
Biomedical EPR Part-B Methodology Instrumentation and Dynamics - Sandra R. Eaton
.pdf130 DEVKUMAR MUSTAFI AND MARVIN W. MAKINEN
location of the sequestered water, we searched for possible hydrogenbonding contacts that could stabilize a sequestered water molecule within van der Waals hard-sphere constraints satisfying the electron-proton distance of 6.65 ± 0.10 Å and lying close to the molecular plane of the spin-label, as required by the dependence of the resonance features on 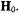 Accordingly, the H’ resonance was best ascribed to a water molecule hydrogen-bonded to the
Accordingly, the H’ resonance was best ascribed to a water molecule hydrogen-bonded to the 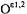 atoms of the Glu166 side chain, as illustrated in Fig. 22. The ENDOR-defined water molecule sits just on the extended van der Waals surface (Lee and Richards, 1971) of the substrate. The water oxygen lies at an approximate 2.9 Å distance from the carbonyl carbon of the scissile ester bond between the substrate and
atoms of the Glu166 side chain, as illustrated in Fig. 22. The ENDOR-defined water molecule sits just on the extended van der Waals surface (Lee and Richards, 1971) of the substrate. The water oxygen lies at an approximate 2.9 Å distance from the carbonyl carbon of the scissile ester bond between the substrate and 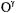 of Ser70 and forms an angle of ~105° with the C = O bond, precisely that expected for O · · · C = O nucleophilic attack (Burgi et al., 1973, 1974).
of Ser70 and forms an angle of ~105° with the C = O bond, precisely that expected for O · · · C = O nucleophilic attack (Burgi et al., 1973, 1974).
Deacylation, as the rate-limiting step for TEM-1 catalyzed hydrolysis of penicillin substrates, is controlled by an ionizing group with 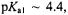 precisely that expected for a glutamyl side chain. The results in Figs. 21 and 22 provide direct structural confirmation that the catalytic role of Glu166 is that of a general-base, activating the water molecule for breakdown of the acylenzyme. Moreover, molecular graphics inspection of the active site shows that the
precisely that expected for a glutamyl side chain. The results in Figs. 21 and 22 provide direct structural confirmation that the catalytic role of Glu166 is that of a general-base, activating the water molecule for breakdown of the acylenzyme. Moreover, molecular graphics inspection of the active site shows that the 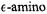 group of Lys73, proposed as a general base catalyst for acylation through the action of a hydrogen bonded water molecule (Strynadka et al., 1992), cannot form a hydrogen-bond to the ENDOR defined water molecule in Fig. 22 (the distance of closest approach being ~4 Å). This observation, therefore, rules against the proposed role of Lys73.
group of Lys73, proposed as a general base catalyst for acylation through the action of a hydrogen bonded water molecule (Strynadka et al., 1992), cannot form a hydrogen-bond to the ENDOR defined water molecule in Fig. 22 (the distance of closest approach being ~4 Å). This observation, therefore, rules against the proposed role of Lys73.
On the basis of electrostatic calculations, it has been shown that Glu166 can serve as the general base catalyst in both acylation and deacylation steps of the reaction, catalyzing extraction of the hydroxyl proton of Ser70 to form the acylenzyme and activating a water molecule as the nucleophile for its breakdown (Atanasov et al., 2000). Thus, ENDOR results provide direct confirmation of the role of Glu166 in catalysis and serve to define the structural basis of  action. Only in a catalytically competent form of the TEM-1 enzyme has it been possible to identify the hydrolytic water molecule. The results again emphasize the need to structurally characterize true intermediates of enzyme-catalyzed reactions instead of enzymeinhibitor complexes or enzymes rendered catalytically incompetent through mutagenesis.
action. Only in a catalytically competent form of the TEM-1 enzyme has it been possible to identify the hydrolytic water molecule. The results again emphasize the need to structurally characterize true intermediates of enzyme-catalyzed reactions instead of enzymeinhibitor complexes or enzymes rendered catalytically incompetent through mutagenesis.
ANGLE-SELECTED ENDOR |
131 |
Figure 21. Comparison of proton ENDOR spectra of the acylenzyme intermediate formed with SLPEN and deuterium enriched wild type (wt) TEM-1  and deuterium enriched Glu166Asn mutant enzyme in protiated cryosolvent buffer. In the lower panel, spectra of the mutant (E166N) and wt enzymes are shown for A and B settings of
and deuterium enriched Glu166Asn mutant enzyme in protiated cryosolvent buffer. In the lower panel, spectra of the mutant (E166N) and wt enzymes are shown for A and B settings of  as lower and upper sets, respectively. Two line pairs observed in the B setting spectra of the wt and E166N mutant species are indicated by stick diagrams, labeled H’ and H”, respectively (also illustrated at higher gain in the upper panel). Comparison of their respective line shapes and positions shows that the feature with the larger splitting, labeled H’, is present in spectra of both the wt and Glul66Asn mutant enzyme while the feature with the smaller splitting, labeled H’, is seen only for the wt enzyme. With permission from Mustafi et al. (2001).
