrane, giving them the opportunity to make additional contacts that enhance binding.
774

Protein Domains and Signal Transduction
Finally, PI(4,5)P2 regulates the actin cytoskeleton. Actin-binding proteins, such as WASP (Wiscott–Aldrich syndrome protein), profilin, cofilin, and gelsolin regulate the assembly and disassembly of filamentous actin. These and
other proteins that promote cytoskeletal remodelling adhere to the plasma membrane by binding PI(4,5)P2. Most use short stretches of basic amino acids that bind the acidic head group. These positively charged regions are not structural homology domains; indeed they may be unstructured prior to binding. (Spectrin, on the other hand, binds PI(4,5)P2 through its PH domain.)
Table 24.2 lists the principal phosphoinositide-binding domains with their preferred targets. Before discussing some of these in further detail, it is important to stress that many may also bind peptide ligands.
PH domains
PH or pleckstrin homology domains are among the most common domains in the human proteome.20 Early studies indicated a general membranetargeting function enabled by interaction with the head groups of membrane phosphoinositides, but although this is undoubtedly an important property of many PH domains, many bind very weakly and some not at all.21
Table 24.2 Phosphoinositide-binding domains
Domain |
Protein |
Specificity |
|
|
|
PH |
PLC 1 |
PI(4,5)P2 |
|
bARK |
PI(4,5)P2, PI(3,4,5)P3 |
|
GRP1 |
PI(3,4,5)P3 |
|
PKB/Akt |
PI(3,4)P2, PI(3,4,5)P3 |
|
Btk |
PI(3,4,5)P3 |
|
Ras GAP1m |
PI(3,4,5)P3 |
|
Ras GAP1IP4BP |
PI(4,5)P2, PI(3,4,5)P3 |
|
FAPP1 |
PI(4)P |
|
TAPP1 |
PI(3,4)P2 |
FYVE |
EEA1 |
PI(3)P |
|
Hrs |
PI(3)P |
|
Fab1/PIKfyve |
PI(3)P |
|
SARA |
PI(3)P |
|
|
|
PX |
p40phox |
PI(3)P |
|
p47phox |
PI(3,4)P2 |
|
SNX1 |
PI(3)P, PI(3,5)P2 |
|
SNX2 |
PI(3)P |
|
SNX3 |
PI(3)P |
|
|
|
ENTH/ANTH |
epsin1 |
PI(4,5)P2 |
|
epsinR |
PI(4)P |
|
AP180 |
PI(4,5)P2 |
Pleckstrin is the major PKC substrate in platelets.22,23 Rather
unusually, it contains two PH domains, one at each end of the molecule.
775
Signal Transduction
EVH1 domains are involved in cytoskeletal reorganization.
PDZ domains bind to the C-terminal residues of some ion channels and receptors.
Ran-binding domains are present in the nuclear pore complex.
FERM domains are present in cytoskeletal proteins.
