
Gale Encyclopedia of Genetic Disorder / Gale Encyclopedia of Genetic Disorders, Two Volume Set - Volume 2 - M-Z - I
.pdf
Rieger syndrome
K E Y T E R M S
Cornea—The transparent structure of the eye over the lens that is continous with the sclera in forming the outermost, protective, layer of the eye.
Craniofacial—Relating to or involving both the head and the face.
Hypoplasia—Incomplete or underdevelopment of a tissue or organ.
Iris—The colored part of the eye, containing pigment and muscle cells that contract and dilate the pupil.
Microcornea—Abnormal smallness of the cornea.
Microdontia—Small teeth.
Myotonia—The inability to normally relax a muscle after contracting or tightening it.
Myotonic dystrophy—A form of muscular dystrophy, also known as Steinert’s condition, characterized by delay in the ability to relax muscles after forceful contraction, wasting of muscles, as well as other abnormalities.
Ocular—A broad term that refers to structure and function of the eye.
Oligodonita—The absence of one or more teeth.
Psychomotor—Movement produced by action of the mind or will.
Stenosis—The constricting or narrowing of an opening or passageway.
the vein at the corner of the eye that drains the water in the eye into the bloodstream (schlemm), and the associated adhesions. Glaucoma can result in damage to the optic disk and gradual loss of vision, causing blindness in approximately 50% of affected individuals.
Additional conditions have sometimes occurred in conjunction with Rieger syndrome. Whether they are separate entities in which the Rieger eye malformations are present or part of Rieger syndrome is not determined. These conditions are:
•Myotonia (a condition in which the muscles do not relax after contracting).
•Myotonic dystrophy (a chronic progressive disease causing muscles to atrophy, slurred speech, failing vision, droopy eyelids, and general muscle weakness).
•Conductive deafness (hearing loss in which sound does not travel well to the inner ear).
•Less than average intellectual function associated with problems in learning and social behavior.
Diagnosis
This disorder can be detected soon after birth if the eye defects are visible. When the eye defects are not visible during the first month of life, Rieger syndrome is usually detected in early childhood when the eye and dental defects become apparent.
Molecular genetic testing for the RIEG1 and RIEG2 genes is not generally available. But since the molecular structure of the genes has been identified, the possibility now exists for DNA-based testing for diagnosis and genetic counseling.
Genetic counseling
Genetic counseling may be beneficial for patients and their families. Only one parent needs to be a carrier in order for the child to inherit the disease. A child has a 50% chance of having the disease if one parent is diagnosed with the disease and a 75% chance of having the disease if both parents have Rieger syndrome.
Prenatal testing
For couples known to be at risk for having a baby with Rieger syndrome, testing may be available to assist in prenatal diagnosis. Prior testing of family members is usually necessary for prenatal testing.
Either chorionic villus sampling (CVS) or amniocentesis may be performed for prenatal testing. CVS is a procedure to obtain a small sample of placental tissue, called chorionic villi tissue, for testing. Examination of fetal tissue can reveal information about the defects that leads Rieger syndrome. Chorionic villus sampling can be performed at 10–12 weeks gestation.
Amniocentesis is a procedure that involves inserting a thin needle into the uterus and the amniotic sac, and withdrawing a small amount of amniotic fluid. DNA can be extracted from the fetal cells contained in the amniotic fluid and tested. Amniocentesis is performed at 16–18 weeks gestation.
Tissue showing the gene mutation for Rieger syndrome Type I or II obtained from CVS or in amniotic fluid is diagnostic.
Related disorders
A number of disorders are similar to Rieger syndrome. Comparisons may be useful for a differential diagnosis. These related disorders include:
•Cat-eye syndrome, a rare disorder marked by a cleft along the eyeball affecting the iris, the membrane that
1002 |
G A L E E N C Y C L O P E D I A O F G E N E T I C D I S O R D E R S |
covers the white of the eyeball (choroid), and/or the retina and causing a vertical pupil; abnormalities such as small polyps or pits near the front of the outer ear; and absence of the opening, duct, or canal of the anus. Other symptoms may include mild mental deficiency and heart defects.
•Ectodermal dysplasias, a group of hereditary syndromes affecting the skin, its derivatives, and some other organs. Symptoms include predisposition to respiratory infection, eczema, poorly functioning sweat glands, abnormal hair and nails, and difficulties with the nasal passages and ear canals.
•Eye, anterior segment dysgenesis, a rare congenital disorder resulting in abnormal tissue development of the outer eye segment. In less severe cases, the back of the outer surface of the cornea is nontransparent (embryotoxin). Symptoms include ocular abnormalities and malformations of the teeth, abdominal wall, skeleton, and heart.
It is generally thought that Axenfeld anomaly, marked by defects limited to the outer part of the field of vision of the eye, should not be considered a separate entity of Rieger syndrome.
Treatment and management
A physician familiar with the range of problems seen in individuals with Rieger syndrome is important for appropriate health supervision. Treatment should include assistance finding support resources for the family and the individual with Rieger syndrome.
