
Учебники / Gene Therapy of Cochlear Deafness - Present Concepts and Future Aspects Ryan 2009
.pdf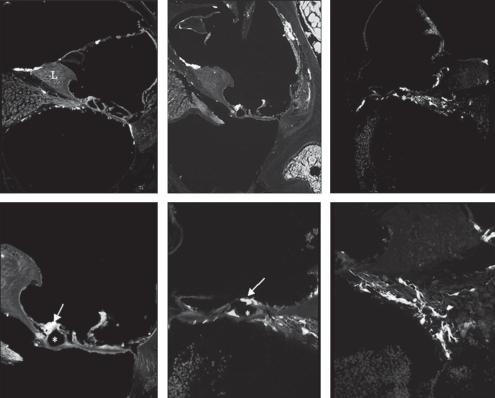
SV |
SV |
RM |
SM |
SM |
|
SM |
|
|
L |
|
ST |
|
ST |
|
ST |
|
|
|
|
||
|
|
|
|
|
|
a |
|
b |
c |
||
|
|
|
|
|
L |
SG
d |
|
e |
|
f |
Fig. 4. GFP expression following in vivo transduction of the murine cochlea with AAV1-CAG-hrGFP. Representative fluorescent images of paraffin-embedded cochlea stained with anti-GFP antibody. 1 × 109 genomic particles of AAV1-CAG-hrGFP virus were injected directly into the cochlea of 4-month- old CD1 mice via cochleostomy. Mice were sacrificed after 4 weeks. Cochleae were fixed with 4% paraformaldehyde and paraffin embedded. Ten-micrometer-thick sections were stained with fluorescent tagged anti-GFP antibody (bright green cells). d High magnification image of b. e, f High magnification images of c. Note the presence of GFP-positive cells within what appears to be hair cells and support cells. SV = Scala vestibuli; SM = scala media; ST = scala tympani; RM = Reissner’s membrane; L = limbus; SL = spiral ligament; SG = spiral ganglion (arrow); * tunnel of Corti; inner hair cells = arrows.
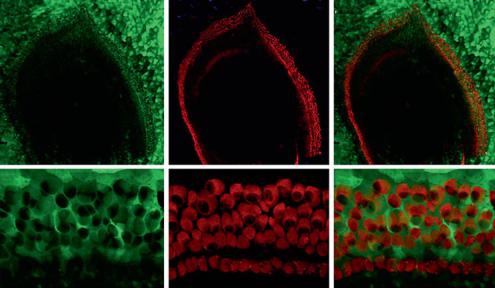
inner phalangeal and border cells. Interestingly, we also observed a loss of outer hair cells within the AAV-GFAP-eGFP-injected cochlea while the contralateral uninjected ear remained intact.
The brain lipid-binding protein (BLBP) promoter has also shown selectivity for astrocytes. Therefore, we also examined the potential for the BLBP promoter to drive support cell-specific expression following AAV1/2 injection (fig. 6). We observed strong expression within inner hair cells, Hansen’s cells. A similar loss of outer hair cells was observed in cochleae transduced with AAV1/2-BLBP-eGFP as that seen with AAV1/2-GFAP-eGFP. We are currently investigating the possible causes for this hair cell loss.
94 |
Luebke · Rova · Von Doersten · Poulsen |
a |
|
b |
|
c |
d |
|
e |
|
f |
Fig. 5. Transduction of E13 cochlear explants with AAV1-GFAP-eGFP. a–c Low magnification of fluorescent images of GFP (a), myosin 6 (b) and merge (c) in an E13 cochlear explant after 5 days in culture. Note the significant number of labeled cells within the sensory epithelium, but unlike the CAG promoter, the labeled cells appear to be supporting cells. d–f High magnification images of GFP (d), myosin 6 (e) and merge (f). * Location of inner pillar cells; + possible border cells or inner phalangeal cells immediately surrounding the inner hair cells. Micrograph obtained in collaboration with Dr. Matthew Kelley, NIDCD.
A number of additional promoters exist that may provide strong support for cellselective expression. Unlike the human CMV promoter described above, the murine CMV promoter exhibits selectivity for astrocytes [40] and when used with adenoviral vectors transduced Hensen and Claudius cells, yet has not yet been examined with AAV vectors. Recently, Furness and Lawton [41] reported that the astrocytic glutamate transporter, GLAST, is expressed only in border cells and inner phalangeal cells of mature guinea pigs. Indeed, we have confirmed that a similar pattern of GLAST expression is observed in P0 cochlear explant cultures (unpublished data). The GLAST promoter is currently being characterized and it seems reasonable that it could be used to drive transgene expression within this limited subpopulation of support cells. Alternatively, the promoter sequences, which drive other support cellspecific genes, such as Jagged-1 and Notch-1, may also prove useful for support cellspecific expression.