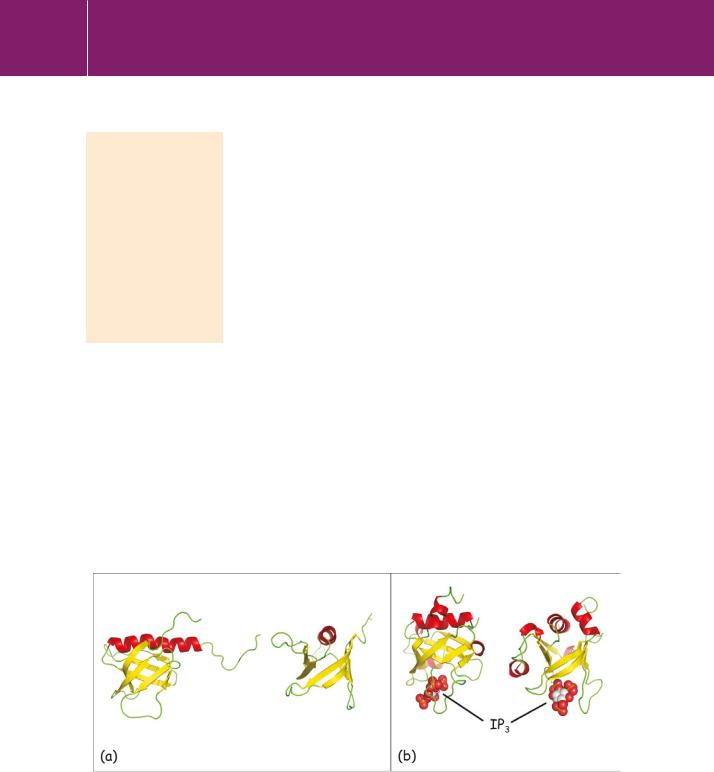
PH domains have ~120 amino acid residues. One of the clearest examples of the core structure is provided by the PH domain of GRK2 (G protein receptor kinase/ -adrenergic receptor kinase), depicted in Figure 24.4a. It consists of a-sandwich, formed by seven antiparallel strands. The arrangement resembles a twisted barrel and it is stabilized by a C-terminal -helix that is packed against one end. Despite low sequence homology among PH domains,
this core framework, termed the PH domain superfold, is highly conserved. Moreover, it has been adopted by other domains, none of which have any sequence similarity with PH domains. These include the PTB domains shown in Figure 24.2, the EVH1, PDZ, and Ran-binding domains, and also part of the FERM domain. Some of these also bind phosphoinositides, but often at sites that do not correspond to those used by PH domains.
The PH domain of PLC 1 is unusual in that it has relatively high affinity (KD 10 6 mol L21) and specificity for PI(4,5)P2.24 The head-group phosphates bind to basic residues in the loops between the strands (mostly 1/ 2 and3/ 4) as illustrated in Figure 24.4b. These help to locate the phospholipase at membrane regions containing PI(4,5)P2, which is also its substrate. However, this is not the whole story. Following this recognition event, hydrophobic contacts, probably involving side chains in the domain’s C-terminal
region, provide an additional attachment, causing it to insert into the lipid bilayer.25 Binding to PI(4,5)P2 accelerates this interaction, which otherwise is phosphoinositide-independent.
As mentioned previously, other PH domains that bind PI(4,5)P2 do so only weakly and unselectively, yet many of the proteins that bear them still localize to membranes. This is because these PH domains make additional hydrophobic contacts that do not involve phosphoinositides. Alternatively,
FIG 24.4 PH domains of GRK2 and PLC 1
(a) Two views of the GRK2 PH domain. The structure on the right is rotated 90° about the y axis. (b) Two similar views of the PLC 1 PH domain, showing a bound IP3 molecule (spheres) representing the head group of PI(4,5)P2 (2bcj,26 1mai27).
776

Protein Domains and Signal Transduction
they may attach to other membrane-associated proteins.21 Then again, additional contacts may be made by residues outside the PH domain, for instance by other membrane targeting domains. (An example of both of these mechanisms is provided by GRK2.)
The few PH domains that bind specifically to PI(3,4)P2 and PI(3,4,5)P3 have affinities in the submicromolar range. These are present in proteins that can sense the production of these phosphoinositides against a high background of PI(4,5)P2. Such effectors include PKB and PDK1, as well as the guanine nucleotide exchange factors for the GTPase Arf (GRP1, ARNO, and cytohesin) and the non-receptor protein tyrosine kinase Btk. All are recruited to the membrane by 3-phosphorylated polyphosphoinositides.
PH domains may also take part in protein–protein interactions. The bestcharacterized example is provided by GRK2 that phosphorylates activated-adrenergic receptors. Its PH domain (Figure 24.4) binds both to PI(4,5)P2 and to the -subunit of the G protein activated by the receptor. Receptor phosphorylation leads to desensitization and down-regulation of signalling. The mechanism is described in Chapter 4 (see page 98).