Treatment of Rieger syndrome is focused on treating the symptoms expressed. Depending on what they are, these treatments may include:
•Drug therapy for glaucoma, usually a topical beta blocker in the form of eye drops. Laser surgery may be performed on those patients in whom the pressure in the eye is not relieved by medications.
•Prostheses (false teeth) or other orthodontic interventions for dental malformations.
•Other surgical management of congenital anomalies includes repair of an umbilical hernia that does not close by itself and plastic surgery for craniofacial abnormalities.
Prognosis
Prognosis depends upon the severity of the disease. Eye defects may lead to severly impaired vision or blindness. Rieger syndrome does not generally lead to a shortened life span.
Resources
BOOKS
Jorde, Lynn B. et al., eds. Medical Genetics. 2nd ed. St. Louis: Mosby, 1999.
PERIODICALS
Alward, W. L. “Axenfeld-Rieger syndrome in the age of molecular genetics.” American Journal of Ophthalmology 130 (2000): 107–15.
Amendt, B. A., E. V. Semina, and W. L. Alward. “Rieger syndrome: a clinical, molecular, and biochemical analysis.” Cellular and Molecular Life Sciences 11 (2000): 1652–66.
Craig, J. E., and D. A. Mackey. “Glaucoma genetics: Where are we? Where will we go?” Current Opinions in Ophthalmology 10 (1999): 126–34.
ORGANIZATIONS
Blind Children’s Fund. 4740 Okemos Rd., Okemos, MI 488641637. (517) 347-1357. http://www.blindchildrensfund
.org .
National Association for Parents of the Visually Impaired. PO Box 317, Watertown, MA 02472. (617) 972-7441 or (800) 562-6265. http://www.spedex.com/napvi .
National Association for Visually Handicapped. 22 West 21st Street, New York, NY 10010. (212) 889-3141. http://www
.navh.org .
National Eye Institute. Bldg. 31 Rm 6A32, 31 Center Dr., MSC 2510, Bethesda, MD 20892-2510. (301) 496-5248. 2020 @nei.nih.gov. http://www.nei.nih.gov .
National Foundation for Ectodermal Dysplasias. PO Box 114, 410 East Main St., Mascoutah, IL 62258-0114. (618) 566-2020. Fax: (618) 566-4718. http://www.nfed
.org .
National Organization for Rare Disorders (NORD). PO Box 8923, New Fairfield, CT 06812-8923. (203) 746-6518 or (800) 999-6673. Fax: (203) 746-6481. http://www
.rarediseases.org .
Vision Community Services. 23 A Elm St., Watertown, MA 02472. (617) 926-4232 or (800) 852-3029. http://www
.mablind.org .
OTHER
OMIM—Online Mendelian Inheritance in Man. http:// www. ncbi . nlm . nih . gov:80/entrez/query. fcgi?db= OMIM
Rarelinks: A site for parents and caregivers dealing with Rieger syndrome. http://rarelinks4parents.homestead.com/ index.html
Jennifer F. Wilson, MS
Riley-Day syndrome see Familial dysautonomia
syndrome Rieger
G A L E E N C Y C L O P E D I A O F G E N E T I C D I S O R D E R S |
1003 |
RNA (Ribonucleic acid)
I RNA (Ribonucleic acid)
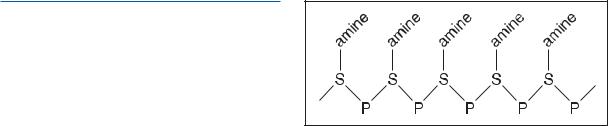
Ribonucleic acid (RNA) conveys genetic information and catalyzes important biochemical reactions. Similar, but not identical, to a single strand of deoxyribonucleic acid (DNA), in some lower organisms, RNA replaces DNA as the genetic material. As with DNA, RNA follows specific base pairing rules, except that in RNA the base uracil replaces the base thymine (i.e., instead of an adenine-thymine or A-T pairing, there is an adenine-uracil or A-U pairing). Accordingly, when RNA acts as a carrier of genetic information, uracil replaces thymine in the genetic code.
In humans, messenger RNA (mRNA) is the product of transcription and acts to convey genetic information from the nucleus to the protein assembly complex at the ribosome. The ribsome is composed of ribosomal RNA (rRNA) and other proteins. Transfer RNAs (tRNA) act to catalyze the translation process by acting as carriers of specific amino acids. Because tRNAs bind to specific sites on the strand of mRNA, the sequence of amino acids subsequently inserted into the synthesized protein is both specific and genetically determined by the nucleotide sequence in DNA from which the mRNA strand was originally transcribed.
Other forms of RNA perform important roles in other biochemical reactions. Regardless of function, RNA is a biopolymer made up of ribonucleotide units and is present in all living cells and some viruses. The chemical units of RNA are ribonucleotide monomers consisting of a ribose sugar (C5H10O5) phosphorylated at the third carbon (C3) and linked to one of four bases through a type of chemical linkage formed between a sugar and a base by a condensation reaction (glycosidic bond). The four bases found in RNA are adenine (A), guanine (G), cytosine (C), and uracil (U). Other bases may also be found, although they are generally modified versions of these four (e.g., methylated bases are found in parts of tRNA).
