
Gale Encyclopedia of Genetic Disorder / Gale Encyclopedia of Genetic Disorders, Two Volume Set - Volume 2 - M-Z - I
.pdf
specially trained to deal with issues of disabilities in children is often helpful in assessing problem areas and encouraging healthy development of self-esteem. Support groups and community organizations for people with Poland anomaly or other disabilities often prove useful as well.
After growth development is advanced enough (usually late adolescence or early adulthood), reconstructive plastic surgery may be offered, primarily to correct cosmetic appearance. The goal of reconstruction is to restore the natural contour of the chest wall while stabilizing the chest wall defect. Chest wall reconstruction must be tailored to the requirements of each patient, but often involves moving and grafting ribs and muscles from other parts of the body to reconstruct the chest wall and breast. In addition, bioengineered cartilage or breast implants can be used to help give the chest a more normal appearance. Hand abnormalities are treated according to the severity, and requires individual consultation with a reconstructive plastic surgeon.
Prognosis
The prognosis for people with Poland anomaly is excellent. Reconstructive surgery is safe and cosmetic corrections achieved can be significant. Associated symptoms of heart and kidney defects as well as cancer association are rare, but indicate that patients with Poland anomaly should be followed closely by a physician familiar with the condition.
Resources
BOOKS
Canale, S.T. Campbell’s Operative Orthopaedics. St. Louis:
Mosby, 1998.
Sabiston, D.C. Textbook of Surgery. Philadelphia: W.B.
Saunders, 1997.
PERIODICALS
Urschel, H.C. “Poland’s syndrome.” Chest Surgery Clinics of North America 10 (May 2000): 393–403.
ORGANIZATIONS
National Organization for Rare Disorders (NORD). PO Box 8923, New Fairfield, CT 06812-8923. (203) 746-6518 or (800) 999-6673. Fax: (203) 746-6481. http://www
.rarediseases.org .
WEBSITES
“Poland anomaly.” In Online Mendelian Inheritance in Man.
http://www3.ncbi.nlm.nih.gov/htbin-post/Omim/ dispmim?173800 .
Oren Traub, MD, PhD
Poland syndactyly see Poland anomaly
Poland syndrome see Poland anomaly
I Polycystic kidney disease
Definition
Polycystic kidney disease (PKD) is one of the most common of all life-threatening human genetic disorders. It is an incurable genetic disorder characterized by the formation of fluid-filled cysts in the kidneys of affected individuals. These cysts multiply over time. It was originally believed that the cysts eventually caused kidney failure by crowding out the healthy kidney tissue. It is now thought that the kidney damage seen in PKD is actually the result of the body’s immune system. The immune system, in its attempts to rid the kidney of the cysts, instead progressively destroys the formerly healthy kidney tissue.
Description
A healthy kidney is about the same size as a human fist. PKD cysts, which can be as small as the head of a pin or as large as a grapefruit, can expand the kidneys until each one is bigger than a football and weighs as much as 38 lbs (17 kg).
There are two types of PKD: infantile PKD, which generally shows symptoms prior to birth; and adult onset PKD. Individuals affected with infantile PKD are often stillborn. Among the liveborn individuals affected with infantile PKD, very few of these children survive to the age of two. The adult onset form of PKD is much more common. The time and degree of symptom onset in the adult form of PKD can vary widely, even within a single family with two or more affected individuals. Symptoms of this form of PKD usually start to appear between the ages of 20 and 50. Organ deterioration progresses more slowly in adult onset PKD than it does in the infantile form; but, if left untreated, adult onset PKD also eventually leads to kidney failure.
Genetic profile
Polycystic kidney disease is expressed as both a recessive and a dominant trait. A recessive genetic trait will not cause disease in a child unless it it inheritied from both parents. A dominant genetic trait can be inherited from just one parent. Those people affected with autosomal dominant PKD (ADPKD) have the much more common adult onset form. Those with autosomal recessive PKD (ARPKD) have the infantile form.
disease kidney Polycystic
G A L E E N C Y C L O P E D I A O F G E N E T I C D I S O R D E R S |
931 |

Polycystic kidney disease
K E Y T E R M S
Biopsy—The surgical removal and microscopic examination of living tissue for diagnostic purposes.
Cancer—A disease caused by uncontrolled growth of the body’s cells.
Computed tomography (CT) scan—An imaging procedure that produces a three-dimensional picture of organs or structures inside the body, such as the brain.
Cyst—An abnormal sac or closed cavity filled with liquid or semisolid matter.
Diuretics—Medications that increase the excretion of urine.
Kidney—Either of two organs in the lumbar region that filter the blood, excreting the end products of the body’s metabolism in the form of urine and regulating the concentrations of hydrogen, sodium, potassium, phosphate and other ions in the body.
Magnetic resonance imaging (MRI)—A technique that employs magnetic fields and radio waves to create detailed images of internal body structures and organs, including the brain.