as lower and upper sets, respectively. Two line pairs observed in the B setting spectra of the wt and E166N mutant species are indicated by stick diagrams, labeled H’ and H”, respectively (also illustrated at higher gain in the upper panel). Comparison of their respective line shapes and positions shows that the feature with the larger splitting, labeled H’, is present in spectra of both the wt and Glul66Asn mutant enzyme while the feature with the smaller splitting, labeled H’, is seen only for the wt enzyme. With permission from Mustafi et al. (2001).
132 |
DEVKUMAR MUSTAFI AND MARVIN W. MAKINEN |
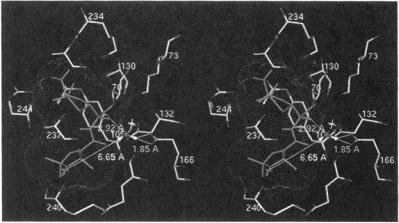
Figure 22. Stereo view of active site of TEM-1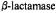 illustrating the ENDOR-defined location of the H’ proton assigned to a sequestered water molecule in the active site. The ENDOR-determined distance from the unpaired electron to one of the water protons and structural relationships to the carboxylate oxygens of Glu166 and to the carbonyl carbon of the ester bond of SLPEN formed with the side chain of Ser70 are indicated. The dotted surface represents the extended van der Waals surface of the spin-labeled acyl group showing that the water molecule is accommodated sterically by the substrate. The star just above the ENDOR-defined water molecule indicates the position of an X-ray-defined water molecule in the free enzyme (Jelsch et al., 1993). Reprinted from Mustafi et at. (2001) with permission.
illustrating the ENDOR-defined location of the H’ proton assigned to a sequestered water molecule in the active site. The ENDOR-determined distance from the unpaired electron to one of the water protons and structural relationships to the carboxylate oxygens of Glu166 and to the carbonyl carbon of the ester bond of SLPEN formed with the side chain of Ser70 are indicated. The dotted surface represents the extended van der Waals surface of the spin-labeled acyl group showing that the water molecule is accommodated sterically by the substrate. The star just above the ENDOR-defined water molecule indicates the position of an X-ray-defined water molecule in the free enzyme (Jelsch et al., 1993). Reprinted from Mustafi et at. (2001) with permission.
4.FUTURE PERSPECTIVES AND CONCLUDING REMARKS
In this review we have emphasized characterization of metal-bound and hydrogen-bonded solvent in small molecule and macromolecule environments to highlight the precision and level of structural detail that can be achieved through application of angle-selected ENDOR. The studies reviewed also show that comparable precision can be achieved in defining structure and conformation of active site residues and of the substrate in cryokinetically isolated reaction intermediates of enzymes. In this respect, ENDOR spectroscopy applied in conjunction with cryosolvent methods offers many advantages that cannot be achieved through application of other spectroscopic methods capable of three-dimensional structure determination, for instance, multi-dimensional NMR. While the latter is well suited for defining the relative spatial distribution of atoms in a protein in solution (at present 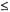 30 kDa) through nuclear Overhauser measurements, given the covalent bonding structure of the constituent amino acid residues, the uncertainties are significantly larger than in ENDOR, up to 50%, for internuclear separations
30 kDa) through nuclear Overhauser measurements, given the covalent bonding structure of the constituent amino acid residues, the uncertainties are significantly larger than in ENDOR, up to 50%, for internuclear separations 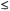 5.0 Å (Zhao and Jardetzky, 1994; Gradwell and
5.0 Å (Zhao and Jardetzky, 1994; Gradwell and
ANGLE-SELECTED ENDOR |
133 |
Feeney, 1996; Zabell and Post, 2002). Furthermore, the time required for NMR data collection of macromolecules in solution and the viscosity of cryosolvent mixtures at low temperatures are incompatible with structural analysis of true intermediates of enzyme-catalyzed reactions or of other chemically labile systems.