The single nucleotides (monomers) of RNA form a linear chain by linking their phosphate groups and sugars in phosphodiester bonds. RNA does not form a double stranded alpha-helix as does DNA. In some parts of the RNA molecule, there is folding into alpha-helical-like regions. Corresponding to their unique functions, messenger RNA (mRNA), ribosomal RNA (rRNA), and transfer RNA (tRNA) all have different threedimensional structures. In higher eukaryotic organisms, different RNAs are found distributed throughout the cell—in the nucleus, cytoplasm, and also in cytoplasmic organelles such as mitochondria and, in plants, chloroplasts.
The molecular structure of RNA. (Gale Group)
The nucleus is the chief site of RNA synthesis and the source of all cytoplasmic RNA, while mitochondria and chloroplasts synthesize their RNA from their own DNA. rRNA is synthesized by the nucleoli within the nucleus, while the high molecular weight precursor to cytoplasmic mRNA, sometimes termed heterogeneous nuclear or hnRNA, is transcribed on the DNA chromatin. Low molecular weight RNA also occurs in the nucleus and consists partly of tRNA and partly of RNA, which has a regulatory function in gene activation. The cytoplasm contains tRNA and rRNA in the ribosomes and mRNA in polysomes, or polyribosomes. The latter are the structural units of protein biosynthesis, consisting of several ribosomes attached to a strand of mRNA.
The function of mRNA is to transcribe the information held in DNA. In the cells of eukaryotic organisms, the first transcriptional product is the long, heterogenous nuclear RNA, or hnRNA. This contains both the nucleotide sequences eventually transcribed into polypeptides and large tracts of sequences not translated. Non-translated sequences are termed introns (or intervening sequences). Removal of introns, and other untranslated portions of the molecule, edits hnRNA into mRNA molecules. After editing removes as much as 90% of hnRNA, the resulting mRNA molecules are transported into the cytoplasm.
rRNA is located within ribosomes, the sites of protein biosynthesis. Ribosomes are large ellipsoid cytoplasmic organelles consisting of RNA and protein.
tRNA, the smallest known functional RNA, is essential for protein biosynthesis. Its purpose is to transfer a specific amino acid from the cytoplasm and incorporate it into the growing polypeptide chain on the polysome. Different tRNAs contain between 70 and 85 nucleotides. The most characteristic feature of tRNA is that it contains the anticodon, a sequence of three nucleotides specific for the mRNA codon sequence. There is at least one tRNA per cell bearing the anticodon for each of the 20 amino acids. The aminoacyl-tRNA (the tRNA carrying the amino acid) binds to the large subunit of a ribosome, where antiparallel basepairing occurs between the anti-
1004 |
G A L E E N C Y C L O P E D I A O F G E N E T I C D I S O R D E R S |

codon of the tRNA and the complementary codon of the associated mRNA. The specificity of this base pairing ensures that the amino acid inserts into the correct position in the growing protein polypeptide chain. During translation, the deacylated tRNA (i.e., with its amino acid removed) is released from the ribosome and becomes available once again for recharging with its amino acid.
DNA-dependent RNA synthesis is the process of RNA sythesis on a template of DNA. According to the rules of base pairing, the base sequence of DNA determines the synthesis of a complementary base sequence in RNA. Assisted (catalyzed) by the enzyme RNA polymerase, the growing RNA chain releases from the template so that the process can start again, even before the previous molecule is complete. Termination codons and a termination factor known as rho-factor end the synthesis process. In certain viruses, RNA-dependent RNA synthesis occurs, with the viral RNA acting as a template for the synthesis of new RNA.
Judyth Sassoon, ARCS, PhD
I Roberts SC phocomelia
Definition
Roberts SC phocomelia is a rare genetic condition that causes severe abnormalities in arm and leg bones. Other abnormalities, such as mental retardation, may also be present.
Description
Roberts SC phocomelia was first described in the year 1919. In the past, Roberts SC phocomelia syndrome was described as two separate syndromes: Roberts syndrome, and SC or pseudo-thalidomide syndrome. More recent examination, however, indicated that they are the same disorder. The term “pseudo-thalidomide” was originally used to describe individuals with limb shortening, as the medication thalidomide is known to cause limb abnormalities in the babies of women taking it during pregnancy.
Phocomelia is a condition in which the hands and feet are present, but the arms and legs are absent. The hands and feet are attached directly to the body. Usually there is greater shortening in the arms than in the legs. People with Roberts SC phocomelia syndrome have varying degrees of hypomelia, which means that the limbs are not fully developed. Some are born without the upper bones of the arms or the legs. This is referred to as
tetraphocomelia. Some people, though, have a less severe form of limb shortening.
