
Biomedical EPR Part-B Methodology Instrumentation and Dynamics - Sandra R. Eaton
.pdf
140 DEVKUMAR MUSTAFI AND MARVIN W. MAKINEN
Kuczera, J., Yin, D., and Karplus, M. (1998). All-Atom Empirical Potential for Molecular Modeling and Dynamics Studies of Proteins. J. Phys. Chem. B102, 3586-3616.
Makinen, M. W. and Fink, A. L. (1977). Reactivity and Cryoenzymology of Enzymes in the Crystalline State. Annu. Rev. Biophys. Bioeng. 6, 301-343.
Makinen, M. W., Kuo, L. C., Dymowski, J. J., and Jaffer, S. (1979). Catalytic Role of the Metal Ion of Carboxypeptidase A in Ester Hydrolysis. J. Biol. Chem. 254, 356-366.
Makinen, M. W. and Mustafi, D. (1995). The Vanadyl Ion: Molecular Structure of Coordinating Ligands by Electron Paramagnetic Resonance and Electron Nuclear Double Resonance Spectroscopy in Metal Ions in Biological Systems, Vol. 31, H. Sigel and A. Sigel, eds., Marcel Dekker, Inc., New York, pp. 89-127.
Makinen, M. W. (1998). Electron Nuclear Double Resonance Determined Structures of Enzyme Reaction Intermediates: Structural Evidence for Substrate Destabilization.
Spectrochim. Acta. Part A, Mol. Biomol. Spectrosc. 54, 2269-2281.
Makinen, M. W., Mustafi, D., and Kasa, S. (1998). ENDOR of Spin Labels for Structure Determination: From Small Molecules to Enzyme Reaction Intermediates in Biological Magnetic Resonance, Vol. 14, L. J. Berliner, ed., Plenum Press, New York, pp. 181-249.
Makinen, M. W. and Brady, M. J. (2002). Structural Origins of the Insulin-Mimetic Activity of Bis(acetylacetonato)oxovanadium(IV). J. Biol. Chem. 277, 12215-12220.
Massova, I. and Mobashery, S. (1998). Kinship and Diversification of Bacterial PenicillinBinding Proteins and  Antimicrob. Agents Chemother. 42, 1-17.
Antimicrob. Agents Chemother. 42, 1-17.
Matthews, B. W. (1968). Solvent Content of Protein Crystals. J. Mol. Biol. 33, 491-497. McConnell, H. M. and Chestnut, D. B. (1958). Theory of Isotropic Hyperfine Interactions
in |
Radicals. J. Chem. Phys. 28, 107-117. |
|
Mustafi, D. and Makinen, M. W. (1988). ENDOR-Determined Solvation Structure of |
in |
|
Frozen-Solutions. Inorg. Chem. 27, 3360-3368.
Mustafi, D., Boisvert, W. E., and Makinen, M. W. (1990a). Structure and Conformation of the Nitroxyl Spin-Label Ethyl 3-(2,2,5,5-tetramethylpyrrolinyl-1-oxyl)-propen-2-oate Determined by Electron Nuclear Double Resonance: Comparison with the Structure of a Spin-Label Substrate of Carboxypeptidase A. Biopolymers 29, 45-55.
Mustafi, D., Sachleben, J. R., Wells, G. B., and Makinen, M. W. (1990b). Structure and Conformation of Spin-Labeled Amino Acids in Frozen Solutions Determined by Electron Nuclear Double Resonance. 1. Methyl N-(2,2,5,5-Tetramethyl-1-Oxypyrrolinyl-3-Carbon- yl)-L-Alanate, a Molecule with a Single Preferred Conformation. J. Am. Chem.Soc. 112, 2558-2566.
Mustafi, D., Wells, G. B., Joela, H., and Makinen, M. W. (1990c). Assignment of Proton ENDOR Resonances of Nitroxyl Spin-Labels in Frozen Solution. Free Radical Res. Commun. 10, 95-101.
Mustafi, D., Joela, H., and Makinen, M. W. (1991). The Effective Position of the Electronic Point Dipole of the Nitroxyl Group of Spin Labels Determined by ENDOR Spectroscopy.
J.Magn. Reson. 91, 497-504.
Mustafi, D., Telser, J., and Makinen, M. W. (1992). Molecular Geometry of Vanadyl Adenine Nucleotide Complexes Determined by EPR, ENDOR, and Molecular Modeling. J. Am. Chem. Soc. 114, 6219-6226.