Ultrasonogram—A procedure where high-fre- quency sound waves that cannot be heard by human ears are bounced off internal organs and tissues. These sound waves produce a pattern of echoes, which are then used by the computer to create sonograms or pictures of areas inside the body.
Uremic poisoning—Accumulation of waste products in the body.
There are mutations on at least three genes that cause adult onset PKD. Approximately 85% of these cases are known to arise from mutations in the PKD1 gene that has been mapped to a region on the short arm of chromosome 16 (16p13.3-p13.12). Another 10–15% of cases of adult onset PKD are thought to be caused by mutations in the PKD2 gene that has been mapped to a region on the long arm of chromosome 4 (4q21-q23). As of early 2001, it is thought that the remainder of the cases of PKD are caused by mutations in the PKD3 gene, which has not yet been mapped. This unidentified “PKD3 gene” may, in fact, be more than one gene.
Adult onset PKD is transmitted from parents to their offspring as a non-sex linked (autosomal) dominant trait.
This means that if either parent carries this genetic mutation, there is a 50% chance that their child will inherit this disease. In the case of two affected parents, there is a 75% probability that their children will be affected with adult onset PKD.
Infantile PKD is caused by a non-sex linked (autosomal) recessive genetic mutation that has been mapped to a region on the short arm of chromosome 6 (6p21). Both parents must be carriers of this mutation for their children to be affected with infantile PKD. In the case of two carrier parents, the probability is 25% that their child will be affected by infantile PKD.
Demographics
One of the most common of all life-threatening genetic diseases, PKD affects more than 60,000 Americans. Over 12.5 million people worldwide are affected with PKD. Approximately one in every 400 to 1000 people is affected with ADPKD. Another one in 10,000 are affected with ARPKD. PKD is observed in both males and females. PKD is also observed with equal probability among ethnic groups.
Signs and symptoms
A baby born with infantile PKD has floppy, low-set ears, a pointed nose, a small chin, and folds of skin surrounding the eyes (epicanthal folds). Large, rigid masses can be felt on the back of both thighs (flanks), and the baby usually has trouble breathing.
In the early stages of adult onset PKD, many people show no symptoms. Generally, the first symptoms to develop are: high blood pressure (hypertension); general fatigue; pain in the lower back or the backs of the thighs; headaches; and/or urinary tract infections accompanied by frequent urination.
As PKD becomes more advanced, the kidneys’ inability to function properly becomes more pronounced. The cysts on the kidney may begin to rupture and the kidneys tend to be much larger than normal. Individuals affected with PKD have a much higher rate of kidney stones than the rest of the population at this, and later stages, of the disease. Approximately 60% of those individuals affected with PKD develop cysts in the liver, while 10% develop cysts in the pancreas.
Because the kidneys are primarily responsible for cleaning the blood, individuals affected with PKD often have problems involving the circulatory system. These include: an underproduction of red blood cells which results in an insufficient supply of oxygen to the tissues and organs (anemia); an enlarged heart (cardiac hyper-
932 |
G A L E E N C Y C L O P E D I A O F G E N E T I C D I S O R D E R S |
trophy) probably caused by long term hypertension; and a leakage of the valve between the left chambers (auricle and ventricle) of the heart (mitral valve prolapse). Less common (affecting approximately 5% of PKD patients) are brain aneurysms. An aneurysm is an abnormal and localized bulging of the wall of a blood vessel. If an aneurysm within the brain leaks or bursts, it may cause a stroke or even death.
Other health problems associated with adult onset PKD include: chronic leg or back pain; frequent infections; and, herniations of the groin and abdomen, including herniation of the colon (diverticular disease). A herniation, or hernia, is caused when a tissue, designed to hold the shape of an underlying tissue, becomes weakened at a particular spot. The underlying tissue pushes against this weakened area until the area is no longer able to hold back the underlying tissue and the area forms an abnormal bulge through which the underlying tissue projects. Diverticular disease is caused by a weakening of the muscles that hold the shape of the organs of the digestive tract. These muscles weaken allowing these organs, particularly one section of the colon, to form sac-like projections that can trap feces and become infected, or rupture.
In the final stages of PKD, the major symptom is kidney (renal) failure. Renal failure is indicated by an increase of nitrogen (in the form of urea) in the blood (uremia, or uremic poisoning). Uremia is a rapidly fatal condition without treatment.
Diagnosis
Many patients who have PKD do not have any symptoms. Their condition may not be discovered unless tests that detect it are performed for other reasons.
The cyst covered kidney on the left is substantially larger than the normal kidney on the right. (Photo Researchers, Inc.)
When symptoms of PKD are present, the diagnostic procedure begins with a family medical history and physical examination of the patient. If several family members have PKD, there is a strong likelihood that the patient has it too. If the disease is advanced, the doctor will be able to feel the patient’s enlarged kidneys. Heart murmur, high blood pressure, and other signs of cardiac impairment can also be detected.