Application of cw ENDOR is associated, nonetheless, with inherent limitations despite the high precision afforded for structure analysis. Threedimensional structure determination by cw ENDOR is most straightforwardly carried out with I = 1/2 nuclei. Assignments of hydrogen resonances are generally dependent on carrying out synthetic chemical procedures for site-specific incorporation of deuterium to be used in parallel experiments. Such added chemical complexity is often time-consuming and arduous. In addition, signal-to-noise in data collection becomes an important consideration since the intensity of resonance features is dependent not only on the electron-nucleus distance, anisotropy of relaxation processes, and the number of nuclei contributing to the resonance, but also on the nuclear moment. Because of this latter factor, use of 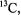 an important nuclide in multi-dimensional NMR experiments, has been limited in cw ENDOR, and most cw ENDOR studies have been restricted to
an important nuclide in multi-dimensional NMR experiments, has been limited in cw ENDOR, and most cw ENDOR studies have been restricted to 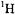 and
and 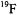 for structural analysis of the type described here. While improved resolution of overlapping resonance features may be achieved by cw ENDOR instrumentation applied at higher microwave frequencies through greater separation of g-values, structure analysis is not likely to be significantly improved unless the number of magnetic nuclei in the molecule of interest used for structure analysis can be increased.
for structural analysis of the type described here. While improved resolution of overlapping resonance features may be achieved by cw ENDOR instrumentation applied at higher microwave frequencies through greater separation of g-values, structure analysis is not likely to be significantly improved unless the number of magnetic nuclei in the molecule of interest used for structure analysis can be increased.
Because the number of electron-nucleus distances determined in cw ENDOR experiments is relatively small, structure analysis at present rests heavily on bond distance and valence angle information determined independently for large fragments of the molecular complex when ENDORdetermined electron-nucleus distances are applied as constraints in torsion angle search calculations. These fragments provide the molecular scaffolding to which the “ENDOR-active” nuclei are covalently attached. Improvement in structure analysis beyond that achieved hitherto with cw ENDOR, therefore, requires a means to increase significantly the number of independently determined electron-nucleus distances. Of most importance, therefore, are pulsed EPR and ENDOR methods that can provide a means to increase the number of electron-nucleus distances through detection of 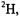
and since these nuclides are not easily employed in cw ENDOR. Two recent reviews describe pulsed EPR and ENDOR spectroscopy and their applications to biological systems (Cammack et al., 1999; Prisner et al., 2001). Also, a series of investigations have been reported in which pulsed EPR and ENDOR methods have been applied to
134 |
DEVKUMAR MUSTAFI AND MARVIN W. MAKINEN |
characterize structures of enzyme active sites and to determine proteinligand interactions (Gurbiel, et al, 1996; Gromov et al., 1999; Walsby et al., 2001). Pulsed methods have also been used to characterize structured solvent in macromolecular systems (Goldfarb, et al., 1996; DeRose et al., 1996).
With respect to the objective of increasing the number of dipolar electron-nucleus distances through a combination of cw and pulsed EPR and ENDOR methods, the goal in structural analysis should be to decrease as much as possible the dependence of the analysis on stereochemical data that define the molecular scaffold to which the “ENDOR-active” nuclei are covalently attached. For instance, while the conformation of SLCEP in Fig. 14 was assigned successfully through use of ENDOR-determined distance constraints, the analysis relies heavily on input from X-ray diffraction studies providing bond distances and valence angles of atoms that are not ENDOR-active. In this respect, it should be noted that direct detection of 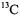 NMR in the vicinity of paramagnetic centers is favored through paramagnetic relaxation processes in contrast to
NMR in the vicinity of paramagnetic centers is favored through paramagnetic relaxation processes in contrast to 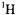 (Banci et al., 1991). On this basis it may be possible to take advantage of the differential relaxation characteristics of
(Banci et al., 1991). On this basis it may be possible to take advantage of the differential relaxation characteristics of 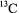 and
and 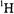 with stochastic ENDOR (Brueggeman and Niklas, 1994). This is essentially a pulsed ENDOR technique. While approaches to extract geometrical information based on this differential property have yet to be developed, it has been shown that NMR detection of
with stochastic ENDOR (Brueggeman and Niklas, 1994). This is essentially a pulsed ENDOR technique. While approaches to extract geometrical information based on this differential property have yet to be developed, it has been shown that NMR detection of 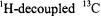 near paramagnetic centers in proteins can be used to identify residue connectivities (Machonkin et al., 2002).
near paramagnetic centers in proteins can be used to identify residue connectivities (Machonkin et al., 2002).
The three-dimensional structure and conformation of a (diamagnetic) chemotactic tripeptide uniformly enriched with 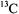 and
and 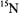 has been determined on the basis of simulated annealing calculations restrained by inter-nuclear distance and torsion angle measurements obtained through solid-state, magic-angle spinning NMR experiments (Rienstra et al., 2002). Extension of a similar approach should be feasible for paramagnetic solidstate systems through a combination of cw and pulsed EPR and ENDOR methods. This combined approach need not be restricted only to macromolecules with naturally occurring paramagnetic sites. Applications of ENDOR structural probes such as nitroxyl spin-labels and the
has been determined on the basis of simulated annealing calculations restrained by inter-nuclear distance and torsion angle measurements obtained through solid-state, magic-angle spinning NMR experiments (Rienstra et al., 2002). Extension of a similar approach should be feasible for paramagnetic solidstate systems through a combination of cw and pulsed EPR and ENDOR methods. This combined approach need not be restricted only to macromolecules with naturally occurring paramagnetic sites. Applications of ENDOR structural probes such as nitroxyl spin-labels and the 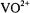 cation would be of clear advantage in view of the success with which they have been employed in both small molecule and macromolecular systems hitherto. On this basis, combined application of cw and pulsed ENDOR methods is likely to yield a significantly enhanced basis for structure assignments.
cation would be of clear advantage in view of the success with which they have been employed in both small molecule and macromolecular systems hitherto. On this basis, combined application of cw and pulsed ENDOR methods is likely to yield a significantly enhanced basis for structure assignments.