Mustafi, D., Boisvert, W. E., and Makinen, M. W. (1993). Synthesis of Conjugated Polyene Carbonyl Derivatives of Nitroxyl Spin-Labels and Determination of Their Molecular Structure and Conformation by Electron Nuclear Double Resonance. J. Am. Chem. Soc. 115, 3674-3682.
Mustafi, D. and Makinen, M. W. (1994). Catalytic Conformation of Carboxypeptidase A. Structure of a True Enzyme Reaction Intermediate Determined by Electron Nuclear Double Resonance. J. Biol. Chem. 269, 4587-4595.

ANGLE-SELECTED ENDOR |
141 |
Mustafi, D. and Nakagawa, Y. (1994). Characterization of Calcium-Binding Sites in the Kidney Stone Inhibitor Glycoprotein Nephrocalcin with Vanadyl Ions: Electron Paramagnetic Resonance and Electron Nuclear Double Resonance Spectroscopy. Proc. Natl. Acad. Sci. USA 91, 11323-11327.
Mustafi, D. and Joela, H. (1995). Origin of the Temperature Dependent Isotropic Hyperfine Coupling of the Vinylic Proton of Oxypyrrolinyl Nitroxyl Spin-Labels. J. Phys. Chem. 99, 11370-11375.
Mustafi, D. and Makinen, M. W. (1995). Structure, Conformation, and Probable Mechanism of Hydrolysis of a Spin-Labeled Penicillin Revealed by Electron Nuclear
DoubleResonance Spectroscopy. J. Am. Chem. Soc. 117, 6739-6746. |
|
Mustafi, D. and Nakagawa, Y. (1996). Characterization of |
Sites in the Kidney |
Stone Inhibitor Glycoprotein Nephrocalcin Using Vanadyl Ions: Different Metal Binding Properties in Strong and Weak Inhibitor Proteins Revealed by EPR and ENDOR. Biochemistry 35, 14703-14709.
Mustafi, D., Knock, M, M., Shaw, R. W., and Makinen, M. W. (1997). Conformational Changes in Spin-Labeled Cephalosporin and Penicillin upon Hydrolysis Revealed by Electron Nuclear Double Resonance Spectroscopy. J. Am. Chem. Soc. 119, 12619-12628.
Mustafi, D., Nakagawa, Y., and Makinen, M. W. (2000). ENDOR Studies of |
Probing |
|||
Protein-Metal Ion Interactions in Nephrocalcin. Cell. Mol. Biol. 46, 1345-1360. |
|
|||
Mustafi, D., Sosa-Peinado, A., and Makinen, M. W. (2001). ENDOR |
Structural |
|||
Characterization of a Catalytically Competent Acylenzyme Reaction Intermediate of |
||||
Wild-Type TEM-1 |
Confirms Glutamate-166 |
as the |
Base |
Catalyst. |
Biochemistry 40, 2397-2409, |
|
|
|
|
Mustafi, D., Sosa-Peinado, A., |
Gupta, V., Gordon, D. J., and |
Makinen, |
M. W. (2002). |
|
Structure of Spin-Labeled Methylmethanethiolsulfonate in Solution and Bound to TEM-1  Determined by Electron Nuclear Double Resonance Spectroscopy.
Determined by Electron Nuclear Double Resonance Spectroscopy.
Biochemistry 41, 797-808.
Nakagawa, Y., Margolis, H. C., Yokoyama, S., Kezdy, F. J., Kaiser, E. T., and Coe, F. L. (1981). Purification and Characterization of a Calcium Oxalate Monohydrate Crystal Growth Inhibitor from Human Kidney Tissue Culture Medium. J. Biol. Chem. 256, 39363944.
Nakagawa, Y., Abram, V., Kezdy, F. J., Kaiser, E. T., and Coe, F. L. (1983). Purification of the Principal Inhibitor of Calcium Oxalate Monohydrate Crystal Growth in Human Urine. J.Biol. Chem. 258, 12594-12600.
Nakagawa, Y., Otsuki, T., and Coe, F. L. (1985). Elucidation of the Multiple Forms of Nephrocalcin by P-31 NMR. FEBS Lett. 250, 187-190.
Neu, H. C. (1992). The Crisis in Antibiotic Resistance. Science 257, 1064-1073.
Paetzel, M., Danel, F., de Castro, L., Mosimann, S. C., Page, M. G. P., and Strynadka, N. C.
