
Gale Encyclopedia of Genetic Disorder / Gale Encyclopedia of Genetic Disorders, Two Volume Set - Volume 2 - M-Z - I
.pdf
only some cells of the body have the extra chromosome error. The condition in which only some of the cells in the body have the extra chromosome is called mosaicism.
Seventy-five to 80% of the cases of Patau syndrome are caused by a trisomy of chromosome 13. Some of these cases are the result of a total trisomy, while others are the result of a partial trisomy. Partial trisomy generally causes less severe physical symptoms than full trisomy. Ten percent of these cases are of the mosaic type, in which only some of the body’s cells have the extra chromosome. The physical symptoms of the mosaic form of Patau syndrome depends on the number and type of cells that carry the trisomy.
Most cases of trisomy are not passed on from one generation to the next. Usually they result from a malfunction in the cell division (mitosis) that occurs after conception. At least 75% of the cases of Patau syndrome are caused by errors in chromosome replication that occur after conception. The remaining 25% are caused by the inheritance of translocations of chromosome 13 with other chromosomes within the parental chromosomes. In these cases, a portion of another chromosome switches places with a portion of chromosome 13. This leads to errors in the genes on both chromosome 13 and the chromosome from which the translocated portion originated.
Demographics
Patau syndrome occurs in approximately one in 10,000 live births. In many cases, miscarriage occurs and the fetus does not survive to term. In other cases, the affected individual is stillborn. As appears to be the case in all trisomies, the risks of Patau syndrome seem to increase with the mother’s age, particularly if she is over 30 when pregnant. Male and female children are equally affected, and the syndrome occurs in all races.
Signs and symptoms
The severity and symptoms of Patau syndrome vary with the type of chromosomal anomaly, from extremely serious conditions to nearly normal appearance and functioning. Full trisomy 13, which is present in the majority of the cases, results in the most severe and numerous internal and external abnormalities. Commonly, the forebrain fails to divide into lobes or hemispheres (holoprosencephaly) and the entire head is unusually small (microcephaly). The spinal cord may protrude through an opening in the vertebrae of the spinal column (myelomeningocele). Children who survive infancy have profound mental retardation and may experience seizures.
K E Y T E R M S
Aminocentesis—A procedure performed at 16-18 weeks of pregnancy in which a needle is inserted through a woman’s abdomen into her uterus to draw out a small sample of the amniotic fluid from around the baby. Either the fluid itself or cells from the fluid can be used for a variety of tests to obtain information about genetic disorders and other medical conditions in the fetus.
Chorionic villus sampling (CVS)—A procedure used for prenatal diagnosis at 10-12 weeks gestation. Under ultrasound guidance a needle is inserted either through the mother’s vagina or abdominal wall and a sample of cells is collected from around the fetus. These cells are then tested for chromosome abnormalities or other genetic diseases.
Chromosome—A microscopic thread-like structure found within each cell of the body and consists of a complex of proteins and DNA. Humans have 46 chromosomes arranged into 23 pairs. Changes in either the total number of chromosomes or their shape and size (structure) may lead to physical or mental abnormalities.
Karyotyping—A laboratory procedure in which chromosomes are separated from cells, stained, and arranged so that their structure can be studied under the microscope.
Mosaicism—A genetic condition resulting from a mutation, crossing over, or nondisjunction of chromosomes during cell division, causing a variation in the number of chromosomes in the cells.
Translocation—The transfer of one part of a chromosome to another chromosome during cell division. A balanced translocation occurs when pieces from two different chromosomes exchange places without loss or gain of any chromosome material. An unbalanced translocation involves the unequal loss or gain of genetic information between two chromosomes.
Trisomy—The condition of having three identical chromosomes, instead of the normal two, in a cell.
Ultrasound—An imaging technique that uses sound waves to help visualize internal structures in the body.
syndrome Patau
G A L E E N C Y C L O P E D I A O F G E N E T I C D I S O R D E R S |
891 |

Patau syndrome
A severe complication that may result in infants with Patau syndrome is synopthamia, in which the eyes are fused together in the center of the face. (Photo Researchers, Inc.)