In addition to the limb abnormalities, 80% of individuals with the syndrome have a small head (microcephaly). In addition, most people with the syndrome have some degree of mental retardation. Most also have facial problems affecting the development of the upper lip (cleft lip) and incomplete development of the palate (the roof of the mouth).
Genetic profile
Roberts SC phocomelia is inherited in an autosomal recessive fashion. This is a pattern in which the child receives one nonfunctioning (abnormal) gene from each parent. When a woman and man who both carry one abnormal gene for Roberts SC phocomelia have children, there is a 25% chance that they will each pass along the gene for the syndrome. People who are termed “carriers” are not affected by the disorder, as they have only one copy of the gene that causes Roberts SC phocomelia. The chances are 50% that they will have a child who is also a carrier of the disorder. The chances are 25% that they will have a baby who is neither a carrier nor affected with Roberts SC phocomelia.
The specific gene that causes the syndrome is not yet known, and there is no direct genetic test to identify a potential carrier of the disease.
In many of the individuals who have been diagnosed with Roberts SC phocomelia, a unique feature may be observed on some of their chromosomes. The exact association of this unusual observation with the syndrome is not yet understood.
Demographics
The exact number of people with the syndrome is not known, as some infants who die before or shortly after birth are never diagnosed or are diagnosed incorrectly. The syndrome affects males and females equally. There is no specific country or region of the world where the disorder is more common.
Signs and symptoms
In the bones of the lower arm (radius and ulna), limb shortening or absence of limbs is evident in approximately 97% of people with the syndrome. The upper arm (humerus) is affected 77% of the time. A missing or shortened thighbone (femur) occurs in about 65% of affected individuals. The bones in the lower leg (tibia and fibula) are shortened or absent in 77% of those with the disorder.
phocomelia SC Roberts
G A L E E N C Y C L O P E D I A O F G E N E T I C D I S O R D E R S |
1005 |

Roberts SC phocomelia
K E Y T E R M S
Amniocentesis—A procedure performed at 16–18 weeks of pregnancy in which a needle is inserted through a woman’s abdomen into her uterus to draw out a small sample of the amniotic fluid from around the baby. Either the fluid itself or cells from the fluid can be used for a variety of tests to obtain information about genetic disorders and other medical conditions in the fetus.
Amniotic fluid—The fluid that surrounds a developing baby during pregnancy.
Cell—The smallest living units of the body which group together to form tissues and help the body perform specific functions.
Genetic test—Testing of chromosomes and genes from an individual or unborn baby for a genetic condition. Genetic testing can only be done if the gene is known.
Ultrasound evaluation—A procedure which examines the tissue and bone structures of an individual or a developing baby.
It is often very hard to flex or bend the knees, ankles, wrists, and/or elbows. While the feet and hands are almost always present, there may be fewer than normal fingers and toes, or shortened fingers. Sometimes the fingers are fused together (syndactyly).
People with the syndrome are smaller than other babies the same age, both before and after birth. Babies with Roberts SC phocomelia syndrome may have thin hair that is often described as silvery in color. In addition, most people with Roberts SC phocomelia syndrome are born with a cleft lip (a failure of the upper lip to close completely) and cleft palate (an opening in the roof of the mouth). Other abnormalities that may occur include a small and underdeveloped chin, a short neck, heart and kidney problems, prominent and widely spaced eyes, and unusually shaped ears.
Diagnosis
This disorder has been diagnosed during pregnancy at 12 weeks, through a test called an ultrasound evaluation. In these incidences, developmental problems with the growth and formation of both the arms and legs were noted. Sometimes the syndrome cannot be diagnosed by ultrasound until later in the pregnancy, when the limb shortening or absence becomes more obvious, and some-
times it cannot be diagnosed by ultrasound at all. Other abnormalities that might be seen by ultrasound include cleft lip, increased distance between the eye sockets, and extra fluid in some of the structures of the brain (hydrocephalus). Excess amniotic fluid levels, kidney problems, and an opening in the spine (spina bifida) have also been found. However, an exact diagnosis of the syndrome cannot be made by ultrasound evaluation alone.
Checking for the unusual chromosome feature is done through amniocentesis, a procedure that collects the developing fetus’s cells for evaluation. But this test is not typically recommended, because not all affected individuals have this chromosome finding. In addition, the chromosome is not always evident in the cells from the amniotic fluid.
As of 2001 there was no accurate prenatal test to diagnose the syndrome during pregnancy.
After a baby is born with characteristics of Roberts SC phocomelia syndrome, a diagnosis can be made through a complete physical examination. In addition, analysis of the baby’s chromosomes may also be useful. The chromosomes can be analyzed through a blood or tissue sample.
Treatment and management
At this time there is no treatment available for individuals with Roberts SC phocomelia syndrome. The shortness or absence of limbs makes it difficult for any type of limb-lengthening therapies to be useful in most instances.
Prognosis
The majority of severely affected individuals will die in the womb, or during or shortly after birth. Those who survive will have very obvious growth deficiency as well as mental retardation. Babies who are not as severely affected, with less dramatic limb shortening and no facial cleft, have a better overall prognosis.