J. (2000). Crystal Structure of the Class D |
OXA-10. Nature Struct. Biol. 7, |
918-925. |
|
Page, M. I. (1987). The Mechanisms of Reactions of |
Antibiotics in Adv. Phys. Org. |
Chem., Vol. 23, D. Bethell, ed., Harcourt Brace Jovanovich Publishers, London, pp. 165270.
Pearlman, D. A., Case, D. A., Caldwell, J. W., Ross, W. S., Cheatham, T. E., Debolt, S., Ferguson, D., Seibel, G., and Kollman, P. (1995). AMBER, a Package of ComputerPrograms for Applying Molecular Mechanics, Normal-Mode Analysis, MolecularDynamics and Free-Energy Calculations to Simulate the Structural and Energetic Properties of Molecules. Comput. Phys. Commun. 91, 1-41.
142 DEVKUMAR MUSTAFI AND MARVIN W. MAKINEN
Pfannebecker, V., Klos, H., Hubrich, M., Volkmer, T., Heuer, A., Wiesner, U., and Spiess, H. W. (1996). Determination of End-to-End Distances in Oligomers by Pulsed EPR. J. Phys. Chem. 100, 13428-13432.
Phillips, D. C. (1967). The Hen Egg-White Lysozyme Molecule. Proc. Natl.Acad. Sci. USA 57, 484-495.
Pinavaia, T. J., Marshall, C. L., Mettler, C. L., Fisk, C. L., Miles, H. T., and Becker, E. D. (1978). Alkali Metal Ion Specificity in the Solution Ordering of a Nucleotide, 5'- Guanosine Monophosphate. J. Am. Chem. Soc. 100, 3625-3627.
Prisner, T., Rohrer, M., and MacMillan, F. (2001). Pulsed EPR Spectroscopy: Biological Applications. Ann. Rev. Phys. Chem. 52, 279-313.
Rakowsky, M. H., Zecevic, A., Eaton, G. R., and Eaton, S. S. (1998). Determination of HighSpin Iron(III)-Nitroxyl Distances in Spin-Labeled Porphyrins by Time-Domain EPR. J. Magn. Reson. 131, 97-110.
Rienstra, C. M., Tucker-Kellogg, L., Jaroniec, C. P., Hohwy, M., Reif, B., McMahon, M. T., Tidor, B., Lozano-Perez, T., and Griffin, R. G. (2002). De novo Determination of Peptide Structure with Solid-State Magic-Angle Spinning NMR Spectroscopy. Proc. Natl. Acad. Sci.USA 99, 10260-10265.
Rist, G. H. and Hyde, J. S. (1968). Ligand ENDOR of Cu-8-Hydroxyquinolate Substituted into a Single Crystal and a Powder of Phthalimide. J. Chem. Phys. 49, 2449-2451.
Rist, G. H. and Hyde, J. S. (1970). Ligand ENDOR of Metal Complexes in Powders. J. Chem. Phys. 52, 4633-4643.
Rodgers, D. W. (1997). Practical Cryocrystallography. Methods Enzymol. 276, 183-203. Rudin, M., Schweiger, A., and Gunthard, H. H. (1982). On the Electronic-Structure of N,N'-
Ethylene-bis(acetylacetonatiminato)Co(II), Co(II)Acacen. 2. ENDOR and Double ENDOR of Ligand Nuclei. Mol. Phys. 46, 1027-1044.
Saenger, W. (1984). Principles of Nucleic Acid Structure, Springer Verlag, New York. Sander, M. E. and Witzel, H. (1985). Direct Chemical Evidence for the Mixed Anhydride
Intermediate of Carboxypeptidase A in Ester and Peptide Hydrolysis. Biochem. Biophys. Res. Commun. 132, 681-687.
Sander, M. E. and Witzel, H. (1986). Direct Chemical Evidence for an Anhydride Intermediate of Carboxypeptidase A in Ester and Peptide Hydrolysis in Zinc Enzymes, I. Bertini, C. Luchinat, W. Maret, and M. Zeppezauer, eds., Birkhaeuser, Boston, Massachusetts, pp. 207-214.
Scholes, C. P., Lapidot, A., Mascarenhas, R., Inubushi, T., Isaacson, R. A., and Feher, G. (1982). Electron Nuclear Double Resonance (ENDOR) from Heme and Histidine Nitrogens in Single-Crystals of Aquometmyoglobin. J. Am. Chem. Soc. 104, 2724-2735.