Incomplete development of the optic (sight) and olfactory (smell) nerves often accompany the brain abnormalities described above. The eyes may be unusually small (microphthalmia) or one eye may be absent (anophthalmia). The eyes are sometimes set close together (hypotelorism) or even fused into a single structure. Incomplete development of any structures in the eye (coloboma) or failure of the retina to develop properly (retinal dysplasia) will also produce vision problems. Individuals with Patau syndrome may be born either partially or totally deaf and many are subject to recurring ear infections.
The facial features of many individuals with Patau syndrome appear flattened. The ears are generally malformed and low-set. Frequently, a child with trisomy 13 has a cleft lip, a cleft palate, or both. Other physical characteristics include loose folds of skin at the back of the neck, extra fingers or toes (polydactyly), permanently flexed (closed) fingers (camptodactyly), notice-
ably prominent heels, “rocker-bottom foot,” and missing ribs. Genital malformations are common in individuals affected with Patau syndrome and include undescended testicles (cryptorchidism), an abnormally developed scrotum, and ambiguous genitalia in males, or an abnormally formed uterus (bicornuate uterus) in females.
In nearly all cases, affected infants have respiratory difficulties and heart defects, including atrial and ventricular septal defects (holes between chambers of the heart); malformed ducts that cause abnormal direction of blood flow (patent ductus arteriosus); holes in the valves of the lungs and the heart (pulmonary and aortic valves); and misplacement of the heart in the right, rather than the left side of the chest (dextrocardia). The kidneys and gastrointestinal system may also be affected with cysts similar to those seen in polycystic kidney disease. These abnormalities are frequently severe and life-threatening.
Partial trisomy of the distal segment of chromosome 13 generally results in less severe, but still serious, symptoms and a distinctive facial appearance including a short upturned nose, a longer than usual area between the nose and upper lip (philtrum), bushy eyebrows, and tumors made up of blood capillaries on the forehead (frontal capillary hemangiomata). Partial trisomy of the proximal segment of chromosome 13 is much less likely to be fatal and has been associated with a variety of facial features including a large nose, a short upper lip, and a receding jaw. Both forms of partial trisomy also result in severe mental retardation.
Beyond one month of age, other symptoms that are seen in individuals with Patau syndrome are: feeding difficulties and constipation, reflux disease, slow growth rates, curvature of the spine (scoliosis), irritability, sensitivity to sunlight, low muscle tone, high blood pressure, sinus infections, urinary tract infections, and ear and eye infections.
Diagnosis
Patau syndrome is detectable during pregnancy through the use of ultrasound imaging, amniocentesis, and chorionic villus sampling (CVS). At birth, the newborn’s numerous malformations indicate a possible chromosomal abnormality. Trisomy 13 is confirmed by examining the infant’s chromosomal pattern through karyotyping or another procedure. Karyotyping involves the separation and isolation of the chromosomes present in cells taken from an individual. These cells are generally extracted from cells found in a blood sample. The 22 non-sex linked chromosomes are identified by size, from largest to smallest, as chromosomes 1 through 22. The sex determining chromosomes are
892 |
G A L E E N C Y C L O P E D I A O F G E N E T I C D I S O R D E R S |

also identified. Patau syndrome is confirmed by the presence of three, rather than the normal two, copies of chromosome 13.
Treatment and management
Some infants born with Patau syndrome have severe and incurable birth defects. However, children with better prognoses require medical treatment to correct structural abnormalities and associated complications. For feeding problems, special formulas, positions, and techniques may be used. Tube feeding or the placement of a gastric tube (gastrostomy) may be required. Structural abnormalities such as cleft lip and cleft palate can be corrected through surgery. Special diets, hearing aids, and vision aids can be used to mitigate the symptoms of Patau syndrome. Physical therapy, speech therapy, and other types of developmental therapy will help the child reach his or her potential.
Since the translocation form of Patau syndrome is genetically transmitted, genetic counseling for the parents should be part of the management of the disease.
Prognosis
Approximately 45% of infants with trisomy 13 die within their first month of life; up to 70% in the first six months; and over 70% by one year of age. Survival to adulthood is very rare. Only one adult is known to have survived to age 33.