Other examples of PH domains that participate in protein–protein interactions include those of GRK3, phospholipase C- 2, and the non-receptor protein tyrosine kinase Btk. (Note that the PH domain of PLC 2 does not bind to phosphoinositides.)
Other phosphoinositide-binding domains
FYVE, PX, and ENTH/ANTH domains bind phosphoinositide headgroups and exhibit preferences that enable the tethering of proteins to specific membranes (Table 24.2). Whereas PI(4,5)P2 is predominant on the inner leaflet of the plasma membrane, other phosphoinositides are enriched on the cytosolic faces of organelles, where they take part in the recruitment of components that form the machinery that controls the vesicular transport of proteins. For example, PI(3)P, PI(3,5)P2, and PI(4)P are markers of early
endosomes, late endosomes, and the Golgi apparatus, respectively. In general, the vesicle-mediated trafficking of soluble or transmembrane proteins between compartments is a complex process. Different components control each stage and in many cases these are recruited through the interaction of a phosphoinositide-binding domain with a particular membrane.28 The initial event involves the induction of curvature at specific points in the membrane of the vesicle-forming compartment. This is followed by the construction of a protein coat to support a vesicle bud, the selection of cargo and then scission of the vesicle and its delivery to the correct destination.
For example, the early or sorting endosome, the receiving compartment for endocytic vesicles arriving from the plasma membrane, is characterized by the presence of PI(3)P which acts as an anchor for the C-terminal FYVE domain of
777

Signal Transduction
EEA-1 (early endosome antigen-1) also binds class III PI 3-kinase, which makes PI(3)P from phosphatidylinositol, helping to retain compartment identity.
Multivesicular bodies are endosomal organelles with internal vesicles. They are elements of the degradation pathway.
the protein EEA-1, a marker of early endosomes.29 The FYVE domain structure ( 80 residues, sometimes called a FYVE finger domain) is stabilized by two Zn2 ions, coordinated by the side chains of 8 cysteine residues (Figure 24.5; zinc fingers are discussed below). It binds 3-phosphorylated inositides with high specificity,30 though the interaction is dependent on the presence of the small GTPase Rab5, as well as sequences adjacent to the FYVE domain.31
The FYVE domain is also present in PI(3)P-5-kinase (also known as PIKfyve). This lipid kinase is tethered to the membranes of late endosomes, where it phosphorylates PI(3)P to form PI(3,5)P2, a marker of multivesicular bodies and late endosomes.
PX domains (phox homology domains) also bind to 3-phosphorylated inositol lipids. They were originally detected in p40phox and p47phox, subunits of the NADPH oxidase complex responsible for the respiratory burst in neutrophils.33 p40phox binds to PI(3)P on the surface of endosomes. PX domains also occur in the sorting nexins, a large protein family with members that direct the
trafficking of membrane proteins, such as receptors, and soluble cargo between cellular compartments. For instance, SNX1 (sorting nexin 1) binds PI(3)P and forms part of a complex that regulates the sorting of specific proteins from the early endosomal compartment to the trans-Golgi network. SNX3 also binds PI(3)P, but directs cargo from the early endosome to the recycling endosome.
A key property of SNX1 is the presence of a BAR domain (Bin, amphiphysin, Rvs). These banana-shaped domains act as curvature sensors, binding to highly curved membranes. Furthermore, they are also able to impose curvature upon membranes and thus take part in vesicle formation. This occurs in regions where the nexin has been recruited by PI(3)P. (It remains to be shown if the imposition of curvature is a general property of the sorting nexins.)
FIG 24.5 The FYVE domain of EEA-1.
The structure shows a bound molecule of inositol 1,3-bisphosphate, corresponding to the head group of PI(3)P (spheres). Also indicated are two Zn2 ions (turquoise spheres) and the residues that coordinate these ions. The two N-terminal cysteines are coloured red and the two C-terminal cysteines are blue. Side chains are not shown (1hyi32).
778