Urinalysis and a blood test called creatine clearance can indicate how effectively the kidneys are functioning. Scanning procedures using intravenous dye reveal kidney enlargement or deformity and scarring caused by cysts. Ultrasound and computed tomography scans (CT scans) can reveal kidney enlargement and the cysts that caused it. CT scans can highlight cyst-damaged areas of the kidneys. A sampling of the kidney cells (biopsy) may be performed to verify the diagnosis.
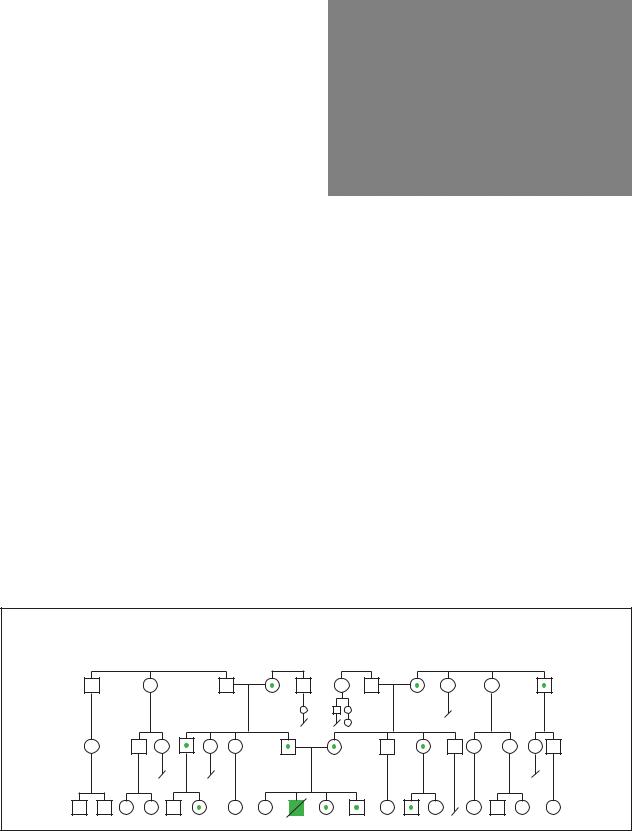
Polycystic Kidney Disease |
Autosomal Recessive |
(Gale Group)
disease kidney Polycystic
G A L E E N C Y C L O P E D I A O F G E N E T I C D I S O R D E R S |
933 |
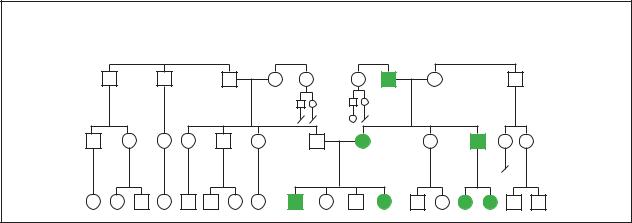
Polycystic kidney disease
Polycystic Kidney Disease
Autosomal Dominant
(Gale Group)
Treatment and management
There is no way to prevent cysts from forming or becoming enlarged, or to prevent PKD from progressing to kidney failure. Treatment goals include preserving healthy kidney tissue; controlling symptoms; and preventing infection and other complications.
If adult PKD is diagnosed before symptoms become evident, urinalysis and other diagnostic tests are performed at six-week intervals to monitor the patient’s health status. If results indicate the presence of infection or another PKD-related health problem, aggressive antibiotic therapy is initiated to prevent inflammation that can accelerate disease progression; iron supplements or infusion of red blood cells are used to treat anemia; and surgery may be needed to drain cysts that bleed, cause pain, have become infected, or interfere with normal kidney function.
Lowering high blood pressure can slow loss of kidney function. Blood-pressure control, which is the cornerstone of PKD treatment, is difficult to achieve. Therapy may include anti-hypertensive medications, diuretic medications, and/or a low-salt diet. As kidney function declines, some patients need dialysis and/or a kidney transplant.
There is no known way to prevent PKD, but certain lifestyle modifications can help control symptoms. People who have PKD should not drink heavily or smoke. They should not use aspirin, non-steroidal antiinflammatory drugs (NSAIDs), or other prescription or over-the-counter medications that can impair kidney function. Individuals affected with PKD should eat a balanced diet, exercise regularly, and maintain a weight appropriate for their height, age, and body type. Regular medical monitoring is also recommended.
Prognosis
There is no known cure for PKD. Those affected with infantile PKD generally die before the age of two. In adults, untreated disease can be rapidly fatal or continue to progress slowly, even after symptoms of kidney failure appear. About half of all adults with PKD also develop kidney failure. Unless the patient undergoes dialysis or has a kidney transplant, they usually do not survive more than four years after diagnosis.
Although medical treatment can temporarily alleviate symptoms of PKD, the expanding cysts continue to increase pressure on the kidneys. Kidney failure and uremic poisoning (accumulation of waste products the body is unable to eliminate) generally cause death about 10 years after symptoms first appear.