Resources
BOOKS
Fleischer, A., et al. Sonography in Obstetrics and Gynecology, Principles & Practice. Stamford: 1996.
Jones, Kenneth. Smith’s Recognizable Patterns of Human
Malformations. 5th ed. Philadelphia: W.B. Saunders Company, 1997.
PERIODICALS
Camlibel, T. “Roberts SC Phocomelia with Isolated Cleft Palate, Thrombocytopenia, and Eosinophilia.” Genetic Counseling 10, no. 2 (1999): 157–61.
McDaniel, L. D. “Novel Assay for Roberts Syndrome Assigns Variable Phenotypes to One Complementation Group.”
1006 |
G A L E E N C Y C L O P E D I A O F G E N E T I C D I S O R D E R S |

American Journal of Medical Genetics 93, no. 3 (2000): 223–9.
WEBSITES
“Entry 268300: Roberts syndrome; RBS.” Online Mendelian Inheritance in Man. http://www.ncbi.nlm.nih.gov/ htbin-post/Omim/dispmim?268300 .
“Limb Anomalies.” University of Kansas Medical Center.
http://www.kumc.edu/gec/support/limb.html .
Katherine Susan Hunt, MS
Roberts syndrome see Roberts SC phocomelia
Robin sequence see Pierre-Robin sequence
Robinow dwarfism see Robinow syndrome
I Robinow syndrome
Definition
Robinow syndrome encompasses two different hereditary disorders, both rare, with a similar pattern of physical abnormalities. Typical features of these conditions include mild to moderate short stature, distinctive facial features, skeletal abnormalities, and abnormal development of the genitalia.
Description
A family that included several individuals with a characteristic pattern of facial features, accompanied by short stature (dwarfism), skeletal abnormalities, and underdevelopment (hypoplasia) of the external genitalia (sex organs) was first described in 1969 by Dr. Meinhard Robinow. He named the condition “Fetal face syndrome,” because the facial features are similar to those of a normal fetus. Only later was Dr. Robinow’s name used to identify the syndrome. Other names for the condition include Robinow dwarfism, as well as “acral dysostosis with facial and genital abnormalities.”
Skeletal abnormalities of varying types and severity occur in every case of Robinow syndrome. Most people with the condition have abnormal development of specific bones of the arms and legs resulting in some degree of short stature. Spinal abnormalities are also common. Most females are fertile, but only a few males with the condition have had children.
Genetic profile
Chromosomes are the microscopic structures inside cells that carry the genes. Each cell of the body contains
K E Y T E R M S
Acromelic—The anatomical term used to denote the end of a limb (arm or leg). In the context of Robinow syndrome, it refers to bones of the hands and feet.
Brachymelia—A general medical term used to describe short limbs.
Hypertelorism—A wider-than-normal space between the eyes.
Hypoplasia—Incomplete or underdevelopment of a tissue or organ.
Mesomelic—The anatomical term used to describe the middle of a limb. The bones that constitute the middle of the arm are the radius and ulna, and mesomelic bones of the leg are the tibia and fibula.
Vertebra—One of the 23 bones which comprise the spine. Vertebrae is the plural form.
46 chromosomes in 23 pairs. The exceptions are sperm and eggs, which normally carry 23 chromosomes—one of each pair. The first 22 pairs of chromosomes in humans are known as the autosomes. An inherited condition is autosomal if the abnormal gene that causes it resides on one of the first 22 pairs of chromosomes.
Several years after Dr. Robinow’s first report, it became clear that some families affected by Robinow syndrome have an autosomal dominant pattern of inheritance, while in other families the syndrome is inherited as an autosomal recessive trait. As of 2000, the reason for this genetic discrepancy was unknown.
Dominant inheritance means that an error in only one gene of a pair is enough to produce symptoms of the disorder. In other words, the abnormally functioning gene of the pair is dominant over the normal gene. A person who carries the gene for autosomal dominant Robinow syndrome has a 50% chance of passing it on to each of his or her offspring.
In autosomal recessive inheritance, a person must have errors in both copies of a gene pair in order to be affected. Someone who carries just one copy of the disease gene has another normally functioning gene of that pair to compensate for it. Therefore, a carrier of a single recessive gene typically shows no symptoms of the disorder. If two people who both carry the gene for recessive Robinow syndrome conceive a pregnancy, there is a 25% chance that they will each contribute the Robinow syndrome gene and have an affected child.