Most survivors have profound mental and physical disabilities; however, the capacity for learning in children with Patau syndrome varies from patient to patient. Older children may be able to walk with or without a walker. They may also be able to understand words and phrases, follow simple commands, use a few words or signs, and recognize and interact with others.
Resources
BOOKS
Gardner, R.J. McKinlay, and Grant R. Sutherland. Chromosome
Abnormalities and Genetic Counseling. New York: Oxford
University Press, 1996.
Jones, Kenneth Lyons. Smith’s Recognizable Patterns of
Human Malformation. 5th ed. Philadelphia: W.B.
Saunders Company, 1997.
PERIODICALS
Baty, Bonnie J., Brent L. Blackburn, and John C. Carey. “Natural History of Trisomy 18 and Trisomy 13: I. Growth, Physical Assessment, Medical Histories, Survival, and Recurrence Risk.” American Journal of Medical Genetics 49 (1994): 175–87.
Baty, Bonnie J., et al. “Natural History of Trisomy 18 and Trisomy 13: II. Psychomotor Development.” American
Journal of Medical Genetics 49 (1994): 189–94.
Delatycki, M. and Gardner, R. “Three cases of trisomy 13 mosaicism and a review of the literature.” Clinical Genetics (June 1997): 403–7.
ORGANIZATIONS
Rainbows Down Under—A Trisomy 18 and Trisomy 13 Resource. SOFT Australia, 198 Oak Rd., Kirrawee, NSW 2232. Australia 02-9521-6039. http://members.optushome
.com.au/karens .
Support Organization for Trisomy 18, 13, and Related Disorders (SOFT). 2982 South Union St., Rochester, NY 14624. (800) 716-SOFT. http://www.trisomy.org .
WEBSITES
Pediatric Database (PEDBASE) Homepage. http://www
.icondata.com/health/pedbase/files/TRISOMY1.HTM . “Trisomy 13.” WebMD http://my.webmd.com/content/asset/
adam_disease_trisomy_13 . (February 9, 2001).
Paul A. Johnson
I Patent ductus arteriosus
Definition
Patent ductus arteriosus (PDA) is a heart abnormality that occurs when the ductus arteriosus (the temporary fetal blood vessel that connects the aorta and the pulmonary artery) does not close at birth.
Description
The ductus arteriosus is a temporary fetal blood vessel that connects the aorta and the pulmonary artery before birth. The ductus arteriosus should be present and open before birth while the fetus is developing in the uterus. Since oxygen and nutrients are received from the placenta and the umbilical cord instead of the lungs, the ductus arteriosus acts as a “short cut” that allows blood to bypass the deflated lungs and go straight out to the body. After birth, when the lungs are needed to add oxygen to the blood, the ductus arteriosus normally closes. The closure of the ductus arteriosus ensures that blood goes to the lungs to pick up oxygen before going out to the body. Closure of the ductus arteriosus usually occurs at birth as levels of certain chemicals, called prostagladins, change and the lungs fill with air. If the ductus arteriosus closes correctly, the blood pumped from the heart goes to the lungs, back into the heart, and then out to the body through the aorta. The blood returning from the lungs and moving out of the aorta carries oxygen to the cells of the body.
arteriosus ductus Patent
G A L E E N C Y C L O P E D I A O F G E N E T I C D I S O R D E R S |
893 |

Patent ductus arteriosus
K E Y T E R M S
Aorta—The main artery located above the heart which pumps oxygenated blood out into the body. Many congenital heart defects affect the aorta.
Catheterization—The process of inserting a hollow tube into a body cavity or blood vessel.
Ductus arteriosus—The temporary channel or blood vessel between the aorta and pulmonary artery in the fetus.
Echocardiograph—A record of the internal structures of the heart obtained from beams of ultrasonic waves directed through the wall of the chest.
Electrocardiogram (ECG, EKG)—A test used to measure electrical impulses coming from the heart in order to gain information about its structure or function.
Endocarditis—A dangerous infection of the heart valves caused by certain bacteria.