Medications used to fight cancer and reduce elevated cholesterol levels have slowed the advance of PKD in laboratory animals. They may soon be used to treat adults and children who have the disease. Researchers are also evaluating the potential benefits of anti-inflamma- tory drugs, which may prevent the scarring that destroys kidney function.
Resources
BOOKS
Shaw, Michael, ed. Everything You Need to Know About Diseases. Springhouse, Penn.: Springhouse Corporation, 1996.
PERIODICALS
Koptides, M. and C. Deltas. “Autosomal dominant polycystic kidney disease: Molecular genetics and molecular pathogenesis.” Human Genetics (August 2000): 115–26.
Pei, Y., A. Paterson, K. Wang, N. He, et al. “Bilineal disease and trans-heterozygotes in autosmal dominant polycystic kidney disease.” American Journal of Human Genetics
(February 2001): 355–63.
934 |
G A L E E N C Y C L O P E D I A O F G E N E T I C D I S O R D E R S |

ORGANIZATIONS
Polycystic Kidney Disease Foundation. 4901 Main Street, Kansas City, MO 64112-2634. (800) PKD-CURE.http://www.pkdcure.org/home.htm .
WEBSITES
Brochert, Adam, MD. “Polycystic Kidney Disease.” (September 4, 2000). HealthAnswers. http://www.healthanswers
.com/library/library_fset.asp .
Cooper, Joel R. “Treating Polycystic Kidney Disease. What Does the Future Hold?” Coolware, Inc. http://www
.coolware.com/health/medical_reporter/kidney1.html . Online Mendelian Inheritance in Man (OMIM). http://www
.ncbi.nlm.nih.gov/htbin-post/Omim/dispmim?600595 . (15 February 2001).
Polycystic Kidney Disease Access Center.
http://www.nhpress.com/pkd/ .
Paul A. Johnson
I Polycystic ovary syndrome
Definition
Polycystic ovary syndrome (PCOS), formerly SteinLeventhal syndrome, is a disorder in which women do not experience normal release of eggs from the ovaries, they have an abnormal production of male hormones, and their body is resistant to the effects of the hormone insulin. The disorder results in infertility, abnormal masculinization, and increased risk of developing heart disease and certain cancers.
Description
The normal function of the female reproductive system is complex, requiring the interplay of different organ systems. One set of important organs are the ovaries. The ovaries are two small structures contained in the lower abdomen, on either side of the uterus, that contain small immature eggs, called ova. Ova are stored within the ovaries in individual structures called follicles.
In a monthly cycle, a part of the brain called the pituitary gland secretes two substances into the blood stream—lutenizing hormone (LH) and follicle-stimulat- ing hormone (FSH). As certain levels of LH and FSH build in the blood stream, the follicles of the eggs begin to swell and grow, creating cysts. Eventually, the changing levels of LH and FSH cause one of the ovarian cysts to burst open, releasing a mature egg. This process by which an egg is released from the ovary is called ovulation.
Once a mature egg is released from the ovary, it passes into the fallopian tubes, tube-like structures that
are passageways to the uterus. If sperm cells from the male are present within the fallopian tubes, they will join with the egg in a process called fertilization. The fertilized egg can then pass into the uterus and implant into the thickened wall of the uterus where it can develop into a fetus. If no sperm cells are present, the mature egg goes unfertilized and is lost, along with the thickened later of the uterus, in a monthly process called menstruation.
Polycystic ovary syndrome (PCOS), first described by I. F. Stein and M. L. Leventhal in 1935, is a disorder in which normal ovulation does not occur. The term “polycystic” derives from the fact that the egg-containing cysts in the ovaries do not burst open, resulting in enlarged ovaries containing many swelled cysts. The reason for this problem in ovulation is unclear, however several abnormalities have been characterized in women with PCOS. First, there is a disturbance in the production of LH and FSH by the pituitary, leading to altered levels of the substances in the blood stream. There is also evidence that the ovaries do not respond appropriately to the FH and LSH that is present. Second, there is an abnormal over-production of male hormones, called androgens, by the ovaries and the adrenal gland. Finally, women with PCOS are resistant to the effects of the hormone, insulin. Insulin is a hormone made in the pancreas that is responsible for transport of sugar from the blood into the cells. While these abnormalities have been well characterized, it is unclear whether they cause PCOS, or whether they are a result the disease.
Genetic profile
Women diagnosed with PCOS frequently have relatives with symptoms similar to that seen in the disorder. As a result of these observations, many scientists have proposed that genetic factors play a role in the disease. Over the past few decades, researchers have identified families in which PCOS appears to be inherited with an autosomal dominant or an X-linked pattern. However, these cases are rare and do not hold true for the majority of people with PCOS.