ANGLE-SELECTED ENDOR |
135 |
5.ACKNOWLEDGMENTS
This work has been supported by grants of the National Science Foundation (MCB-0092524) and of the National Institutes of Health (DK57599).
6.REFERENCES
Albanese, N. F. and Chasteen, N. D. (1978). Origin of Electron Paramagnetic Resonance Line Widths in Frozen Solutions of Oxovanadium(IV) Ion. J. Phys. Chem. 82, 910-914.
Ament, S. S., Wetherin, J. B., Moncrief, J. W., Flohr, K., Mochizuk, M., and Kaiser, E.T. (1973). Determination of Absolute-Configuration of (+)3-Carboxy-2,2,5,5-Tetramethyl-1- Pyrrolidinyloxy. J. Am. Chem. Soc. 95, 7896-7897.
Atanasov, B. P., Mustafi, D., and Makinen, M. W. (2000). Protonation of the  Nitrogen is the Trigger Event in the Catalytic Action of Class A
Nitrogen is the Trigger Event in the Catalytic Action of Class A  Proc. Natl. Acad. Sci. USA 97, 3160-3165.
Proc. Natl. Acad. Sci. USA 97, 3160-3165.
Atherton, N. M. (1993). Principles of Electron Spin Resonance, Royal Society of Chemistry, London, U. K. Atherton, N. M. and Shackleton, J. F. (1980). Proton ENDOR of 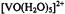 in
in  Mol. Phys. 39, 1471-1485.
Mol. Phys. 39, 1471-1485.
Atherton, N. M. and Shackleton, J. F. (1984). Proton Hyperfine Couplings in
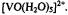 The Validity of the Point-Dipole Approximation. Chem. Phys. Lett. 103, 302-304.
The Validity of the Point-Dipole Approximation. Chem. Phys. Lett. 103, 302-304.
Attanasio, D. (1986). Structural Information from ENDOR Spectroscopy: The Frozen Solution Proton Spectra of Some VO(IV) Complexes. J. Phys. Chem. 90, 4952-4957.
Attanasio, D. (1989). Structural Information from Powder ENDOR Spectroscopy. J. Chem. Soc., Faraday Trans. 1 85, 3927-3937.
Audet, P., Simard, C., and Savoie, R. (1991). A Vibrational Spectroscopic Study of the SelfAssociation of 5'-GMP in Aqueous Solution. Biopolymers 31, 243-251.
Baker, J. M., Davies, E. R., and Hurrell, J. P. (1968). Electron Nuclear Double Resonance in Calcium Fluoride Containing  and
and  in Tetragonal Sites. Proc. Royal Soc.(London) A308, 403-431.
in Tetragonal Sites. Proc. Royal Soc.(London) A308, 403-431.
Ballhausen, C. J. and Gray, H. B. (1962). The Electronic Structure of the Vanadyl Ion. Inorg. Chem. 1, 111-122.
Ballhausen, C. J., Djurinskij, B. F., and Watson, K. J. (1968). Polarized Absorption Spectra of
3 Crystalline Polymorphs of |
J. Am. Chem. Soc. 90, 3305-3309. |
|
Banci, L., Bertini, I., and Luchinat, C. (1991). Nuclear and Electronic Relaxation, VCH |
||
Publishers, Weinheim, Germany. |
|
|
Bauer, R. S. and Berliner, L. J. (1979). Spin Label Investigations of |
Active- |
|
Site Structure in Single-Crystals. J. Mol. Biol. 128, 1-19.
Benner, S. A. (1988). Stereoelectronic Analysis of Enzymatic Reactions in Mechanistic Principles of Enzyme Activity, J. Liebman and A. Grunberg, eds., VCH Publishers, Inc., New York, pp. 27-74.
Blackburn, E. H. (2000). Telomere States and Cell Fates. Nature 408, 53-56.
Blinder, S. M. (1960). Orientation Dependence of Magnetic Hyperfine Structure in Free Radicals. J. Chem. Phys. 33, 748-752.