Oxygenated blood—Blood carrying oxygen through the body.
Pulmonary artery—An artery that carries blood from the heart to the lungs.
Pulmonary edema—A problem caused when fluid backs up into the veins of the lungs. Increased pressure in these veins forces fluid out of the vein and into the air spaces (alveoli). This interferes with the exchange of oxygen and carbon dioxide in the alveoli.
In some infants, the ductus arteriosus remains open (or patent) and the resulting heart defect is known as patent ductus arteriosus (PDA). In most cases, a small PDA does not result in physical symptoms. If the PDA is larger, health complications may occur.
In an average individual’s body, the power of blood being pumped by the heart and other forces leads to a certain level of pressure between the heart and lungs. The pressure between the heart and lungs of an individual affected by PDA causes some of the oxygenated blood that should go out to the body (through the aorta) to return back through the PDA into the pulmonary artery. The pulmonary artery takes the blood immediately back to the lungs. The recycling of the already oxygenated blood forces the heart to work harder as it tries to supply enough oxygenated blood to the body. In this case, the left side of the heart usually grows larger as it works harder and must contain all of the extra blood moving
back into the heart. This is known as a left-to-right or aor- tic-pulmonary shunt.
As noted, the size of the PDA determines how much harder the heart has to work and how much bigger the heart becomes. If the PDA is large, the bottom left side of the heart is forced to pump twice as much blood because it must supply enough blood to recycle back to the lungs and move out to the body. As the heart responds to the increased demands for more oxygenated blood by pumping harder, the pulmonary artery has to change in size and shape in order to adapt to the increased amount and force of the blood. In some cases, the increase in size and shape changes the pressure in the pulmonary artery and lungs. If the pressure in the lungs is higher than that of the heart and body, blood returning to the heart will take the short cut back into the aorta from the pulmonary artery through the PDA instead of going to the lungs. This backward flowing of blood does not carry much oxygen. If blood without much oxygen is being delivered to the body, the legs and toes will turn blue or cyanotic. This is called a shunt reversal.
When a PDA results in a large amount of blood being cycled in the wrong order, either through a left-to- right shunt or shunt reversal, the overworked, enlarged heart may stop working (congestive heart failure) and the lungs can become filled with too much fluid (pulmonary edema). At this time, there is also an increased risk for a bacterial infection that can inflame the lining of the heart (endocarditis). These three complications are very serious.
Genetic profile
PDA can be a result of an environmental exposure before birth, inheriting a specific changed or mutated gene or genes, a symptom of a genetic syndrome, or be caused by a combination of genetic and environmental factors (multifactorial).
Environmental exposures that can increase the chance for a baby to be affected by PDA include fetal exposure to rubella before birth, preterm delivery, and birth at a high altitude location.
PDA can be an inherited condition running in families as isolated PDA or part of a genetic syndrome. In either case, there are specific gene changes or mutations that lead to an abnormality in the elastic tissue forming the walls of the ductus arteriosus. The genes causing isolated PDA have not been identified, but it is known that PDA can be inherited through a family in an autosomal dominant pattern or an autosomal recessive pattern.
Every person has approximately 30,000 genes, which tell our bodies how to grow and develop correctly. Each gene is present in pairs since one is inherited from
894 |
G A L E E N C Y C L O P E D I A O F G E N E T I C D I S O R D E R S |
Normal Circulation |
|
|
Patent Ductus Arteriosus |
|||||
Closed |
|
|
Open |
|||||
ductus |
|
|
ductus |
|||||
|
|
|
Aorta |
|||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Pulmonary artery
arteriosus ductus Patent
Failure of the temporary fetal blood vessel that connects the aorta and the pulmonary artery (ductus arteriosus) to close after birth results in patent ductus arteriosus. This open duct interferes with proper blood flow through the aorta. (Gale Group)
the mother, and one is inherited from the father. In an autosomal dominant condition, only one changed or mutated copy of the gene for PDA is necessary for a person to have PDA. If a parent has an autosomal dominant form of PDA, there is a 50% chance for each child to have the same or similar condition.