Current theories suggest that different genetic changes may result in PCOS or that multiple genetic factors are needed for the full manifestation of the disease. Abnormalities in several genes have been associated with PCOS, including mutations in the genes for follistatin (locus 5p14), 17-beta-hydroxysteroid dehydrogenase (locus 9p22), and a cytochrome P450 enzyme (locus 15q23-q24). Each of these genes plays a different role in the response to LH and FSH, or in the conversion of male hormones to female hormones, although their relationship to PCOS is unclear. Ongoing research is likely to identify further genetic mutations that are associated with PCOS.
syndrome ovary Polycystic
G A L E E N C Y C L O P E D I A O F G E N E T I C D I S O R D E R S |
935 |

Polycystic ovary syndrome
K E Y T E R M S
Acanthosis nigricans—A skin condition characterized by darkly pigmented areas of velvety wart-like growths. Acanthosis nigricans usually affects the skin of the armpits, neck, and groin.
Androgens—A group of steroid hormones that stumulate the development of male sex organs and male secondary sexual characteristics.
Diabetes—An inability to control the levels of sugar in the blood due to an abnormality in the production of, or response to, the hormone insulin.
Fallopian tube—Either of a pair of tubes that conduct ova from the ovaries to the uterus.
Follicle—A pouch-like depression.
Follicle-stimulating hormone (FSH)—A hormone that stimulates estrogen in females and stimulates sperm production in males.
Hirsuitism—The presence of coarse hair on the face, chest, upper back, or abdomen in a female as a result of excessive androgen production.
Hormone—A chemical messenger produced by the body that is involved in regulating specific bodily functions such as growth, development, and reproduction.
Infertility—Inability in a woman to become pregnant.
Insulin—A hormone produced by the pancreas that is secreted into the bloodstream and regulates blood sugar levels.
Lutenizing hormone (LH)—A hormone secreted by
the pituitary gland that regulates the menstrual cycle and triggers ovulation in females. In males it stimulates the testes to produce testosterone.
Masculinization—Development of excess body and facial hair, deepening of the voice, and increase in muscle bulk in a female due to a hormone disorder.
Menstruation—Discharge of blood and fragments of the uterine wall from the vagina in a monthly cycle in the absence of pregnancy.
Mutation—A permanent change in the genetic material that may alter a trait or characteristic of an individual, or manifest as disease, and can be transmitted to offspring.
Ova—Another name for the egg cells that are located in the ovaries.
Ovary—The female reproductive organ that produces the reproductive cell (ovum) and female hormones.
Ovulation—The monthly process by which an ovarian follicle or cyst ruptures, releasing a mature egg cell.
Pituitary gland—A small gland at the base of the brain responsible for releasing many hormones, including luteinizing hormone (LH) and follicl-stim- ulating hormone (FSH).
Uterus—A muscular, hollow organ of the female reproductive tract. The uterus contains and nourishes the embryo and fetus from the time the fertilized egg is implanted until birth.
Demographics |
Signs and symptoms |
Estimates of the prevalence of PCOS in the general population have ranged from 2-20% with recent studies suggesting that 3-6% of women of reproductive age are affected by the disorder. This makes PCOS one of the most common hormone disorders in women of reproductive age.
It is unclear whether this disease is distributed uniformly among different geographical areas and ethnic groups, however, studies performed in 1999 show the prevalence of this disorder in the United States is just over 3% in African-American females and almost 5% in Caucasian females. The prevalence of PCOS in Greek women was shown to be higher, nearly 7%.
The first signs of PCOS tend to manifest at puberty. As a result of the failure to ovulate normally, young women with PCOS may fail to menstruate or menstruate only erratically. A small percentage of women may have normal menstrual cycles. Women affected with PCOS often experience infertility, an inability to become pregnant. Additionally, women with PCOS tend to gain weight, and 70% eventually become obese.
The overproduction of androgens leads to changes in the body that are more typical of male development. For example, approximately 70% of women with PCOS will show hair growth on the face, chest, stomach, and thighs (hirsuitism). Simultaneously, they show thinning of the
936 |
G A L E E N C Y C L O P E D I A O F G E N E T I C D I S O R D E R S |

hair more typical of male-pattern baldness. Other male characteristics, such as deep voice, acne, and increased sex drive may also be present, and affected women often have decreased breast size.
Women with PCOS do not respond appropriately to the hormone, insulin. As a result, 15% of women with PCOS may develop high levels of sugar in the blood later in life, a condition known as diabetes. Resistance to insulin is also associated with dark, warty skin growths in the groin and armpits, known as acanthosis nigricans.
Untreated PCOS is a risk factor for the development of several dangerous conditions. The hormone abnormalities in PCOS place women at considerable risk for endometrial cancer and possibly breast cancer. The risk of endometrial cancer is three times higher in women with PCOS than in normal women, and small studies suggest that the risk of breast cancer may by three to four times higher. PCOS also results in increased risk of high blood pressure, diabetes, and high cholesterol, all of which contribute to heart disease and stroke.
Diagnosis
A diagnostic search for PCOS is usually initiated when women experience an absence of menstrual periods for at least six months, an inability to become pregnant, and/or abnormal hair growth or acne. A comprehensive physical exam performed at that time may reveal excessive body hair, low voice, acanthosis nigricans, or obesity. Enlarged ovaries are also identifiable on pelvic examination in about 50% of patients.