Boles, M. O., Girven, R. J., and Gane, P. A. C. (1978). Structure of Amoxycillin Trihydrate and a Comparison with Structures of Ampicillin. Acta Crystallogr. B34, 461-466.
136 DEVKUMAR MUSTAFI AND MARVIN W. MAKINEN
Bolin, K. A., Hanson, P., Wright, S. J., and Millhauser, G. L. (1998). An NMR Investigation of the Conformational Effect of Nitroxide Spin Labels on Ala-rich Helical Peptides. J. Magn. Reson. 131, 248-253.
Bolin, K. A. and Millhauser, G. L. (1999). Alpha and 3(10): The Split Personality of Polypeptide Helices. Accts. Chem. Res. 32, 1027-1033.
Brooks, B. R., Bruccoleri, R. E., Olafson, B. D., States, D. J., Swaminathan, S., and Karplus, M., (1983). CHARMM: A Program for Macromolecular Energy, Minimization, and Dynamics Calculations. J. Comput. Chem. 4, 187-217.
Brueggeman, W. and Niklas, J. R. (1994). Stochastic ENDOR. J. Magn. Reson. Series A 108, 25-29.
Burgi, H. B., Dunitz, J. D., and Shefter, E. (1973). Geometrical Reaction Coordinates. 2. Nucleophilic Addition to a Carbonyl Group. J. Am. Chem. Soc. 95, 5065-5067.
Burgi, H. B., Dunitz, J. D., and Shefter, E. (1974). Chemical-Reaction Paths. 4. Aspects of O C=O Interactions in Crystals. Acta Crystallogr. B30, 1517-1527.
Bush, K., Jacoby, G. A., and Medeiros, A. A. (1995). A Functional Classification Scheme for
 and Its Correlation with Molecular Structure. Antimicrob. Agents. Chemother. 39, 1211-1233.
and Its Correlation with Molecular Structure. Antimicrob. Agents. Chemother. 39, 1211-1233.
Cammack, R., Gay, E., and Shergill, J. K. (1999). Studies of Hyperfine Interactions in [2Fe2S] Proteins by EPR and Double Resonance Spectroscopy. Coord. Chem. Rev. 192, 10031022.
Chasteen, N. D. (1981). Vanadyl(IV) EPR Probes. Inorganic and Biochemical Aspects. in Biological Magnetic Resonance, Vol. 3, L. J. Berliner and J. Reuben, eds., Plenum Press, New York, pp. 53-119.
Chasteen, N. D. (1983). The Biochemistry of Vanadium. Structure & Bonding (Berlin) 53, 105-138.
Chasteen, N. D., Ed. (1990). Vanadium in Biological Systems, Kluwer Academic, Boston, Massachusetts.
Christianson, D. W. and Lipscomb, W. N. (1986). X-Ray Crystallographic Investigation of Substrate Binding to Carboxypeptidase A at Subzero Temperature. Proc. Natl. Acad. Sci. USA 83, 7568-7572.
Christianson, D. W. and Lipscomb, W. N. (1989). Carboxypeptidase A. Accts. Chem. Res. 22, 62-69.
Cornman, C. R., Zovinka, E. P., Boyajian, Y. D., Geiserbush, R. M., Boyle, P. D., and Singh, P. (1995). Structural and EPR Studies of Vanadium Complexes of Deprotonated Amide Ligands. Effects on the V-51 Hyperfine Coupling-Constant. Inorg. Chem. 34, 4213-4219.
Cornman, C. R., Geiser-Bush, K. M., Rowley, S. P., and Boyle, P. D. (1997). Structural and Electron Paramagnetic Resonance Studies of the Square Pyramidal to Trigonal Bipyramidal Distortion of Vanadyl Complexes Containing Sterically Crowded Schiff Base Ligands. Inorg.Chem. 36, 6401-6408.
Damblon, C., Raquet, X., Lian, L. Y., Lamotte-Brasseur, J., Fonze, E., Charlier, P., Roberts, G. C. K., and Frère, J. M. (1996). The Catalytic Mechanism of  NMR Titration of an Active Site Lysine Residue of the TEM-1 Enzyme. Proc. Natl. Acad. Sci. USA 93, 1747-1752.
NMR Titration of an Active Site Lysine Residue of the TEM-1 Enzyme. Proc. Natl. Acad. Sci. USA 93, 1747-1752.
Davis, T. D., Christofferson, R. E., and Maggiora, G. N. (1975). Ab Initio Calculations on Large Molecules Using Molecular Fragments. Nitroxide Spin Label Characterizations. J. Am. Chem. Soc. 97, 1347-1354.
DeRose, V. J., Liu, K. E., Lippard, S. J., and Hoffman, B. M. (1996). Investigation of the Dinuclear Fe Center of Methane Monooxygenase by Advanced Paramagnetic Resonance Techniques: On the geometry of DMSO binding. J. Am. Chem. Soc. 118, 121-134.