In summary, both adenoviral and AAV vectors show great promise for inner ear gene therapy. Due to the ototoxicity observed with 2nd-generation adenoviral vectors and the lack of toxicity experienced with AAV vectors, we expect AAV vectors will gain wider acceptance in future cochlear gene transfer studies. As our findings and others have shown, issues such as viral toxicity, viral titer load needed, viral serotype,
Adenoviral and AAV-Mediated Gene Transfer |
95 |
a |
|
e |
|
i |
b |
|
f |
|
j |
c |
|
g |
|
k |
d |
|
h |
|
l |
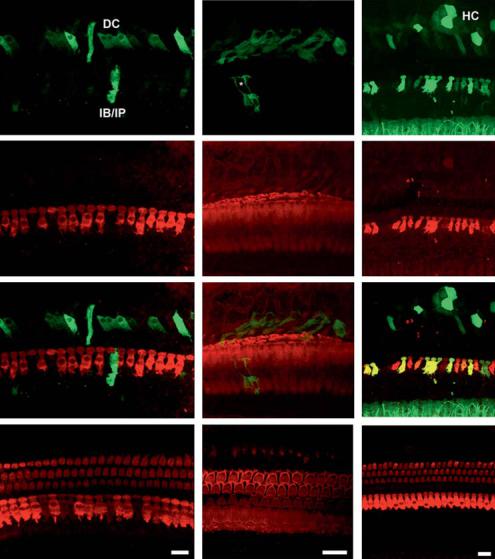
Fig. 6. Guinea pig cochleae transduced in vivo with AAV1/2 using astrocyte-specific promoters. Whole mount images (60×) of a left ear (a GFP; b myosin VIIa; c merge) treated with 109 gp of AAV1/2- GFAP-eGFP and the untreated control (d merge). GFP expression (a) appears to be limited to support cells, most likely Dieter’s cells (DC), inner border cells/inner phalangeal cells (IB/IP), and therefore does not colocalize with the myosin VIIa counterstain (c). Treated ears had a noticeable loss of outer hair cells and inner hair cells show signs of disruption as well (b). e–g 100× images of an animal from the same treatment group, GFP (e), phalloidin (f) and merged (g) images, respectively. *The site of an inner hair cell flanked by inner border or inner phalangeal cells expressing GFP (e). Again, many of the hair cells appear to be lost and the stereocilia are poorly marked by the phalloidin counterstain (f). h The right control ear (merged image). i–k 60× images from an animal infused with 1010 gp of AAV1/2-BLBP-eGFP. GFP (i) is found in some of the inner hair cells and in support cells as well, DC and Hensen cells (HC). The ganglia within the spiral limbus also appear to be expressing GFP (bottom of frame). j Myosin VIIa staining shows nearly complete loss of outer hair cells and some inner hair cells. l The right control ear (merge). GFP expression has been augmented with mouse anti-GFP antibodies. All scale bars represent 20 μm unless otherwise noted.
96 |
Luebke · Rova · Von Doersten · Poulsen |
and choice of promoter, vary with respect to viral vector (adenovirus vs. AAV), in vitro or in vivo infection, animal species and animal age. All of these factors can affect the efficiency of gene transfer and must be considered in cochlear gene therapy studies.
Acknowledgments
We would like to thank Dr. Matthew Kelley for technical support with embryonic culturing experiments. This work was supported by NIH grants DC003086 and DC007217 and DoD grant 0014– 07–1–1097.
References
 1 Derby ML, Sena-Esteves M, Breakefield XO, Corey DP: Gene transfer into the mammalian inner ear using HSV-1 and vaccinia virus vectors. Hear Res 1999;134:1–8.
1 Derby ML, Sena-Esteves M, Breakefield XO, Corey DP: Gene transfer into the mammalian inner ear using HSV-1 and vaccinia virus vectors. Hear Res 1999;134:1–8.
 2 Van de Water TR, Staecker H, Halterman MW, Federoff HJ: Gene therapy in the inner ear. Mechanisms and clinical implications. Ann NY Acad Sci 1999;884:345–360.
2 Van de Water TR, Staecker H, Halterman MW, Federoff HJ: Gene therapy in the inner ear. Mechanisms and clinical implications. Ann NY Acad Sci 1999;884:345–360.
 3 Han JJ, Mhatre AN, Wareing M, Pettis R, Gao WQ, Zufferey RN, Trono D, Lalwani AK: Transgene expression in the guinea pig cochlea mediated by a lentivirusderived gene transfer vector. Hum Gene Ther 1999;10:1867–1873.
3 Han JJ, Mhatre AN, Wareing M, Pettis R, Gao WQ, Zufferey RN, Trono D, Lalwani AK: Transgene expression in the guinea pig cochlea mediated by a lentivirusderived gene transfer vector. Hum Gene Ther 1999;10:1867–1873.