Scott, W. R. P., Hunenberger, P. H., Tironi, I. G., Mark, A. E., Billeter, S. R., Fennen, J., Torda, A. E., Huber, T., Kruger, P., and van Gunsteren, W. F. (1999). The GROMOS Biomolecular Simulation Program Package. J. Phys.Chem. A103, 3596-3607.
Smith, T. S., LoBrutto, R., and Pecoraro, V. L. (2002). Paramagnetic Spectroscopy of Vanadyl Complexes and Its Applications to Biological Systems. Coord. Chem. Rev. 228, 1-18.
Snetsinger, P. A., Chasteen, N. D., Cornelius, J. B., and Singel, D. J. (1992). Probing the Iron Center of the Low-Spin Cyanide Adduct of Transferrin by ESEEM Spectroscopy. J. Phys. Chem. 96, 7917-7922.
Sosa-Peinado, A., Mustafi, D., and Makinen, M. W. (2000). Overexpression and Biosynthetic Deuterium Enrichment of TEM-1  for Structural Characterization by Magnetic Resonance Methods. Protein Expres. Purif. 19, 235-245.
for Structural Characterization by Magnetic Resonance Methods. Protein Expres. Purif. 19, 235-245.
Stryer, L. (1988). Biochemistry, 3rd edit., W. H. Freeman and Company, New York.

ANGLE-SELECTED ENDOR |
143 |
Strynadka, N. C. J., Adachi, H., Jensen, S. E., Johns, K., Sielecki, A., Betzel, C., Sutoh, K., and James, M. N. J. (1992). Molecular Structure of the Acylenzyme Intermediate in 
 Hydrolysis at 1.7 Å Resolution. Nature 359, 700-705.
Hydrolysis at 1.7 Å Resolution. Nature 359, 700-705.
Swartz, H. M. and Halpern, H. (1998). EPR Studies of Living Animals and Related Model Systems (In Vivo EPR) in Biological Magnetic Resonance, Vol. 14, L. J. Berliner, ed., Plenum Press, NewYork, pp. 367-404.
Sweet, R. M. and Dahl, L. F. (1970). Molecular Architecture of the Cephalosporins. Insights into Biological Activity Based on Structural Investigations. J. Am. Chem. Soc. 92, 54895507.
Teng, T. Y. and Moffat, K. (1998). Cooling Rates during Flash Cooling. J. Appl. Crystallogr. 1, 252-257.
Togni, A., Rist, G., Rihs, G., and Schweiger, A. (1993). EPR, H-1 and C-13 ENDOR, N-14 ESEEM, and X-Ray Crystallographic Studies of Oxovanadium(IV) Bis((1R)-3-(Hepta- fluorobutyryl)Camphorate) - a Catalyst for Asymmetric Hetero-Diels-Alder Reactions. J. Am. Chem. Soc. 115, 1908-1915.
Turley, J. W. and Boer, F. P. B. (1972). The Crystal Structure of the Nitroxide Free Radical 2,2,5,5-Tetramethyl-3-carbamidopyrroline-1-oxyl. Acta Crystallogr. B28, 1641-1644.
van Ormondt, D. and Visser, H. (1968). Ligand ENDOR of  in
in 
Phys. Lett. A26, 343-344.
Van Zele, C. J., Cunningham, M. A., and Makinen, M. W. (2001). Validation of Nitroxyl Spin-Label Force-Field Parameters through Molecular Dynamics Simulations. J. Comput. Chem. 22, 1113-1123.
Vocadlo, D. J., Davies, G. J., Laine, R., and Withers, S. G. (2001). Catalysis by Hen EggWhite Lysozyme Proceeds via a Covalent Intermediate. Nature 412, 835-838.
Walsby, C. J., Hong, W.,Broderick, W. E., Cheek, J., Ortillo, D., Broderick, J. B., and Hoffman, B. M. (2002). Electron Nuclear Double Resonance Spectroscopic Evidence that S-Adenosylmethionine Binds in Contact with the Catalytically Active [4Fe-4S](+) Cluster of Pyruvate Formate-Lyase Activating Enzyme. J. Am. Chem. Soc. 124, 3143-3151.
Weiner, P. K. and Kollman, P. A. (1981). AMBER - Assisted Model-Building with Energy Refinement - a General Program for Modeling Molecules and Their Interactions. J. Comput. Chem. 2, 287-303.
Wells, G. B. and Makinen, M. W. (1988). ENDOR Determined Molecular Geometries of Spin-Labeled Fluoroanilides in Frozen Solution. J. Am. Chem. Soc. 110, 6343-6352.