ANGLE-SELECTED ENDOR |
137 |
Deslongchamps, P. (1983). Stereoelectronic Effects in Organic Chemistry, Pergamon Press, |
|
Oxford, U.K. |
|
di Matteo, A. and Barone, V. (1999). |
Development and Validation of Effective |
Computational Strategies for the Study of Metal Nitroxide Complexes. J. Phys. Chem. A103, 7676-7685.
Dikanov, S. A., Liboiron, B. D., Thompson, K. H., Vera, E., Yuen, V. G., McNeill, J. H., and Orvig, C. (1999). In vivo Electron Spin-Echo Envelope Modulation (ESEEM) Spectroscopy: First Observation of Vanadyl Coordination to Phosphate in Bone. J. Am. Chem. Soc. 121, 11004-11005.
Dikanov, S. A., Liboiron, B. D., and Orvig, C. (2002). Two-Dimensional (2D) Pulsed Electron Paramagnetic Resonance Study of 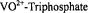 Interactions: Evidence for Tridentate Triphosphate Coordination, and Relevance to Bone Uptake and Insulin Enhancement by Vanadium Pharmaceuticals. J. Am. Chem. Soc. 124, 2969-2978.
Interactions: Evidence for Tridentate Triphosphate Coordination, and Relevance to Bone Uptake and Insulin Enhancement by Vanadium Pharmaceuticals. J. Am. Chem. Soc. 124, 2969-2978.
Dodge, R. P., Tempelton, D.H., and Zalkin, A. (1961). Crystal Structure of Vanadyl Bisacetylacetonate. Geometry of Vanadium in Fivefold Coordination. J. Chem. Phys. 35, 55-67.
Dorio, M. M. and Freed, J. H., Eds. (1979). Multiple Electron Resonance Spectroscopy,
Plenum Press, New York.
Douzou, P. (1977). Cryobiochemistry, Academic Press, New York.
Fattah, J., Twyman, J. M., Heyes, S. J., Watkin, D. J., Edwards, A. J., Prout, K., and Dobson, C. M. (1993). Combination of CP MAS NMR and X-Ray Crystallography. Structure and Dynamics in a Low-Symmetry Molecular-Crystal, Potassium Penicillin-V. J. Am. Chem. Soc. 115, 5636-5650.
Feher, G. (1956). Observation of Nuclear Magnetic Resonances via the Electron Spin Resonance Line. Phys. Rev. 103, 834-835.
Feher, G. (1957). Electronic Structure of F-Centers in KCl by the Electron Spin Double Resonance Technique. Phys. Rev. 105, 1122-1123.
Feher, G., Isaacson, R. A., Scholes, C. P., and Nagel, R. (1973). Electron Nuclear Double Resonance (ENDOR) Investigation on Myoglobin and Hemoglobin. Ann. N. Y. Acad. Sci. 222, 86-101.
Fields, R. A. and Hutchison, Jr., C. A. (1985). The Determination of Hydrogen Coordinates in Lanthanum Nicotinate Dihydrate Crystals by 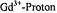 Double Resonance. J. Chem. Phys. 82, 1711-1722.
Double Resonance. J. Chem. Phys. 82, 1711-1722.
Fink, A. L. and Geeves, M. A. (1979). Cryoenzymology: The Study of Enzyme Catalysis at Subzero Temperatures. Methods Enzymol. 63, 336-370.
Fink, A. L. and Cartwright, S. J. (1981). Cryoenzymology. CRC Crit. Rev. Biochem. 11, 145207.
Flohr, K. and Kaiser, E. T. (1972). Enantiomeric Specificity in Chymotrypsin-Catalyzed Hydrolysis of 3-Carboxy-2,2,5,5-Tetramethylpyrrolidin-1-Oxy para-Nitrophenyl Ester. J. Am. Chem. Soc. 94, 3675.
Garman, E. F. and Schneider, T. R. (1997). Macromolecular Cryocrystallography. J. Appl. Crystallogr. 30, 211-237.
Gellert, M., Lipsett, M. N., and Davies, D. R. (1962). Helix Formation by Guanylic Acid.
Proc. Natl. Acad. Sci. USA 48, 2013-2018.
Gersmann, H. R. and Swalen, J. D. (1962). Electron Paramagnetic Resonance Spectra of Copper Complexes. J. Chem. Phys. 36, 3221-3233.
Gochev, G. P. and Yordanov, N. D. (1993). Polycrystalline ENDOR Crystallography, a New Methodological Approach. J. Magn. Reson. A102, 180-182.