Blood tests can be performed that may yield results consistent with PCOS, including abnormal levels of LH and FSH (typically in a ratio of 3:1), abnormally high levels of androgens (testosterone, DHEA, DHEAS), abnormally high levels of insulin, and abnormally low levels of a substance called sex hormone-binding globulin. In addition, a physician may perform a diagnostic test called a “progesterone challenge”. In this test, a physician administers a hormone called progesterone to the patient to determine if it will provoke menstruation. If menstruation does occur in response to the progesterone, it is likely that a patient has PCOS.
Finally, an ultrasound examination of the ovaries may be performed to determine if large cystic follicles can be documented. With this approach, the diagnosis of PCOS is based on the finding of more than eight enlarged follicles in the ovary.
Treatment and management
There is no cure for PCOS, thus treatment focuses on several goals, including the restoration of the menstrual
Females affected with Stein-Leventhal syndrome often have excessive facial hair, known as hirsutism. (Photo Researchers, Inc.)
cycle, blocking the effect of androgens, reducing insulin resistance, lowering the risk of cancer and heart disease, and possibly restoring ovulation and fertility.
In patients who do not desire pregnancy, hormones can be administered in the form of birth control pills, which may result in normal menstrual cycles, decreased hair growth and acne, and a lower risk of developing endometrial cancer. Although women will note a decrease in hair growth after approximately six months of treatment with birth control pills, additional cosmetic hair removal therapy is often necessary. In women who do not respond appropriately to birth control pills, another medication known as luprolide (Lupron) can be used, but with more long term side effects (e.g., hot flushes, bone demineralization, atrophic vaginitis).
Other types of medication can be used to block the effects of androgens. When these medications are taken with birth control pills, 75% of women report decreased body hair growth. The most commonly used medications to block androgen effects are spironolactone (Aldactone), flutamide (Eulexin), and cyproterone (Cyprostat).
Treatment with medications that restore the body’s normal response to insulin has been shown to decrease LH and androgen levels. Recent studies have demonstrated that such agents restore the menstrual cycle in 68-95% of patients treated for as short a time as four to six months. One of the most commonly used medications to improve the effects of insulin is metformin (Glucophage).
In patients who are trying to become pregnant, a physician can administer medications that will cause ovulation. The main medication used to induce ovulation is clomiphene citrate (Clomid). Ovulation is successful in approximately 75% of women treated with clomiphene, but only 30-40% of women will successfully become
syndrome ovary Polycystic
G A L E E N C Y C L O P E D I A O F G E N E T I C D I S O R D E R S |
937 |

Porphyrias
pregnant. Another medication, follitropin alpha (Gonal- F), has achieved pregnancy rates of 58-82%, but may cause more side effects and frequently results in more than one baby per pregnancy.
Some women who do not respond to medications may undergo surgery to remove portions of the ovary. For reasons that are not completely understood, removal of a portion of the ovary may result in some degree of normal menstrual cycles.
While medications and surgery may provide a degree of symptomatic relief for some women, other simultaneous strategies can increase their benefits. Behavior modifications, including weight reduction, diet, and exercise, are recommended for all women with PCOS. As little as a 7% reduction in body weight can lead to a significant decrease in androgen levels and to the resumption of ovulation in obese women with PCOS. Cosmetic techniques, including electrolysis (destruction of the hair follicle using electricity) and laser therapy, may be used to decrease hair growth. Finally, women should be seen regularly for full physical examinations including pelvic exams to aid in the early detection of ovarian, breast, and uterine cancer and should be managed by an interdisciplinary health care team including a primary care physician, obstetrician/gynecologist and reproductive endocrinologist.
Prognosis
While PCOS is one of the most common hormone disorders in young women, proper diagnosis and treatment has greatly increased the quality of life in these individuals. Roughly half of women with PCOS will be able to achieve pregnancy, and about three-fourths will see reduction in masculine traits such as hair growth with proper medical treatment. Initiation of vigorous exercise and a restricted diet may result in even better outcomes. It should be noted that patients with PCOS are at higher risk of developing diabetes, heart disease, and certain cancers and should be seen regularly by a physician. Barring these developments, lifespan in patients with PCOS is approximately the same as the general population.
Resources
BOOKS
“Disorders of Ovarian Function” In Williams Textbook of Endocrinology, edited by J. D. Wilson. Philadelphia: W.B. Saunders, 1998, pp 781-801.
“Hypofunction of the Ovaries.” In Nelson Textbook of Pediatrics, edited by R.E. Behrman. Philadelphia: W.B. Saunders, 2000, pp 1752-1758.
Kistner’s Gynecology and Women’s Health, edited by K. J. Ryan. St. Louis: Mosby, 1999.