PDA can also be inherited in an autosomal recessive manner. A recessive condition occurs when a child receives two changed or mutated copies of the gene for a particular condition, such as PDA (one copy from each parent). Individuals with a single changed or mutated copy of a gene for a recessive condition, are known as carriers, and have no health problems related to the condition. In fact, each person carries between five and 10 genes for harmful, recessive conditions. However, when two people who each carry a changed or mutated copy of the same gene for a recessive condition meet, there is a chance, with each pregnancy, for the child to inherit the two changed or mutated copies from each parent. In this case, the child would have PDA. For two known carriers, there is a 25% risk with each child to have a child with PDA, a 50% chance to have a child who is a carrier, and a 25% chance to have a child who is neither affected nor a carrier.
Most cases of PDA occur as the result of multifactorial inheritance, which is caused by the combination of genetic factors and environmental factors. The combined factors lead to isolated abnormalities in the elastic tissue forming the walls of the ductus arteriosus. Family
studies can provide different recurrence risks depending on the family member affected by multifactorial PDA. If an individual is affected by isolated, multifactorial PDA, they have a 2–4% chance of having a child affected by PDA. If a couple has one child with isolated, multifactorial PDA, there is a 3% chance that another of their children could be affected by PDA. If a couple has two children affected by isolated, multifactorial PDA, there is a 10-25% chance that they could have another child affected by PDA.
Unless a specific pattern of inheritance, preterm delivery, or known exposure is found through the examination of a detailed pregnancy and family history, the multifactorial family studies are used to estimated the possible risk of recurrence of PDA in a family.
Demographics
PDA is a very common heart disorder. Though an exact incidence of PDA is difficult to determine, one review in 1990 found that approximately 8% of live births were found to be affected by PDA. PDA can occur in full-term infants, but is seen most frequently in preterm infants, infants born at a high altitude, and babies whose mothers were affected by German measles (rubella) during pregnancy. PDA is two to three times more common in females than males. PDA occurs in individuals of every ethnic origin and does not occur more frequently in any one country or ethnic population.
G A L E E N C Y C L O P E D I A O F G E N E T I C D I S O R D E R S |
895 |

Pedigree analysis
Signs and symptoms
The main sign of PDA is a constant heart murmur that sounds like the hum of a refrigerator or other machinery. This murmur is usually heard by the doctor using a stethoscope. Otherwise, there are no specific symptoms of PDA, unless the ductus arteriosus size is large. Children and adults with a large ductus arteriosus can show difficulty in breathing during moderate physical exercise, an enlarged heart, and failure to gain weight. In some cases, heart failure and pulmonary congestion can indicate a PDA.
Diagnosis
Diagnosis is most often made by detecting the characteristic “machinery” heart murmur heard by a doctor through a stethoscope. Tests such as a chest x ray, echocardiograph, and ECG are used to support the initial diagnosis. Other indications of PDA include failure to gain weight, frequent chest infections, heavy breathing during mild physical exertion, congestive heart failure, and pulmonary edema. Prenatal ultrasounds are unable to detect PDA because the heart defect does not occur until the time of birth.
Treatment and management
The treatment and management of PDA depends upon the size of the PDA and symptoms being experienced by the affected individual. In some cases, a PDA can correct itself in the first months of life. In preterm infants experiencing symptoms, the first step in correcting a PDA is treatment through medications such as indomethacin. In preterm infants whose PDA is not closed through medication, full term infants affected by PDA, and adults, surgery is an option for closing the ductus arteriosus. In 2000 and 2001, researchers have developed and reviewed alternatives to surgical closure such as interventional cardiac catheterization and video-assisted thorascopic surgical repair. A cardiologist can help individuals determine the best method for treatment based on their physical symptoms and medical history.
Prognosis
Adults and children can survive with a small opening remaining in the ductus arteriosus. Treatment, including surgery, of a larger PDA is usually successful and frequently occurs without complications. Proper treatment allows children and adults to lead normal lives.
Resources
BOOKS
Alexander, R.W., R. C. Schlant, and V. Fuster, eds. The Heart. 9th ed. New York: McGraw-Hill, 1998.