138 DEVKUMAR MUSTAFI AND MARVIN W. MAKINEN
Goldfarb, D., Bernardo, M., Thomann, H., Kroneck, P. M. H., and Ullrich, V. (1996). Study of Water Binding to Low-Spin Fe(III) in Cytochrome P450 by Pulsed ENDOR and Fourpulse ESEEM Spectroscopies. J. Am. Chem. Soc. 118, 2686-2693.
Gradwell, M. J. and Feeney, J. (1996). Validation of the Use of Intermolecular NOE Constraints for Obtaining Docked Structures of Protein-Ligand Complexes. J. Biomol. NMR 7, 48-58.
Gromov, I., Marchesini, A., Farver, O., Pecht, I., and Goldfarb, D. (1999). Azide Binding to the Trinuclear Copper Center in Laccase and Ascorbate Oxidase. Eur. J. Biochem. 266, 820-830.
Gurbiel, R. J., Doan, P. E., Gassner, G. T., Macke, T. J., Case, D. A., Ohnishi, T., Fee, J. A., Ballou, D. P., and Hoffman, B. M. (1996). Active Site Structure of Rieske-Type Proteins: Electron Nuclear Double Resonance Studies of Isotopically Labeled Phthalate Dioxygenase from Pseudomonas cepacia and Rieske Protein from Rhodobacter capsulatus and Molecular Modeling Studies of a Rieske Center. Biochemistry 35, 78347845.
Happe, J. A. and Morales, M. (1966). Nitrogen-15 Nuclear Magnetic Resonance Evidence
that |
Does Not Complex with the Nitrogen Atoms of Adenosine Triphosphate. J. Am. |
|
Chem. Soc. 88, 2077-2078. |
|
|
Hayat, H. and Silver, B. L. (1973). Oxygen-17 and Nitrogen-14 |
Polarization Parameters |
|
and Spin Density Distribution in Nitroxyl Group. J. Phys. Chem. 77, 72-78.
Henderson, T. A., Hurst, G. C., and Kreilick, R. W. (1985). Angle-Selected ENDOR Spectroscopy. 2. Determination of Proton Coordinates from a Polycrystalline Sample of Bis(2,4-pentanedionato)copper(II). J. Am. Chem. Soc. 107, 7299-7303.
Herrmann, T., Guntert, P., and Wuthrich, K. (2002). Protein NMR Structure Determination with Automated NOE Assignment Using the New Software CANDID and the Torsion Angle Dynamics Algorithm DYANA. J. Mol. Biol. 319, 209-227.
Hoffman, B. M., Martinsen, J., and Venters, R. A. (1984). General Theory of Polycrystallline ENDOR Patterns. g and Hyperfine Tensors of Arbitrary Symmetry and Relative Orientation. J. Magn. Reson. 59, 110-123.
Hoffman, B. M., Venters, R. A., and Martinsen, J. (1985). General Theory of Polycrystalline ENDOR Patterns. Effects of Finite EPR and ENDOR Component Linewidths. J. Magn. Reson. 62, 537-542.
Hoffman, B. M. and Gurbiel, R. J. (1989). Polycrystalline ENDOR Patterns from Centers of Axial EPR Spectra. General Formulas and Simple Analytic Expressions for Deriving Geometric Information from Dipolar Couplings. J. Magn. Reson. 82, 309-317.
Hubbell, W. L., Gross, A., Langen, R., and Lietzow, M. A. (1998). Recent Advances in SiteDirected Spin-Labeling of Proteins. Curr. Opin. Struct. Biol. 8, 649-656.
Hurst, G. C., Henderson, T. A., and Kreilick, R. W. (1985). Angle Selected ENDOR
Spectroscopy. 1. Theoretical Interpretation of |
ENDOR Shifts from Randomly Oriented |
Transition Metal Complexes. J. Am. Chem. Soc. 107, 7294-7299. |
|
Hutchison, Jr., C. A. and McKay, D. B. (1977). Determination of Hydrogen Coordinates in |
|
Lanthanum Nicotinate Dihydrate Crystals by |
Double-Resonance. J. Chem. |
Phys. 66, 3311-3330. |
|
Hutchison, Jr., C. A. and Orlowski, T. E. (1980). The Determination of Deuterium Atom Coordinates and Nuclear-Quadrupole Interactions in Lanthanum Nicotinate Dihydrate Crystals by 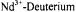 Double-Resonance. J. Chem. Phys. 73, 1-14.
Double-Resonance. J. Chem. Phys. 73, 1-14.
Iijima, H., Dunbar, J. B., and Marshall, G. R. (1987). Calibration of Effective van der Waals Atomic Contact Radii for Proteins and Peptides. Proteins: Struct., Func., Genet. 2, 330-
339.
ANGLE-SELECTED ENDOR |
139 |
Improta, R., di Matteo, A., and Barone, V. (2000). Effective Modeling of Intrinsic and Environmental Effects on the Structure and Electron Paramagnetic Resonance Parameters of Nitroxides by an Integrated Quantum Mechanical/Molecular Mechanics/Polarizable Continuum Model Approach. Theor. Chem. Accts. 104, 273-279.