syndrome Robinow
G A L E E N C Y C L O P E D I A O F G E N E T I C D I S O R D E R S |
1007 |
Robinow syndrome
|
|
|
|
|
|
|
|
|
|
|
Robinow Syndrome |
|
|
|
|||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Small genitalia |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||
Chest deformity (pectus excavatum) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||
|
|
|
Short forearms |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||
|
|
|
Broad thumbs |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
teeth |
|
|
|
|
|
|
|
||||||||||||
|
|
|
Large head |
Misaligned |
|
|
|
|
|
|
|
|
|
|
|
||||||||||||
|
|
Prominent forehead |
Small genitalia |
|
|
|
|||||||||||||||||||||
|
Triangle-shaped mouth |
Short stature |
|
|
|
||||||||||||||||||||||
Wrist abnormality (Madelung deformity) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Small chin |
|
|
|
|||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Prominent forehead |
|
|
|
|||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Small genitalia |
|
|
|
|||||||||
|
|
|
|
|
|
|
|
|
|
|
Chest deformity (pectus excavatum) |
|
|
|
|||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||
(Gale Group) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||
Mutations in the ROR2 gene are responsible for recessive Robinow syndrome. As of 2000, the exact function of the protein encoded by the ROR2 gene had not been determined, and the gene responsible for dominant Robinow syndrome had not been located.
Demographics
Both the dominant and recessive forms of Robinow syndrome are rare. Dominant Robinow syndrome does not appear to occur more frequently in any particular ethnic group. A significant proportion of recessive Robinow syndrome cases, however, have occurred in Czechoslovakia, Turkey, and the Middle East. In addition, some children with recessive Robinow syndrome have parents who are genetically related (consanguineous), such as first cousins. Parental consanguinity is sometimes seen in rare, autosomal recessive conditions, since people who are genetically related are more likely to carry the same recessive gene(s).
Signs and symptoms
The signs and symptoms of Robinow syndrome can be grouped into those that involve the face, those that affect the skeleton, and those affecting the genitalia. There is a good deal of overlap of symptoms between the dominant and recessive forms. In general, however, peo-
ple with recessive Robinow syndrome tend to be more severely affected.
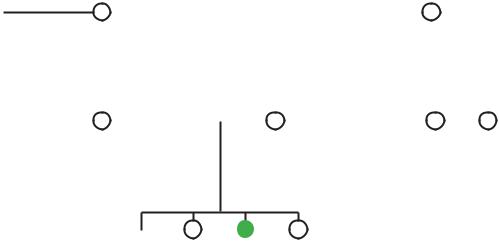
The facial features of Robinow syndrome include a flat nasal bridge, slightly upturned nose, triangularshaped mouth, protruding forehead (frontal bossing), wide space between the eyes (hypertelorism), wide eye openings, low-set ears, long philtrum (groove from nose to upper lip), small lower jaw (micrognathia), excessive growth of the gums, and crowding of teeth.
People with Robinow syndrome have what is known as acromesomelic brachymelia. Acromesomelic refers to bones at the end (acro) and in the middle (meso) of the limbs. Brachymelia is the medical term for short limbs. Thus, short limbs in Robinow syndrome are due to shortened bones in the hands, feet, lower arms, and lower legs. Dominant Robinow syndrome is associated with normal height to borderline short stature, while recessive Robinow syndrome always results in short stature. Abnormalities of the spine often involve misshapen or fused vertebrae (socalled segmentation defects), as well as scoliosis. Vertebral abnormalities are more frequent and more pronounced in the recessive form of Robinow syndrome. Ribs may be fused together or abnormally shaped, and this may lead to pectus excavatum (sunken breastbone).
Males with Robinow syndrome typically have a hypoplastic penis, and may have undescended testicles
1008 |
G A L E E N C Y C L O P E D I A O F G E N E T I C D I S O R D E R S |

(cryptorchidism). Females can have a small clitoris and hypoplastic labia. A dysfunctional sex-steroid response- and-feedback mechanism may be partly to blame for some of the signs of Robinow syndrome, particularly the genital anomalies.
Physical anomalies found less frequently in Robinow syndrome include heart defects, kidney abnormalities, cleft lip/palate, and hearing loss. Most individuals with Robinow syndrome have normal intelligence, but a few have mild mental retardation.
Diagnosis
The diagnosis of Robinow syndrome is made by physical examination. Several other genetic syndromes have some of the same physical signs as Robinow syndrome, which can make arriving at the correct diagnosis more difficult. However, the pattern of skeletal abnormalities in Robinow syndrome has a distinct appearance when seen on x rays, which may help in confirming the diagnosis. Testing of the ROR2 gene is theoretically possible for recessive Robinow syndrome, but would only be offered on a research basis, if at all. As of 2000, there were no laboratory tests available to aid in the diagnosis of dominant Robinow syndrome.
Treatment and management
There is no cure for either type of Robinow syndrome. Future research may help to determine if some type of hormone therapy can be used to treat the short stature and/or hypoplastic genitalia. Otherwise, no specific protocol is recommended for managing children and adults with either form of the condition. An orthopedic surgeon might be needed to address any problems that arise related to skeletal abnormalities, especially in the spine. Special educational intervention would be indicated for anyone with learning disabilities or mental retardation.
People with pronounced physical signs of the condition (short stature and facial features) may have difficulties with their self-image. Males with a hypoplastic penis can present a special problem. In those cases, added psychological and social support is particularly important. Genetic counseling should be offered to individuals/ families affected by Robinow syndrome to help them understand the condition, its inheritance, and any testing (including prenatal) that might be available.