PERIODICALS
Hunter, M.H. “Polycystic Ovary Syndrome: It’s Not Just Infertility.” American Family Physician 62(September 2000): 1079-1088.
ORGANIZATIONS
Polycystic Ovarian Syndrome Association. PO Box 80517, Portland, OR 97280. (877) 775-PCOS. http://www.pcosupport.org .
WEBSITES
“Polycystic Ovary Syndrome 1.” OMIM—Online Mendelian Inheritance in Man. http://www.ncbi.nlm.nih.gov/ entrez/dispomim.cgi?id=184700 .
Oren Traub, MD, PhD
Polyps and spots syndrome see Peutz-
Jeghers syndrome
Polysplenia syndrome see Asplenia
Pompe disease see Acid maltase deficiency
I Porphyrias
Definition
The porphyrias are disorders in which the body produces too much porphyrin and insufficient heme (an ironcontaining non-protein portion of the hemoglobin molecule). Porphyrin is a foundation structure for heme and certain enzymes. Excess porphyrins are excreted as waste in the urine and stool. Overproduction and overexcretion of porphyrins causes low, unhealthy levels of heme and certain important enzymes creating various physical symptoms.
Description
Biosynthesis of heme is a multistep process that begins with simple molecules and ends with a large, complex heme molecule. Each step of the chemical pathway is directed by its own task-specific protein, called an enzyme. As a heme precursor molecule moves through each step, an enzyme modifies the precursor in some way. If a precursor molecule is not modified, it cannot proceed to the next step, causing a build-up of that specific precursor.
This situation is the main characteristic of the porphyrias. Owing to a defect in one of the enzymes of the heme biosynthesis pathway, protoporphyrins or porphyrins (heme precursors) are prevented from proceeding
938 |
G A L E E N C Y C L O P E D I A O F G E N E T I C D I S O R D E R S |
further along the pathway. These precursors accumulate at the stage of the enzyme abnormality causing an array of physical symptoms in an affected person. Specific symptoms depend on the point at which heme biosynthesis is blocked and which precursors accumulate. In general, the porphyrias primarily affect the skin and the nervous system. Symptoms can be debilitating or life threatening in some cases. Porphyria is most commonly an inherited condition. It can also, however, be acquired after exposure to poisonous substances.
Heme
Heme is produced in several tissues in the body, but its primary biosynthesis sites are the liver and bone marrow. Heme synthesis for immature red blood cells, namely the erythroblasts and the reticulocytes, occurs in the bone marrow.
Although production is concentrated in the liver and bone marrow, heme is utilized in various capacities in virtually every tissue in the body. In most cells, heme is a key building block in the construction of factors that oversee metabolism and transport of oxygen and energy. In the liver, heme is a component of several vital enzymes, particularly cytochrome P450. Cytochrome P450 is involved in the metabolism of chemicals, vitamins, fatty acids, and hormones; it is very important in transforming toxic substances into easily excretable materials. In immature red blood cells, heme is the featured component of hemoglobin. Hemoglobin is the red pigment that gives red blood cells their characteristic color and their essential ability to transport oxygen.
Heme biosynthesis
The heme molecule is composed of porphyrin and an iron atom. Much of the heme biosynthesis pathway is dedicated to constructing the porphyrin molecule. Porphyrin is a large molecule shaped like a four-leaf clover. An iron atom is placed at its center point in the last step of heme biosynthesis.
The production of heme may be compared to a factory assembly line. At the start of the line, raw materials are fed into the process. At specific points along the line, an addition or adjustment is made to further development. Once additions and adjustments are complete, the final product rolls off the end of the line.
The heme “assembly line” is an eight-step process, requiring eight different and properly functioning enzymes:
1.delta-aminolevulinic acid synthase
2.delta-aminolevulinic acid dehydratase
3.porphobilogen deaminase
4.uroporphyrinogen III cosynthase
5.uroporphyrinogen decarboxylase
6.coproporphyrinogen oxidase
7.protoporphyrinogen oxidase
8.ferrochelatase
The control of heme biosynthesis is complex. Various chemical signals can trigger increased or decreased production. These signals can affect the enzymes themselves or the production of these enzymes, starting at the genetic level. For example, one point at which heme biosynthesis may be controlled is at the first step. When heme levels are low, greater quantities of delta-aminolevulinic acid (ALA) synthase are produced. As a result, larger quantities of heme precursors are fed into the biosynthesis pathway to step up heme production.
Porphyrias
Under normal circumstances, when heme concentrations are at an appropriate level, precursor production decreases. However, a glitch in the biosynthesis path- way—represented by a defective enzyme—means that heme biosynthesis does not reach completion. Because heme levels remain low, the synthesis pathway continues to churn out precursor molecules in an attempt to correct the heme deficit.
The net effect of this continued production is an abnormal accumulation of precursor molecules and development of some type of porphyria. Each type of porphyria corresponds with a specific enzyme defect and an accumulation of the associated precursor. Although there are eight steps in heme biosynthesis, there are only seven types of porphyrias; a change in ALA synthase activity does not have a corresponding porphyria.