Jelsch, C., Maury, L., Masson, J. M., and Samama, J. P. (1993). Crystal Structure of
Escherichia coli TEM1 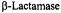 at 1.8 Å Resolution. Proteins: Struct., Func., Genet.
at 1.8 Å Resolution. Proteins: Struct., Func., Genet.
16, 364-383.
Jiang, F. S. and Makinen, M. W. (1995). NMR and ENDOR Conformational Studies of the Vanadyl Guanosine 5'-Monophosphate Complex in Hydrogen-Bonded Quartet Assemblies. Inorg. Chem. 34, 1736-1744.
Jiang, F. S., Tsai, S. W., Chen, S., and Makinen, M. W. (1998). ENDOR-Determined Structure of a Complex of  with a Spin-Labeled Transition-State Inhibitor Analogue. J. Phys. Chem. B102, 4619-4627.
with a Spin-Labeled Transition-State Inhibitor Analogue. J. Phys. Chem. B102, 4619-4627.
Joela, H., Mustafi, D., Fair, C. C., and Makinen, M. W. (1991). Structure and Confirmation of Spin-Labeled Methyl L-Phenylalanate and L-Phenylalanine Determined by Electron Nuclear Double resonance spectroscopy. J. Phys. Chem. 95, 9135-9144.
Jost, P. C. and Griffith, O. H. (1978). The Spin-Labeling Technique. Methods Enzymol. 49, 369-418.
Kang, C., Zhang, X. H., Ratliff, R., Moyzis, R., and Rich, A. (1992). Crystal-Structure of 4- Stranded Oxytricha Telomeric DNA. Nature 356, 126-131.
Kevan, L. and Kispert, L. D. (1976). Electron Spin Double Resonance Spectroscopy, John Wiley and Sons, New York.
Khramtsov, V. V. and Weiner, L. (1988). Proton Exchange in Stable Nitroxyl Radicals: pH Sensitive Spin Probes. Chap. 2 in Imidazoline Nitroxides, Vol. II, L. B. Volodarsky, ed., CRC Press, Boca Raton, Florida, pp. 37-80.
Khramtsov, V. V. and Volodarsky, L. B. (1998). Use of Imidazoline Nitroxides in Studies of Chemical Reactions in Biological Magnetic Resonance, Vol. 14, L. J. Berliner, ed., Plenum Press, New York, pp. 109-180.
Kivelson, D. and Lee, S.-K. (1964). ESR Studies and the Electronic Structure of Vanadyl Ion Complexes. J. Chem. Phys. 41, 1896-1903.
Knox, J. R. (1995). Extended-Spectrum and Inhibitor-Resistant TEM-Type  Mutations, Specificity, and Three-Dimensional Structure. Antimicrob. Agents Chemother. 39, 2593-2601.
Mutations, Specificity, and Three-Dimensional Structure. Antimicrob. Agents Chemother. 39, 2593-2601.
Kretsinger, R. H. and Nockolds, C. E. (1973). Carp Muscle Calcium-Binding Protein. II. Structure Determination and General Description. J. Biol. Chem. 248, 3313-3326.
Kurreck, H., Kirste, B., and Lubitz, W. (1988). Electron Nuclear Double Resonance Spectroscopy of Radicals in Solution: Application to organic and Biological Chemistry,
VCH |
Publishers, New York. |
Langen, R., |
Oh, K. J., Cascio, D., and Hubbell, W. L. (2001). Crystal Structures of Spin |
Labeled T4 Lysozyme Mutants: Implications for the Interpretation of EPR Spectra in Terms of Structure. Biochemistry 39, 8396-8405.
Lee, B. and Richards, F. M. (1971). The Interpretation of Protein Structures: Estimation of Static Accessibility. J. Mol. Biol. 55, 379-400.
Machonkin, T. E., Westler, W. M., and Markley, J. L. (2002). C-13{C-13} 2D NMR: A Novel Strategy for the Study of Paramagnetic Proteins with Slow Electronic Relaxation Rates. J. Am. Chem. Soc. 124, 3204-3205.
MacKerell, A. D., Bashford, D., Bellott, M., Dunbrack, R. L., Evanseck, J. D., Field, M. J., Fischer, S., Gao, J., Guo, H., Ha, S., Joseph-McCarthy, D., Kuchnir, L., Kuczera, K., Lau, F. T. K., Mattos, C., Michnick, S., Ngo, T., Nguyen, D. T., Prodhom, B., Reiher, W. E., Roux, B., Schlenkrich, M., Smith, J. C., Stote, R., Straub, J., Watanabe, M., Wiorkiewicz-