 4 Zheng JL, Lewis AK, Gao WQ: Establishment of conditionally immortalized rat utricular epithelial cell lines using a retrovirus-mediated gene transfer technique. Hear Res 1998;117:13–23.
4 Zheng JL, Lewis AK, Gao WQ: Establishment of conditionally immortalized rat utricular epithelial cell lines using a retrovirus-mediated gene transfer technique. Hear Res 1998;117:13–23.
 5 Raphael Y, Frisancho JC, Roessler BJ: Adenoviralmediated gene transfer into guinea pig cochlear cells in vivo. Neurosci Lett 1996;207:137–141.
5 Raphael Y, Frisancho JC, Roessler BJ: Adenoviralmediated gene transfer into guinea pig cochlear cells in vivo. Neurosci Lett 1996;207:137–141.
 6 Dazert S, Aletsee C, Brors D, Gravel C, Sendtner M, Ryan A: In vivo adenoviral transduction of the neonatal rat cochlea and middle ear. Hear Res 2001;151:30–40.
6 Dazert S, Aletsee C, Brors D, Gravel C, Sendtner M, Ryan A: In vivo adenoviral transduction of the neonatal rat cochlea and middle ear. Hear Res 2001;151:30–40.
 7 Mondain M, Restituito S, Vincenti V, Gardiner Q, Uziel A, Delabre A, Mathieu M, Bousquet J, Demoly P: Adenovirus-mediated in vivo gene transfer in guinea pig middle ear mucosa. Hum Gene Ther 1998;9:1217–1221.
7 Mondain M, Restituito S, Vincenti V, Gardiner Q, Uziel A, Delabre A, Mathieu M, Bousquet J, Demoly P: Adenovirus-mediated in vivo gene transfer in guinea pig middle ear mucosa. Hum Gene Ther 1998;9:1217–1221.
 8 Luebke AE, Steiger JD, Hodges BL, Amalfitano A: A modified adenovirus can transfect cochlear hair cells in vivo without compromising cochlear function. Gene Ther 2001;8:789–794.
8 Luebke AE, Steiger JD, Hodges BL, Amalfitano A: A modified adenovirus can transfect cochlear hair cells in vivo without compromising cochlear function. Gene Ther 2001;8:789–794.
 9 Jero J, Mhatre AN, Tseng CJ, Stern RE, Coling DE, Goldstein JA, Hong K, Zheng WW, Hoque AT, Lalwani AK: Cochlear gene delivery through an intact round window membrane in mouse. Hum Gene Ther 2001;12:539–548.
9 Jero J, Mhatre AN, Tseng CJ, Stern RE, Coling DE, Goldstein JA, Hong K, Zheng WW, Hoque AT, Lalwani AK: Cochlear gene delivery through an intact round window membrane in mouse. Hum Gene Ther 2001;12:539–548.
 10 Stöver T, Yagi M, Raphael Y: Transduction of the contralateral ear after adenovirus-mediated cochlear gene transfer. Gene Ther 2000;7:377–383.
10 Stöver T, Yagi M, Raphael Y: Transduction of the contralateral ear after adenovirus-mediated cochlear gene transfer. Gene Ther 2000;7:377–383.
 11 Kho ST, Pettis RM, Mhatre AN, Lalwani AK: Safety of adeno-associated virus as cochlear gene transfer vector: analysis of distant spread beyond injected cochleae. Mol Ther 2000;2:368–373.
11 Kho ST, Pettis RM, Mhatre AN, Lalwani AK: Safety of adeno-associated virus as cochlear gene transfer vector: analysis of distant spread beyond injected cochleae. Mol Ther 2000;2:368–373.
 12 Luebke AE, Foster PK, Muller CD, Peel AL: Cochlear function and transgene expression in the guinea pig cochlea, using adenovirusand adeno-associated virus-directed gene transfer. Hum Gene Ther 2001;12:773–781.
12 Luebke AE, Foster PK, Muller CD, Peel AL: Cochlear function and transgene expression in the guinea pig cochlea, using adenovirusand adeno-associated virus-directed gene transfer. Hum Gene Ther 2001;12:773–781.
13 Lalwani A, Walsh B, Reilly P, Carvalho G, Zolotukhin S, Muzyczka N, Mhatre A: Long-term in vivo cochlear transgene expression mediated by recombinant adeno-associated virus.’ Gene Ther 1998;5:277–281.
 14 Holt JR, Johns DC, Wang S, Chen ZY, Dunn RJ, Marban E, Corey DP: Functional expression of exogenous proteins in mammalian sensory hair cells infected with adenoviral vectors. J Neurophysiol 1999;81:1881–1888.
14 Holt JR, Johns DC, Wang S, Chen ZY, Dunn RJ, Marban E, Corey DP: Functional expression of exogenous proteins in mammalian sensory hair cells infected with adenoviral vectors. J Neurophysiol 1999;81:1881–1888.