Wells, G. B., Mustafi, D., and Makinen, M. W. (1990). Structure and Conformation of SpinLabeled Amino Acids in Frozen Solutions Determined by Electron Nuclear Double Resonance. 2. Methyl N-(2,2,5,5-Tetramethyl-1-Oxypyrrolinyl-3-Carbonyl)-L- Tryptophanate, a Molecule with Multiple Conformations. J. Am. Chem. Soc. 112, 25662574.
Wells, G. B., Mustafi, D., and Makinen, M. W. (1994). Structure at the Active-Site of an
Acylenzyme of |
and Implications for the |
Catalytic Mechanism. An |
Electron Nuclear Double Resonance Study. J. Biol. Chem. 269, 4577-4586. |
||
Williams, R. J. P. (1985). The Symbiosis of Metal and Protein Functions. Eur. J. Biochem. |
||
150, 231-248. |
|
|
Wuthrich, K. and Connick, R. F. (1968). Nuclear Magnetic |
Resonance Studies of the |
|
Coordination of Vanadyl Complexes in Solution and the Rate of Elimination of |
||
Coordinated Water Molecules. Inorg. Chem. 7, 1377-1388. |
|
|
Yim, M. B. and Makinen, M. W. (1986). ENDOR Study of |
Complexes in Frozen |
|
Solutions. J. Magn. Reson. 70, 89-105. |
|
|

144 DEVKUMAR MUSTAFI AND MARVIN W. MAKINEN
Yordanov, N. D. and Zdravkova, M. (1986). H-1 and P-31 ENDOR Studies on Powdered Samples of Magnetically Dilute Copper(II) O,O'-Disubstituted Dithiophosphate Complexes. Chem. Phys. Lett. 127, 487-491.
Yordanov, N. D., Zdravkova, M., and Shopov, D. (1986). An Improved Method for ENDOR Study of Powdered Samples. Chem. Phys. Lett. 124, 191-195.
Zabell, A. P. R. and Post, C. B. (2002). Docking Multiple Conformations of a Flexible Ligand into a Protein Binding Site Using NMR Restraints. Proteins: Struct., Func., Genet. 46, 295-307.
Zdravkova, M. and Yordanov, N. D. (1994). ENDOR Crystallography – Current Practical Applications. Appl. Magn. Reson. 6, 83-105.
Zell, A., Einspahr, H., and Bugg, |
C. E. (1985). Model for Calcium-Binding to |
Acid Residues |
of Proteins – Crystal Structure of Calcium |
 Biochemistry 24, 533-537.
Biochemistry 24, 533-537.
Zhao, D. Q. and Jardetzky, O. (1994). An Assessment of the Precision and Accuracy of Protein Structures Determined by NMR. Dependence on Distance Errors. J. Mol. Biol. 239, 601-607.
Zimmerman, S. B. (1976). X-Ray Study by Fiber Diffraction Methods of a Self-Aggregate of Guanosine-5'-Phosphate with Same Helical Parameters as Poly(Rg). J. Mol. Biol. 106, 663-672.
Chapter 5
Solution-ENDOR of Some Biologically Interesting
Radical Ions
Fabian Gerson and Georg Gescheidt
Department of Chemistry, University of Basel, Klingelbergstrasse 80, CH-4056 Basel,
Switzerland
Abstract: A simple phenomenological treatment of the solution-ENDOR spectroscopy is presented. It is followed by a brief report on such studies carried out on some radical ions belonging to two classes of biologically interesting compounds, quinones and porphyrinoids.