Jaworski, Anna Marie, ed. The Heart of a Mother. Temple, TX.: Baby Hearts Press, 1999.
Kleinman, Mary. What Your Doctor Didn’t Tell you About
Congenital Heart Disease. Salt Lake City: Northwest Publishing Inc., 1993.
Neill, Catherine. The Heart of A Child. Baltimore: Johns Hopkins University, 1992.
ORGANIZATIONS
CHASER (Congenital Heart Anomalies Support, Education, and Resources). 2112 North Wilkins Rd., Swanton, OH 43558. (419) 825-5575. http://www.csun.edu/ ~hfmth006/chaser .
Kids with Heart. 1578 Careful Dr., Green Bay, WI 54304. (800) 538-5390. http://www.execpc.com/~kdswhrt .
WEBSITES
Berger, Sheri. The Congenital Heart Defects Resource Page.
http://www.csun.edu/~hfmth006/chaser/ . (Updated
January 6, 2000).
“Congenital Cardiovascular Disease.” American Heart
Association http://www.americanheart.org/Heart_and_
Stroke_A_Z_Guide/conghd.html . 2000.
“Heart Disorders.” Family Village http://www.familyvillage
.wisc.edu/index.html . (Updated March 24, 2000).
Dawn A. Jacob, MS
PC deficiency see Pyruvate carboxylase deficiency with lactic acidemia
I Pedigree analysis
Definition
A pedigree is a family tree or chart made of symbols and lines that represent a patient’s genetic family history. The pedigree is a visual tool for documenting biological relationships in families and the presence of diseases. Pedigree analysis is an assessment made by a medical professional about genetic risk in a family.
Purpose
Pedigrees are most often constructed by medical geneticists or genetic counselors. People are referred to genetic professionals because of concern about the presence of a genetic condition in a family member. Pedigree analysis can help identify a genetic condition running through a family, aids in making a diagnosis, and aids in determining who in the family is at risk for genetic conditions. During pedigree construction, the family’s beliefs about the cause for a genetic disease or emotional issues related to a diagnosis may be revealed. For
896 |
G A L E E N C Y C L O P E D I A O F G E N E T I C D I S O R D E R S |

instance, family members may experience guilt or shame about passing on a genetic trait. Thus, the communication process involved in taking the family history may allow the health care provider to identify areas in which the patient may need reassurance, education, or emotional support.
Creating a pedigree
Pedigree symbols
A standard set of symbols has been established for use in creating pedigrees. Some of the most commonly used symbols are shown in this entry. When a person is affected with a birth disorder, mental retardation, or other health problems, the individual is shaded or marked. If more than one condition is present in a family, different identifying marks should be made. A key to decipher these markings should also be included on the pedigree. The meaning of each horizontal and vertical line is also shown.
Information obtained
A typical pedigree is made of information about three generations of a family. The consultand is the person seeking genetic evaluation, counseling, or testing. The proband in a family is the person in a family affected with a genetic disorder. Beginning with the consultand, questions should be asked about the health of first, second, and third degree relatives. First-degree relatives are children, parents, and siblings. Second-degree relatives are half siblings, nieces, nephews, aunts and uncles, grandparents, and grandchildren. Third-degree relatives are first cousins. Important information to obtain on both sides of the family includes:
•ages or dates of birth
•presence of any birth disorders, learning problems, chronic illnesses, surgeries, or medical treatments
•presence of specific features of a disease if the condition is suspected in the family
•genetic testing results if previously performed in the family
•cause of death for deceased family members
•pregnancy losses, stillbirths, or infant deaths and causes
•infertility in the family
•ethnic background of the families
•consanguinity
It is important to establish the accuracy of information given by patients. Therefore, medical records are often requested in order to provide accurate risk assessment.
K E Y T E R M S
Autosomal—Relating to any chromosome besides the X and Y sex chromosomes. Human cells contain 22 pairs of autosomes and one pair of sex chromosomes.
Consanguinity—A mating between two people who are related to one another by blood.
Dizygotic twins—Non-identical twins that usually occur when two sperm fertilize two separate eggs during the same time period.