 15 Holt JR: Viral-mediated gene transfer to study the molecular physiology of the Mammalian inner ear. Audiol Neurootol 2002;7:157–160.
15 Holt JR: Viral-mediated gene transfer to study the molecular physiology of the Mammalian inner ear. Audiol Neurootol 2002;7:157–160.
 16 Davis GL: In vitro models of viral-induced congenital deafness. Am J Otol 1981;3:156–160.
16 Davis GL: In vitro models of viral-induced congenital deafness. Am J Otol 1981;3:156–160.
 17 Bueler H: Adeno-associated viral vectors for gene transfer and gene therapy. Biol Chem 1999;380:613– 622.
17 Bueler H: Adeno-associated viral vectors for gene transfer and gene therapy. Biol Chem 1999;380:613– 622.
Adenoviral and AAV-Mediated Gene Transfer |
97 |
 18 Carter PJ, Samulski RJ: Adeno-associated viral vectors as gene delivery vehicles. Int J Mol Med 2000;6:17–27.
18 Carter PJ, Samulski RJ: Adeno-associated viral vectors as gene delivery vehicles. Int J Mol Med 2000;6:17–27.
 19 During MJ, Ashenden LM: Towards gene therapy for the central nervous system. Mol Med Today 1998;4:485–493.
19 During MJ, Ashenden LM: Towards gene therapy for the central nervous system. Mol Med Today 1998;4:485–493.
 20 Flotte T, Carter B, Conrad C, Guggino W, Reynolds T, Rosenstein B, Taylor G, Walden S, Wetzel R: A phase I study of an adeno-associated virus-CFTR gene vector in adult CF patients with mild lung disease. Hum Gene Ther 1996;7:1145–1159.
20 Flotte T, Carter B, Conrad C, Guggino W, Reynolds T, Rosenstein B, Taylor G, Walden S, Wetzel R: A phase I study of an adeno-associated virus-CFTR gene vector in adult CF patients with mild lung disease. Hum Gene Ther 1996;7:1145–1159.
 21 Peel AL, Klein RL: Adeno-associated virus vectors: activity and applications in the CNS. J Neurosci Methods 2000;98:95–104.
21 Peel AL, Klein RL: Adeno-associated virus vectors: activity and applications in the CNS. J Neurosci Methods 2000;98:95–104.
 22 Rabinowitz JE, Samulski RJ: Building a better vector: the manipulation of AAV virions. Virology 2000;278:301–308.
22 Rabinowitz JE, Samulski RJ: Building a better vector: the manipulation of AAV virions. Virology 2000;278:301–308.
 23 Snyder RO: Adeno-associated virus-mediated gene delivery. J Gene Med 1999;1:166–175.
23 Snyder RO: Adeno-associated virus-mediated gene delivery. J Gene Med 1999;1:166–175.
 24 Xiao X, Li J, McCown TJ, Samulski RJ: Gene transfer by adeno-associated virus vectors into the central nervous system. Exp Neurol 1997;144:113–124.
24 Xiao X, Li J, McCown TJ, Samulski RJ: Gene transfer by adeno-associated virus vectors into the central nervous system. Exp Neurol 1997;144:113–124.
 25 Walters RW, Yi SM, Keshavjee S, Brown KE, Welsh MJ, Chiorini JA, Zabner J: Binding of adeno-associ- ated virus type 5 to 2,3-linked sialic acid is required for gene transfer. J Biol Chem 2001;276:20610– 20616.
25 Walters RW, Yi SM, Keshavjee S, Brown KE, Welsh MJ, Chiorini JA, Zabner J: Binding of adeno-associ- ated virus type 5 to 2,3-linked sialic acid is required for gene transfer. J Biol Chem 2001;276:20610– 20616.
 26 Grimm D, Kern A, Rittner K, Kleinschmidt JA: Novel tools for production and purification of recombinant adenoassociated virus vectors. Hum Gene Ther 1998;9:2745–2760.
26 Grimm D, Kern A, Rittner K, Kleinschmidt JA: Novel tools for production and purification of recombinant adenoassociated virus vectors. Hum Gene Ther 1998;9:2745–2760.
 27 Grimm D, Kleinschmidt JA: Progress in adenoassociated virus type 2 vector production: promises and prospects for clinical use. Hum Gene Ther 1999;10:2445–2450.
27 Grimm D, Kleinschmidt JA: Progress in adenoassociated virus type 2 vector production: promises and prospects for clinical use. Hum Gene Ther 1999;10:2445–2450.
 28 Auricchio A, Hildinger M, O’Connor E, Gao GP, Wilson JM: Isolation of highly infectious and pure adeno-associated virus type 2 vectors with a singlestep gravity-flow column. Hum Gene Ther 2001;12:- 71–76.