Prognosis
Any one person with Robinow syndrome may have a good prognosis, depending on how well they cope with their particular symptoms. A child with recessive
Robinow syndrome is more likely to have long-term difficulties than a child with the dominant form of the condition, but no blanket statements can be made. Overall, life span should not be significantly decreased in most cases of Robinow syndrome, since the majority of affected individuals do not have life-threatening complications.
Resources
BOOKS
Jones, Kenneth Lyons. Smith’s Recognizable Patterns of
Human Malformation. 5th ed. Philadelphia: W.B.
Saunders Company, 1997.
ORGANIZATIONS
Robinow Syndrome Foundation. PO Box 1072, Anoka, MN 55303. (612) 434-1152. http://www.robinow.org .
OTHER
“Robinow syndrome.” National Library of Medicine (NLM). MedlinePlus. http://medlineplus.nlm.nih.gov/mesh/ jablonski/syndromes/syndrome_cgi?index=562.htm .
Scott J. Polzin, MS
Robinow-Silverman-Smith syndrome see
Robinow syndrome
Robinow-Sorauf syndrome see Saethre-
Chotzen syndrome
Romano-Ward syndrome see Long-QT syndrome
I Rothmund-Thomson
syndrome
Definition
Rothmund-Thomson syndrome (RTS) is an extremely rare inherited disorder that appears in infancy and features skin degeneration (atrophic dermatosis), clouding of the lenses of the eyes (juvenile cataracts), skeletal abnormalities, short stature, and an increased risk of skin and bone cancers.
Description
Rothmund-Thomson syndrome is usually first apparent between three and six months of age. This disorder is characterized by early sun sensitivity and progressive degeneration or wasting (atrophy) of the skin as well as scarring and abnormal pigmentation of the skin. Other characteristic signs include sparse hair, clouding of
syndrome Thomson-Rothmund
G A L E E N C Y C L O P E D I A O F G E N E T I C D I S O R D E R S |
1009 |

Rothmund-Thomson syndrome
K E Y T E R M S
Alopecia—Loss of hair or baldness.
Atrophic dermatosis—Wasting away of the skin.
Depigmentation—Loss of pigment or skin color.
Dysplastic—The abnormal growth or development of a tissue or organ.
Edema—Extreme amount of watery fluid that causes swelling of the affected tissue.
Frontal bossing—A term used to describe a rounded forehead with a receded hairline.
Hyperpigmentation—An abnormal condition characterized by an excess of melanin in localized areas of the skin, which produces areas that are much darker than the surrounding unaffected skin.
Hypogonadism—Small testes in men and scarse or irregular mentruation for females.
Keratosis—A raised thickening of the outer horny layer of the skin.
Microdontia—Small teeth.
Poikiloderma—A condition characterized by skin atrophy, widening of the small blood vessels (telangiectasia), and pigment changes giving a mottled appearance.
Prognathism—A protruding lower jaw.
Saddle nose—A sunken nasal bridge.
Telangiectasia—An abnormal widening of groups of small blood vessels in the skin.
the lenses of the eyes (juvenile cataracts), short stature, malformations of the face and head, teeth, nails, and bone, and other physical abnormalities. In rare cases, mental retardation may be present.
The syndrome was first described in 1868 by August von Rothmund, a German ophthalmologist, and in both 1923 and 1936 by Matthew S. Thomson, a British dermatologist. Both independently noted a familial disorder with cataracts, saddle nose, and skin degeneration. It is believed that Thomson’s finding was the same disease that was seen long before by Rothmund. Other names for Rothmund-Thomson syndrome include poikiloderma congenita and poikiloderma atrophicans with cataract.
Genetic profile
Rothmund-Thomson is attributed to a mutation in a gene located on chromosome 8. Mutations in the gene
RecQL4 (chromosomal locus 8q24), also called the Rothmund-Thomson gene, have been identified in four patients with Rothmund-Thomson syndrome.
Rothmund-Thomson syndrome is inherited as an autosomal recessive trait. This means that both parents have one copy of the Rothmund-Thomson gene but do not have the disease. Each of their children has a 25% chance of not having the gene, a 50% chance of having one Rothmund-Thomson gene (and, like the parents, being unaffected), and a 25% risk of having both Rothmund-Thomson genes and the disease.
Demographics
There is no specific population group that is at greater risk for this disorder, although it is more common in women (2:1). Evidence of Rothmund-Thomson syndrome has been found to occur in all races and many nationalities. The majority of affected people are from full-term pregnancies. As of the year 2001, a total of approximately 250 cases have been reported in English-speaking medical literature. The number of carriers for Rothmund-Thomson syndrome is unknown.
Signs and symptoms
The major characteristics of Rothmund-Thomson syndrome are skin abnormalities, short stature, juvenile cataracts, small hands, and delayed activities of the ovaries in females or testes in males. Symptoms vary from individual to individual.