1.SOLUTION-ENDOR SPECTROSCOPY
1.1Introduction
For studies of organic radicals, the by far most important multiresonance technique is electron-nuclear double resonance (ENDOR) discovered by Feher in 1956 on a phosphorus-doped silicon system (Feher,1956; Feher, 1998). Several years later, it was applied to radicals in solution by Hyde and Maki (Hyde and Maki, 1964; Hyde, 1965; Hyde, 1974), as well as by Möbius and his colleagues (Biehl et al,, 1971; Möbius and Dinse, 1972; Möbius, 1998) who also introduced TRIPLE-resonance techniques (Biehl et al., 1975; Möbius and Biehl, 1979). The reason for the application of ENDOR spectroscopy to radicals in solution lagging behind that to paramagnetic species in solids was partly due to the lack of interest in the liquid phase by physicists who first used this technique. Even more important were problems of instrumentation. In addition to the conventional EPR apparatus and a special cavity with radiofrequency (RF) coils, the ENDOR technique requires a RF source to saturate the NMR transitions. For liquids, the RF power must be much higher than for solids, and so must be the efficiency of the cooling system (Atherton, 1979). Although ENDOR has
145
146 |
FABIAN GERSON AND GEORG GESCHEIDT |
not attained a popularity comparable to EPR, it is now used by an increased number of research groups, especially since ENDOR accessories have become commercially available from the Varian Associates (Hyde, 1998) in the seventies and from the Bruker GmbH (Schmalbein, 1998) in the eighties. The ENDOR technique has been briefly dealt with in several early monographs on EPR spectroscopy (Ayscough, 1967; Carrington and McLachlan, 1967; Scheffler and Stegmann, 1970; Wertz and Bolton, 1972; Atherton, 1973) and, in some length, in a few books specialized in multiresonance (Kevan and Kispert, 1976; Dorio and Freed, 1979). An excellent introduction into the ENDOR technique, as used for organic radicals in solution, is to be found in a review article (Kurreck et al., 1984) and, in more detail, in a book by same authors (Kurreck et al., 1988). The latter also contains a comprehensive account of the pertinent ENDOR studies up to 1988. The physical fundamentals underlying this doubleresonance technique can be grasped by considering the so called transientENDOR effect in the way presented by Kurreck et al. and adopted in a recent monograph on EPR spectroscopy (Gerson and Huber, 2003). The following treatment is a condensed version of a section in this monograph.
1.2Physical Fundamentals
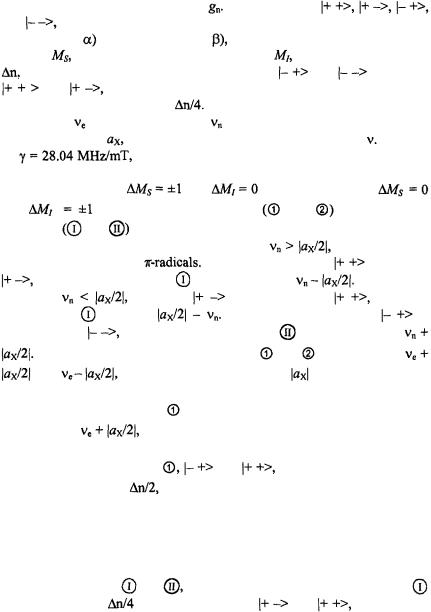
Figure 1. Schemes relevant to the transient-ENDOR effect for a paramagnetic system consisting of one unpaired electron and one magnetic nucleus with I = 1/2 and 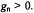 (a)
(a)
Energy levels in absence of the saturation. (b) Effect of saturation of the ESR transition  on
on
the populations. (c) and (d) Effect of the saturation of the NMR transitions  and
and  respectively, on the populations. Reproduced partly from (Kurreck et al., 1988) and (Gerson and Huber, 2003) by permission of VCH Publishers and Wiley-VCH, respectively.
respectively, on the populations. Reproduced partly from (Kurreck et al., 1988) and (Gerson and Huber, 2003) by permission of VCH Publishers and Wiley-VCH, respectively.
SOLUTION ENDOR OF RADICAL IONS |
147 |
The relevant schemes are shown in Figure 1. They depict four Zeemanenergy levels which, at a given field strength, B, of the magnetic field are characteristic of a paramagnetic system consisting of one unpaired electron and one magnetic nucleus X, such as proton, with the spin-quantum number
I = 1/2 and a positive nuclear factor |
The four levels, |
|
|||||
and |
are specified by the signs of the magnetic spin-quantum numbers, |
||||||
+1/2 (spin up; |
or –1/2 (spin down; |
whereby the first sign applies to the |
|||||
number, |
of electron and the second to that, |
of nucleus. The excess, |
|||||
of the electron-spin population in the levels |
and |
relative to |
|||||
|
and |
|
|
required for the EPR absorption, is symbolized by four |
|||
dots, |
each |
dot |
standing for |
The Zeeman splittings are given as |
|||
frequencies, |
|
for the electron and |
for the nucleus, and the hyperfine- |
||||
coupling constant, |
of the nucleus X also has the dimension of |
Division |
|||||
by |
|
|
|
the gyromagnetic ratio of the electron, converts this |
|||
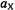 value in MHz into the coupling constant in mT, the unit of B. According
value in MHz into the coupling constant in mT, the unit of B. According
to the selection rules, |
|
and |
for the electron and |
||
and |
for the nucleus, two EPR |
and |
and two NMR |
||
transitions |
and |
are allowed. |
|
|
|
The schemes (a) – (d) in Figure 1 hold for |
which is usually |
||||
the case with protons in |
In this case, the level |
lies below |
|||
and the NMR transition |
has the frequency |
On the other |
|||
hand, for |
|
the level |
is shifted below |
and the |
|
frequency of |
becomes |
In either case, the level |
is |
||
situated below |
so that the NMR transition |
|
has the frequency |
|
|
The frequencies of the EPR transitions |
and |
are throughout |
|
||
and |
respectively, thus differing by |
as expected. |
|
||
In an ENDOR experiment, one EPR transition is selected for further
procedure; it is the transition |
in scheme (a). After having been locked at |