28 Auricchio A, Hildinger M, O’Connor E, Gao GP, Wilson JM: Isolation of highly infectious and pure adeno-associated virus type 2 vectors with a singlestep gravity-flow column. Hum Gene Ther 2001;12:- 71–76.
 29 Auricchio A, O’Connor E, Hildinger M, Wilson JM: A single-step affinity column for purification of serotype-5 based adeno-associated viral vectors. Mol Ther 2001;4:372–374.
29 Auricchio A, O’Connor E, Hildinger M, Wilson JM: A single-step affinity column for purification of serotype-5 based adeno-associated viral vectors. Mol Ther 2001;4:372–374.
 30 Loeb JE, Cordier WS, Harris ME, Weitzman MD, Hope TJ : Enhanced expression of transgenes from adeno-associated virus vectors with the woodchuck hepatitis virus posttranscriptional regulatory element: implications for gene therapy. Hum Gene Ther 1999;10:2295–2305.
30 Loeb JE, Cordier WS, Harris ME, Weitzman MD, Hope TJ : Enhanced expression of transgenes from adeno-associated virus vectors with the woodchuck hepatitis virus posttranscriptional regulatory element: implications for gene therapy. Hum Gene Ther 1999;10:2295–2305.
 31 Paterna JC, Moccetti T, Mura A, Feldon J, Büeler H : Influence of promoter and WHV post-transcrip- tional regulatory element on AAV-mediated transgene expression in the rat brain. Gene Ther 2000; 7:1304–1311.
31 Paterna JC, Moccetti T, Mura A, Feldon J, Büeler H : Influence of promoter and WHV post-transcrip- tional regulatory element on AAV-mediated transgene expression in the rat brain. Gene Ther 2000; 7:1304–1311.
 32 Xiao W, Chirmule N, Berta SC, McCullough B, Gao G, Wilson JM: Gene therapy vectors based on adenoassociated virus type 1. J Virol 1999;73:3994–4003.
32 Xiao W, Chirmule N, Berta SC, McCullough B, Gao G, Wilson JM: Gene therapy vectors based on adenoassociated virus type 1. J Virol 1999;73:3994–4003.
 33 Stone IM, Lurie DI, Kelley MW, Poulsen DJ: Adenoassociated virus-mediated gene transfer to hair cells and support cells of the murine cochlea. Mol Ther 2005;11:843–848.
33 Stone IM, Lurie DI, Kelley MW, Poulsen DJ: Adenoassociated virus-mediated gene transfer to hair cells and support cells of the murine cochlea. Mol Ther 2005;11:843–848.
 34 Bedrosian JC, Gratton MA, Brigande JV, Tang W, Landau J, Bennett J: In vivo delivery of recombinant viruses to the fetal murine cochlea: transduction characteristics and long-term effects on auditory function. Mol Ther 2006;14:328–335.
34 Bedrosian JC, Gratton MA, Brigande JV, Tang W, Landau J, Bennett J: In vivo delivery of recombinant viruses to the fetal murine cochlea: transduction characteristics and long-term effects on auditory function. Mol Ther 2006;14:328–335.
 35 Liu Y, Okada T, Sheykholeslami K, Shimazaki K, Nomoto T, Muramatsu S, Kanazawa, T, Takeuchi K, Ajalli R, Mizukami H, Kume A, Ichimura K, Ozawa K: Specific and efficient transduction of Cochlear inner hair cells with recombinant adeno-associated virus type 3 vector. Mol Ther 2005;12:725–733.
35 Liu Y, Okada T, Sheykholeslami K, Shimazaki K, Nomoto T, Muramatsu S, Kanazawa, T, Takeuchi K, Ajalli R, Mizukami H, Kume A, Ichimura K, Ozawa K: Specific and efficient transduction of Cochlear inner hair cells with recombinant adeno-associated virus type 3 vector. Mol Ther 2005;12:725–733.
 36 Liu Y, Okada T, Nomoto T, Ke X, Kume A, Ozawa K, Xiao S: Promoter effects of adeno-associated viral vector for transgene expression in the cochlea in vivo. Exp Mol Med 2007;39:170–175.
36 Liu Y, Okada T, Nomoto T, Ke X, Kume A, Ozawa K, Xiao S: Promoter effects of adeno-associated viral vector for transgene expression in the cochlea in vivo. Exp Mol Med 2007;39:170–175.
 37 Boeda B, Weil D, Petit C: A specific promoter of the sensory cells of the inner ear defined by transgenesis. Hum Mol Genet 2001;10:1581–1589.
37 Boeda B, Weil D, Petit C: A specific promoter of the sensory cells of the inner ear defined by transgenesis. Hum Mol Genet 2001;10:1581–1589.
 38 Izumikawa M, Minoda R, Kawamoto K, Abrashkin KA, Swiderski DL, Dolan DF, Brough DE, Raphael Y: Auditory hair cell replacement and hearing improvement by Atoh1 gene therapy in deaf mammals. Nat Med. 2005;11:271–276.