Skin abnormalities usually appear in infancy, between three and six months of age. Skin changes begin as red inflamed patches, occasionally with blistering, on the cheeks along with swelling and then spread to other areas of the face, the arms and legs, and buttocks. Skin inflammation eventually subsides and a condition develops known as poikiloderma, characterized by abnormal widening (dilation) of groups of small blood vessels (telangiectasia), skin tissue degradation (atrophy), and patchy areas of abnormally decreased and/or unusually increased brown pigmentation (depigmentation and hyperpigmentation), giving the skin a mottled look. Skin that is exposed to the sun usually shows greater abnormalities. Sun sensitivity typically continues throughout the affected person’s life. Those with extreme sun sensitivity can develop thickening of the skin (keratosis) of the face, hands, and feet, or cancerous skin changes later in life. Affected individuals are at increased risk of developing skin cancers (basal cell carcinoma and squamous cell carcinoma) and bone cancer (osteosarcoma).
1010 |
G A L E E N C Y C L O P E D I A O F G E N E T I C D I S O R D E R S |
There are many other physical abnormalities that affect people with Rothmund-Thomson syndrome. Juvenile cataracts, the clouding of the lenses of the eyes, develop in almost half of the people with RTS between the ages of four and seven. Severe growth delays result in short stature throughout life. Skeletal abnormalities such as unusually small hands and feet are common. Less typical are stubby fingers and toes, underdeveloped (hypoplastic) or absent thumbs, and/or underdeveloped (hypoplastic) or missing forearm bones (ulna and radii). Hypogonadism, the deficient activity of the ovaries in females or testes in males, causes irregular menstruation in females, and delayed sexual development and reduced fertility in both males and females. Facial skeletal abnormalities include a triangularshaped face with a prominent forehead (frontal bossing), a sunken nasal bridge (saddle nose), and a protruding lower jaw (prognathism). Scalp hair is usually thin and fine, although alopecia (balding) occasionally occurs in early childhood. Often the eyebrows and eyelashes are sparse or absent. Dental abnormalities include excessive cavities, unusually small teeth (microdontia), or delayed or failure of teeth to erupt. Dysplastic, or abnormally developed nails are also seen in many people with Rothmund-Thomson syndrome.
Diagnosis
A diagnosis of Rothmund-Thomson syndrome is made based on clinical examination. There are no laboratory diagnostic tests. Mutations of the RecQL4 gene have been found in a few individuals with RTS. However, as of the year 2001, genetic testing is still on a research basis and is not available for diagnostic purposes.
There are no published diagnostic criteria. Diagnosis is usually based on the presence of the characteristic poikilodermatous rash in childhood, along with one or more of the following features: small stature, sparse or absent hair, cataracts, and cancer.
Treatment and management
Essential management of Rothmund-Thomson syndrome includes avoiding sun exposure and diligently using sunscreen that has both UVA and UVB protection.
An ophthalmologic evaluation for the detection and management of cataracts is recommended for affected people on an annual basis up to age 15. Surgical removal of significant cataracts may be necessary.
Because skin cancer is a risk, it is important to monitor the affected individual closely for lesions with
unusual color or texture. They should also be watched carefully for any signs and symptoms of osteosarcoma, a cancerous bone tumor, including bone pain, swelling, or a growing lesion on the arms or legs.
Pulsed-dye laser therapy has been used to treat the widening of small blood vessels (telangiectases). Medications called retinoids can reduce the potential for skin cancer. Keratolytic drugs are used to cause thick skin to swell, soften, and then fall away.
Prognosis
Individuals with Rothmund-Thomson syndrome usually have a normal life span, although an increased risk for bone and skin cancer has been found. Most affected individuals will have normal intelligence, however learning disabilities and mental retardation have been reported in a small number of patients.
Resources
PERIODICALS
Hall, Judith G., et al. “Rothmund-Thomson Syndrome with Severe Dwarfism.” American Journal of Diseases of
Children 134 (1980): 165–169.
Starr D. G., et al. “Non-Dermatological Complications and Genetic Aspects of the Rothmund-Thomson Syndrome.” Clinical Genetics 27 (1985): 102–104.
ORGANIZATIONS
National Organization for Rare Disorders (NORD). PO Box 8923, New Fairfield, CT 06812-8923. (203) 746-6518 or (800) 999-6673. Fax: (203) 746-6481. http://www
.rarediseases.org .
WEBSITES
Plon, Sharon E., MD, PhD, and Lisa L. Wang, MD. (October 6, 1999). “Rothmund-Thomson syndrome.” GeneClinics. University of Washington, Seattle. http://www
.geneclinics.org/profiles/rts .
Nina B. Sherak, MS, CHES
RSH syndrome see Smith-Lemli-Opitz syndrome
RSH/SLO syndrome see Smith-Lemli-Opitz syndrome
Rubinstein syndrome see Rubinstein-Taybi syndrome
syndrome Thomson-Rothmund
G A L E E N C Y C L O P E D I A O F G E N E T I C D I S O R D E R S |
1011 |