Enzymes involved in heme biosynthesis display subtle, tissue-specific variations; therefore, heme biosynthesis may be impeded in the liver, but normal in the immature red blood cells, or vice versa. Incidence of porphyria varies widely between types and occasionally by geographic location. Although certain porphyrias are more common than others, their greater frequency is only relative to other types. All porphyrias are considered to be rare disorders.
In the past, the porphyrias were divided into two general categories based on the location of the porphyrin production. Porphyrias affecting heme biosynthesis in the liver were referred to as hepatic porphyrias. Porphyrias affecting heme biosynthesis in immature red blood cells were referred to as erythropoietic porphyrias (erythropoiesis is the process through which red blood cells are produced). As of 2001, porphyrias are usually
Porphyrias
G A L E E N C Y C L O P E D I A O F G E N E T I C D I S O R D E R S |
939 |

Porphyrias
K E Y T E R M S
Autosomal dominant—A pattern of genetic inheritance where only one abnormal gene is needed to display the trait or disease.
Autosomal recessive—A pattern of genetic inheritance where two abnormal genes are needed to display the trait or disease.
Biosynthesis—The manufacture of materials in a biological system.
Bone marrow—A spongy tissue located in the hollow centers of certain bones, such as the skull and hip bones. Bone marrow is the site of blood cell generation.
Enzyme—A protein that catalyzes a biochemical reaction or change without changing its own structure or function.
Erythropoiesis—The process through which new red blood cells are created; it begins in the bone marrow.
Erythropoietic—Referring to the creation of new red blood cells.
Gene—A building block of inheritance, which contains the instructions for the production of a particular protein, and is made up of a molecular sequence found on a section of DNA. Each gene is found on a precise location on a chromosome.
Hematin—A drug administered intravenously to halt an acute porphyria attack. It causes heme biosynthesis to decrease, preventing the further accumulation of heme precursors.
Heme—The iron-containing molecule in hemoglobin that serves as the site for oxygen binding.
Hemoglobin—Protein-iron compound in the blood that carries oxygen to the cells and carries carbon dioxide away from the cells.
Hepatic—Referring to the liver.
Neuropathy—A condition caused by nerve damage. Major symptoms include weakness, numbness, paralysis, or pain in the affected area.
Porphyrin—A large molecule shaped like a fourleaf clover. Combined with an iron atom, it forms a heme molecule.
Protoporphyrin—A precursor molecule to the porphyrin molecule.
grouped into acute and non-acute types. Acute porphyrias produce severe attacks of pain and neurological effects. Non-acute porphyrias present as chronic diseases.
The acute porphyrias, and the heme biosynthesis steps at which enzyme problems occur, are:
•ALA dehydratase deficiency porphyria (step 2). This porphyria type is very rare. The inheritance pattern appears to be autosomal recessive. In autosomal recessively inherited disorders a person must inherit two defective genes, one from each parent. A parent with only one gene for an autosomal recessive disorder does not display symptoms of the disease.
•Acute intermittent porphyria (step 3). Acute intermittent porphyria (AIP) is also known as Swedish porphyria, pyrroloporphyria, and intermittent acute porphyria. AIP is inherited as an autosomal dominant trait, which means that only one copy of the abnormal gene needs to be present for the disorder to occur. Simply inheriting this gene, however, does not necessarily mean that a person will develop the disease. Approximately five to 10 per 100,000 persons in the United States carry a gene for AIP, but only 10% of these people ever develop symptoms of the disease.
•Hereditary coproporphyria (step 6). Hereditary coproporphyria (HCP) is inherited in an autosomal dominant manner. As with all porphyrias, it is an uncommon ailment. By 1977, only 111 cases of HCP were recorded; in Denmark, the estimated incidence is two in one million people.
•Variegate porphyria (step 7). Variegate porphyria (VP) is also known as porphyria variegata, protocoproporphyria, South African genetic porphyria, and Royal malady (supposedly King George III of England and Mary, Queen of Scots, had VP). VP is inherited in an autosomal dominant manner and is especially prominent in South Africans of Dutch descent. Among that population, the incidence is approximately three in 1,000 persons. It is estimated that there are 10,000 cases of VP in South Africa. Interestingly, it appears that the affected South Africans are descendants of two Dutch settlers who came to South Africa in 1680. Among other populations, the incidence of VP is estimated to be one to two cases per 100,000 persons.
The non-acute porphyrias, and the steps of heme biosynthesis at which they occur, are:
•Congenital erythropoietic porphyria (step 4). Congenital erythropoietic porphyria (CEP) is also called Gunther’s disease, erythropoietic porphyria, congenital porphyria, congenital hematoporphyria, and erythropoietic uroporphyria. CEP is inherited in an autosomal recessive manner. It is a rare disease, esti-
940 |
G A L E E N C Y C L O P E D I A O F G E N E T I C D I S O R D E R S |