38 Izumikawa M, Minoda R, Kawamoto K, Abrashkin KA, Swiderski DL, Dolan DF, Brough DE, Raphael Y: Auditory hair cell replacement and hearing improvement by Atoh1 gene therapy in deaf mammals. Nat Med. 2005;11:271–276.
 39 Rio C, Dikkes P, Liberman MC, Corfas G: Glial fibrillary acidic protein expression and promoter activity in the inner ear of developing and adult mice. J Comp Neurol 2002;442:156–162
39 Rio C, Dikkes P, Liberman MC, Corfas G: Glial fibrillary acidic protein expression and promoter activity in the inner ear of developing and adult mice. J Comp Neurol 2002;442:156–162
 40 Aiba-Masago S, Baba S, Li RY, Shinmura Y, Kosugi I, Arai Y, Nishimura M, Tsutsui Y: Murine cytomegalovirus immediate-early promoter directs astro- cyte-specific expression in transgenic mice. Am J Pathol 1999;154:735–743.
40 Aiba-Masago S, Baba S, Li RY, Shinmura Y, Kosugi I, Arai Y, Nishimura M, Tsutsui Y: Murine cytomegalovirus immediate-early promoter directs astro- cyte-specific expression in transgenic mice. Am J Pathol 1999;154:735–743.
 41 Furness DN, Lawton DM: Comparative distribution of glutamate transporters and receptors in relation to afferent innervation density in the mammalian cochlea. J Neurosci 2003;23:11296–11304.
41 Furness DN, Lawton DM: Comparative distribution of glutamate transporters and receptors in relation to afferent innervation density in the mammalian cochlea. J Neurosci 2003;23:11296–11304.
Anne E. Luebke, PhD
Department of Biomedical Engineering, University of Rochester Medical Center Box 603, 601 Elmwood Ave. MC68547 Rochester, NY 14642 (USA)
Tel. +1 585 273 1635, Fax +1 585 276 5334, E-Mail Anne_Luebke@urmc.rochester.edu
98 |
Luebke · Rova · Von Doersten · Poulsen |

Ryan AF (ed): Gene Therapy of Cochlear Deafness.
Adv Otorhinolaryngol. Basel, Karger, 2009, vol 66, pp 99–115
Cellular Targeting for Cochlear Gene Therapy
Allen F. Ryan Lina M. Mullen Joni K. Doherty
Departments of Surgery/Otolaryngology and Neurosciences, UCSD School of Medicine, La Jolla, Calif., USA
Abstract
Gene therapy has considerable potential for the treatment of disorders of the inner ear. Many forms of inherited hearing loss have now been linked to specific locations in the genome, and for many of these the genes and specific mutations involved have been identified. This information provides the basis for therapy based on genetic approaches. However, a major obstacle to gene therapy is the targeting of therapy to the cells and the times that are required. The inner ear is a very complex organ, involving dozens of cell types that must function in a coordinated manner to result in the formation of the ear, and in hearing. Mutations that result in hearing loss can affect virtually any of these cells. Moreover, the genes involved are active during particular times, some for only brief periods of time. In order to be effective, gene therapy must be delivered to the appropriate cells, and at the appropriate times. In many cases, it must also be restricted to these cells and times. This requires methods with which to target gene therapy in space and time. Cell-specific gene promoters offer the opportunity to direct gene therapy to a desired cell type. Moreover, conditional promoters allow gene expression to be turned off and on at desired times. Theoretically, these technologies offer a mechanism by which to deliver gene therapy to any cell, at any given time. This chapter will examine the potential for such targeting to deliver gene therapy to the inner ear in a precisely controlled manner.
Introduction
While gene therapy has great promise for the treatment of disease, delivery has proven to be one of the greatest challenges to its application [1]. Part of this challenge is providing sufficient levels or durations of gene expression to influence a disease. However, a perhaps more crucial difficulty is targeting therapy to the cells that require treatment, and at the times that are necessary for it to be effective. This is especially important in complex tissues that contain many cell types, such as the inner ear, or during development when gene expression events and cells are changing rapidly. Gene therapy delivered to the wrong cell type, or at the wrong time, could be ineffective or even harmful [2].
Fortunately, tools with which to address these issues exist within the genome itself. All genes contain regulatory elements that control when and where a gene is expressed [3]. Many such regulatory elements, including gene promoters, enhancers and repressors, are cell-specific in that they limit gene expression to a subset of cells in the body. Many of these regulatory sequences also limit expression to a particular period of time. Almost all gene promoters are subject to regulation that controls the level of gene expression. Some regulatory elements also respond to factors that can be delivered to cells in an autocrine manner, permitting control by exogenous application of an appropriate factor. Finally, many viruses contain regulatory elements that control gene expression in their mammalian host cells. Harnessing these natural regulatory mechanisms makes it possible, at least in theory, to tailor gene therapy to any cell at virtually any time, and to regulate the level of transcript expression in a fairly precise manner.