|
its frequency, |
this transition is saturated by an intense microwave |
|
(MW) irradiation. Consequently, as indicated in scheme (b), the populations
in the two levels relevant to |
and |
become equal. Both levels |
then exhibit an excess |
and the intensity of the pertinent EPR signal is |
|
strongly reduced. In the next step, the system is subjected to an intense irradiation with radiofrequency (RF), which is scanned from 0 to higher values. At two frequencies, the NMR transitions become saturated, first  at
at 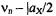 and, subsequently,
and, subsequently,  at
at 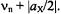 As a result, the populations in the pairs of the affected levels are equalized, as shown in schemes (c) and
As a result, the populations in the pairs of the affected levels are equalized, as shown in schemes (c) and
(d) for the transitions |
and |
respectively. Saturation of the transition |
||
leads to an excess |
in each of the levels |
and |
while such a |
|

148 |
|
FABIAN GERSON AND GEORG GESCHEIDT |
||||
process in |
yields |
in |
and |
Thus, |
either of the two |
|
saturation processes makes the population in the level |
by |
higher |
||||
than in 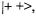 so that in either case the EPR transition
so that in either case the EPR transition  is desaturated, and the intensity of the EPR signal exhibits an increase, the so called ENDOR enhancement. Such an enhancement is, however, not directly verified, but its occurrence is confirmed by the NMR absorptions observed for the transitions
is desaturated, and the intensity of the EPR signal exhibits an increase, the so called ENDOR enhancement. Such an enhancement is, however, not directly verified, but its occurrence is confirmed by the NMR absorptions observed for the transitions  and
and 
1.3Spectra
Figure 2. Schematic presentation of an ENDOR spectrum arising from one nucleus X or a set of equivalent nuclei X with the coupling constant 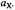 Reproduced from (Gerson and Huber, 2003) by permission of Wiley-VCH.
Reproduced from (Gerson and Huber, 2003) by permission of Wiley-VCH.
The ENDOR signals which arise from the NMR transitions  and
and 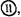 while scanning the RF
while scanning the RF 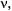 are schematically shown in Figure 2. Contrary to the NMR experiment, their intensity is due to a population excess,
are schematically shown in Figure 2. Contrary to the NMR experiment, their intensity is due to a population excess, 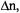 of electron spins, which is by several orders of magnitude larger than the analogous excess number of nuclear spins. Thus, the sensitivity of ENDOR is much higher than that of NMR, although it is lower than that of EPR. It can readily be verified that any magnetic nucleus X or a set of such equivalent nuclei with the coupling constant
of electron spins, which is by several orders of magnitude larger than the analogous excess number of nuclear spins. Thus, the sensitivity of ENDOR is much higher than that of NMR, although it is lower than that of EPR. It can readily be verified that any magnetic nucleus X or a set of such equivalent nuclei with the coupling constant 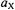 gives rise to a single pair of ENDOR signals, irrespective of the nuclear spin-quantum number I and the nuclear factor
gives rise to a single pair of ENDOR signals, irrespective of the nuclear spin-quantum number I and the nuclear factor 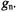 This pair of signals generally appears in a separate NMR frequency range characteristic of X and
This pair of signals generally appears in a separate NMR frequency range characteristic of X and 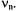 For
For 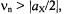 which holds for the ENDOR spectra reproduced in this chapter, the two signals appear at
which holds for the ENDOR spectra reproduced in this chapter, the two signals appear at 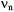
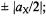 they are centered on the frequency,
they are centered on the frequency, 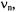 of the “free” nucleus X and separated by the coupling constant
of the “free” nucleus X and separated by the coupling constant 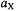 (Figure 2, top). On the other hand, for
(Figure 2, top). On the other hand, for 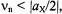 the two signals occur at
the two signals occur at 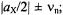 they are centered on
they are centered on 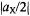 and separated by
and separated by 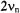 (Figure 2, bottom). The ENDOR signals can be
(Figure 2, bottom). The ENDOR signals can be

SOLUTION ENDOR OF RADICAL IONS |
149 |
recorded as absorption A or as the first derivative  as function of
as function of  depending on whether modulation is applied to the magnetic field or to the frequency. The latter procedure was used to record the ENDOR spectra in Figures 3 – 7 shown in the following sections.
depending on whether modulation is applied to the magnetic field or to the frequency. The latter procedure was used to record the ENDOR spectra in Figures 3 – 7 shown in the following sections.