Mechanisms of Gene Regulation
Gene expression, and the functions of mRNA and proteins that are the products of genes, can be regulated at many stages. An initial step in gene transcription is the exposure of genomic DNA, without which regulatory and transcriptional complexes cannot operate. This occurs primarily through the positioning of nucleosomes, around which the DNA is coiled, to allow access by regulatory and transcriptional proteins. Exposure of DNA is strongly influenced by the acetylation and methylation state of histones [4]. These nuclear proteins make up the nucleosomes and are critically involved in packaging DNA within the nucleus. Acetylation of primarily the N-terminal glycine of histones by acetyltransferases reduces their affinity for DNA, thereby decreasing their density and increasing access for transcription factors (TFs) and RNA polymerase to their binding sites within the gene [5]. Histones can also be affected by amino acid methylation [6]. Histone methylation is most often associated with reduced transcription due to increased histone density and recruitment of silencing RNA and proteins. In its ultimate form this process results in the formation of dense heterochromatin leading to gene silencing. However, some methylation patterns of certain histone residues can reduce nucleosome density and increase transcription [7], thus the arrangement of nucleosomes within a cell’s nucleus helps to determine the genes that can be expressed in that cell.
The identity of cells is in large part determined by the subset of the genome that is transcribed in the cell. Part of determining the repertoire of genes that is available to a cell occurs via selective gene silencing. This can involve via semi-permanent increases in nucleosome density, a process mediated in part by histone methylation and de-acetylation as described above, or the more permanent formation of heterochromatin [6]. However, another major mechanism of silencing involves the methylation of DNA, primarily within gene promoters [8]. Such DNA methylation typically
100 |
Ryan · Mullen · Doherty |
occurs in the CpG islands (clusters of CG dinucleotides) that are associated with transcription start sites of many genes. However, even genes lacking CpG islands can be subject to silencing via methylation, especially in stem cells [9].
If their unmethylated DNA is exposed, the primary active regulation of genes occurs at the stage of transcription into mRNA. Transcription is mediated by RNA polymerase II and other members of the core transcriptional complex, which bind to the DNA at the transcriptional start site. The activity of the transcriptional complex is influenced by various TFs that bind directly to DNA at more diverse locations within the gene. TFs are characterized by protein domains that bind to specific DNA sequences. They also typically contain protein-binding domains. A transactivation domain can interact directly with the transcriptional complex and influence the activation of RNA polymerase II. Alternatively, a TF may bind to another protein that lacks a DNA-binding domain but possesses a transactivation domain. The differential regulation of gene transcription by TFs and associated proteins appears to be especially important during development [10, 11].
Once a gene has been transcribed to mRNA, post-transcriptional regulation is also common. Translation from mRNA to protein can be regulated by a variety of processes. For example, microRNAs destabilize mRNA, leading to its degradation and silencing, and microRNAs also cooperate to regulate genes via combinatorial coding [12]. Differential mRNA splicing can result in a variety of proteins from the same initial RNA transcript. There are also, of course, many forms of post-translational modification of proteins including phosphorylation and cleavage.
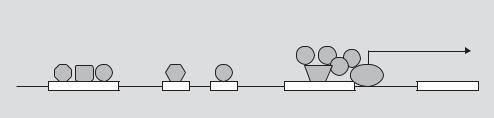
Thus there are multiple layers of potential regulation that can contribute to the diversity in gene influence. Any of these could potentially be used as control mechanisms in gene therapy. However, the most attention has been focused on the interaction between regulatory DNA sequences and TFs [11]. The transcription of mRNA from a gene is largely controlled by cis-acting regulatory elements in the non-coding DNA sequences of the gene. Most such elements are DNA motifs, DNA sequences specifically recognized by the DNA-binding domains of TFs, and to which these proteins bind. Cis-regulatory elements in genes can be broadly divided into two categories: promoters and enhancers/repressors. Promoters serve as the binding site for the transcriptional complex including RNA polymerase II itself and the many associated TFs and TF-binding proteins that make up the core machinery for transcribing DNA into mRNA. Promoters are thus located at the transcriptional start site of a gene (fig. 1).
Enhancers and repressors function to regulate the rate of transcription. Through the binding of TFs and associated proteins, they can determine whether or not a gene is active in a particular cell type, as well as the level of transcription. These elements are often located 5 to the promoter, but they can be many thousands of bases distant. Regulatory sequences can also be present in transcribed but untranslated sequences of the gene such as the 5 -UTR or introns, particularly the first intron. They can also be located 3 to the transcribed sequence. Once TFs and associated proteins are bound,
Targeting Inner Ear Gene Therapy |
101 |
|
|
|
Transcriptional |
|
|
|
|
complex |
|
|
|
|
|
mRNA |
|
|
|
TF TF |
TF |
|
|
|
TBP TF |
|
TF TF TF |
TF |
TF |
Pol II |
|
Enhancer |
|
Enhancer |
Promoter |
Exon |
Fig. 1. Schematic representation of gene regulation. The core promoter of a gene lies immediately upstream of the transcription initiation site at which mRNA production by RNA polymerase II (Pol II) begins. The promoter serves as a binding site for the transcription complex, which consists of Poll II, various transcription factors (TFs), and for promoters containing a TATA box, the TATA-binding protein (TBP). At other locations in the gene, enhancers serve as binding sites for additional TFs and can influence the rate of transcription of the gene. Binding of different combinations of TFs can direct expression to different cell types or times.
these regulators are thought to influence transcription by interacting physically with the transcriptional complex and enhancing or repressing RNA polymerase activity. This interaction is mediated by a transactivation domain that is separate from the DNA-binding domain of the TF, or may be present in a TF-binding protein (fig. 2). A given gene typically has multiple enhancer and/or repressor elements (fig. 1).
TFs bind to the gene’s promoter and to enhancers/repressors, typically in a combination of several factors at each. The combination of TFs that are present in a given cell play a major role in determining what DNA motifs are occupied, and how the gene will respond. Since the same DNA is present in all cells, the combination of TFs expressed by a cell is thus an important determinant of what genes will be expressed, and when they will be transcribed. While both promoters and enhancers/repressors can determine the cellular specificity of genes, enhancers of a gene are most often responsible for cellular specificity if a gene is expressed differently in different cells. A cell that expresses a certain combination of TFs may activate one enhancer, while another cell type expressing different TFs may activate a separate enhancer, which allows the gene to be regulated separately in each cell type. Other cell types that lack appropriate TFs will fail to activate the gene. Alternatively, different combinations of TFs and other regulatory proteins may act differently on the same enhancer. For example, many TFs have negative regulators that can either compete for the same or an overlapping binding site, or mask a transactivation domain and prevent interaction with the transcriptional complex. Such patterns of DNA-regulatory motifs, and the combinations of TFs and other regulatory proteins that bind to them, are sometimes referred to as the second genetic code [13].
The nuclear receptors comprise a special category of TFs. This includes a number of hormone receptors, such as the estrogen receptor, which when activated by their ligands will bind to promoters or enhancers/repressors to activate/silence a gene. Nuclear receptors permit the rapid regulation of gene activity. Binding sites for nuclear
102 |
Ryan · Mullen · Doherty |
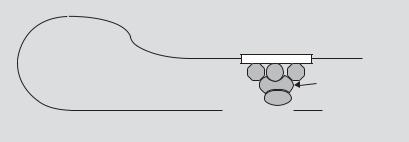
Enhancer
TF TF TF
TAP
TC
|
|
|
Promoter |
|
Exon |
Fig. 2. Schematic representation of the means by which enhancers are thought to influence the transcriptional complex (TC). Transcription factors (TFs) that are bound to their DNA recognition DNA motifs in an enhancer are brought into contact with the TC by the folding of DNA. Either the TFs or a transcription-associated protein (TAP) interact with the TC via a transactivation domain. Many promoters are inactive without the influence of enhancers.
receptors can be engineered into the promoters of a gene therapy vector, allowing it to be regulated. A modified form of the estrogen receptor-binding sequence that is sensitive to tamoxifen is frequently used to produce vectors that are only transcribed in the presence of this molecule [14].
An artificial conditional system has also been engineered using bacterial and viral DNA sequences. For this method, sequence encoding a tetracycline-sensitive DNA-binding protein domain from Escherichia coli is fused to a sequence encoding a transactivating protein domain from Herpes simplex. In the resultant chimeric protein, binding of the DNA-binding domain is dependent upon tetracycline (or related compounds like doxycycline). Different forms of the protein either require (Tet-on) or are inhibited by (Tet-off) tetracycline for DNA binding. Once the DNAbinding domain is bound to its recognition sequence, which can be engineered into a gene-regulatory sequence, the transactivation domain activates the transcriptional complex on the promoter, thus activating the downstream gene. Regulation using the Tet-On and Tet-Off systems is somewhat tighter than with the estrogen receptor [15].
Harnessing Gene Regulation for Gene Therapy
The gene-regulatory processes outlined above can be utilized for gene therapy. For example, a promoter or enhancer that produces expression limited to a given cell type and/or developmental period can be used in a gene therapy vector. This would permit the vector to drive expression of a gene product only in that cell or at that time. Similarly conditional promoters, ones that express only in the presence of a particular nuclear ligand, can be used to turn on gene therapy for a brief period. In practice, there are difficulties with both approaches.
Targeting Inner Ear Gene Therapy |
103 |