Although ENDOR is less sensitive than EPR, this deficiency is amply made good by the enormous increase in spectral resolution. As the width, 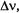 of ENDOR signals (line-width) of ca. 0.3 MHz is comparable to
of ENDOR signals (line-width) of ca. 0.3 MHz is comparable to 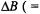
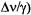 of ca. 0.01 mT, generally achieved for EPR lines in a well-resolved spectrum of an organic radical in fluid solution, the increase in resolution by ENDOR relative to EPR spectroscopy is due to a drastic decrease in the number of lines. With each further set of equivalent nuclei X giving rise to pairs of ENDOR signals, the number of lines grows additively, and not multiplicatively as in EPR spectra. Irrespective of the number
of ca. 0.01 mT, generally achieved for EPR lines in a well-resolved spectrum of an organic radical in fluid solution, the increase in resolution by ENDOR relative to EPR spectroscopy is due to a drastic decrease in the number of lines. With each further set of equivalent nuclei X giving rise to pairs of ENDOR signals, the number of lines grows additively, and not multiplicatively as in EPR spectra. Irrespective of the number 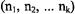 of nuclei in each set (1, 2, ... k) with
of nuclei in each set (1, 2, ... k) with 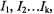 the total number of ENDOR lines for k sets is thus 2k and not
the total number of ENDOR lines for k sets is thus 2k and not 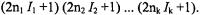
A disadvantage of ENDOR spectroscopy is, that, unlike NMR, the intensity of a signal is not a reliable measure for the number of interacting nuclei giving rise to it. This is because the ENDOR enhancement and, therewith, the intensity of the ENDOR signals depends on whether the nuclear-spin relaxation responsible for the saturation of the NMR transitions
and  can compete with the electron-spin relaxation effective in
can compete with the electron-spin relaxation effective in
saturating the selected EPR transition |
Usually, the electron-spin |
relaxation, which takes care of inversions of electron spins 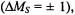 is much more efficient than the nuclear-spin relaxation which causes inversions of nuclear spins
is much more efficient than the nuclear-spin relaxation which causes inversions of nuclear spins 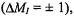 so that the former must be slowed down by appropriate experimental conditions. When cross-relaxation processes with
so that the former must be slowed down by appropriate experimental conditions. When cross-relaxation processes with 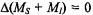 can be neglected, as is often the case with protons in organic and bioorganic radicals, this slowing down is achieved by using viscous solvents and/or low temperatures. The ENDOR experiment is impeded by an enhanced electron-spin relaxation, e.g. in the presence of heavy nuclei (which are often contained in transition metals of bioorganic molecules) or by the dynamic Jahn-Teller effect relevant to radical with an axial symmetry (rotational axis
can be neglected, as is often the case with protons in organic and bioorganic radicals, this slowing down is achieved by using viscous solvents and/or low temperatures. The ENDOR experiment is impeded by an enhanced electron-spin relaxation, e.g. in the presence of heavy nuclei (which are often contained in transition metals of bioorganic molecules) or by the dynamic Jahn-Teller effect relevant to radical with an axial symmetry (rotational axis 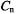 with n equal or larger than 3) in a degenerate ground state. An enhanced electron-spin relaxation is mostly conspicuous in the corresponding EPR spectrum, as hyperfine lines are broadened and difficult to saturate.
with n equal or larger than 3) in a degenerate ground state. An enhanced electron-spin relaxation is mostly conspicuous in the corresponding EPR spectrum, as hyperfine lines are broadened and difficult to saturate.
The ENDOR technique proved to be particularly useful for radicals of low symmetry with a large number of overlapping and/or incompletely resolved EPR lines (Gerson et al., 1975). Because of its lower sensitivity, it requires somewhat larger radical concentration than EPR spectroscopy, and its application to transient radicals is, therefore, more problematic. In order to increase the signal-to-noise ratio, the ENDOR spectra are usually accumulated by repeated recording and addition. Radical ions, which are