Obligate carrier—An individual who, based on pedigree analysis, must carry a genetic mutation for a particular genetic disease. Parents of a child with an autosomal recessive disorder are obligate carriers.
Pedigree patterns
Autosomal dominant inheritance
Pedigree 1 illustrates the occurrence of an autosomal dominant disorder called neurofibromatosis (NF). NF is characterized by growths under the skin called neurofibromas, dark spots on the skin called café au lait spots, and an eye finding called Lisch nodules. NF is caused by a single dominant gene on chromosome 17. Each person who is affected with NF has a 50% chance to pass the gene on to each child. The symptoms of NF are variable so that some family members are affected more seriously than others. The pedigree shows that in autosomal dominant inheritance, multiple generations of a family are affected. This is called vertical transmission of a trait through a family. Males and females are equally likely to be affected. In a particular sibship, about half of the siblings are affected.
Autosomal recessive inheritance
Pedigree 2 illustrates the occurrence of an autosomal recessive disorder called cystic fibrosis (CF) in a family. CF is a chronic respiratory disease characterized by digestive problems and a shortened life span. A person with CF has two genes for the condition on chromosome 7. Each parent is an obligate carrier of a gene for the condition. When both parents are carriers, there is a one in four or 25% chance that each child they have together will be affected. In autosomal recessive inheritance, siblings are most often affected rather than people in successive generations. Since siblings are affected, this is called horizontal transmission of a disease in the family. Males and females are equally likely to be affected in this
analysis Pedigree
G A L E E N C Y C L O P E D I A O F G E N E T I C D I S O R D E R S |
897 |
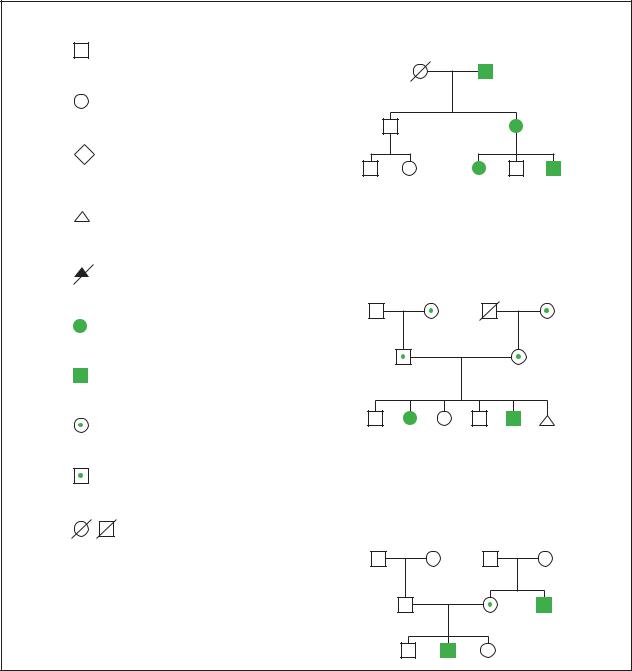
Pedigree analysis
Pedigree Symbols
Male
Female
Gende unknown
Miscarriage
Elective terminated of Pregnancy
Affected female
Affected male
Carrier female
Carrier male
Deceased
Pedigree 1: Neurofibromatosis
Autosomal Dominant Inheritance
Pedigree 2: Cystic Fibrosis
Autosomal Recessive Inheritance
Pedigree 3: Hemophilia
X-Linked Recessive Inheritance
The illustration above identifies several common symbols used to represent individuals in a pedigree chart. The three pedigree charts to the side provide examples of different types of inheritance patterns and the transmission of abnormal genes through three generations in a family. (Gale Group)
type of inheritance and others in the family have an increased chance to be unaffected carriers of the disease.
X-linked recessive inheritance
Pedigree 3 illustrates the occurrence of an X-linked disorder called hemophilia. Hemophilia is characterized by excessive bleeding and bruising. Depending on the
type of hemophilia, a particular blood-clotting factor is deficient. In X-linked recessive inheritance, males are affected with the condition while females are unaffected carriers. In X-linked recessive inheritance, vertical transmission of the disease is seen, with skipping of generations. There is no male-to-male transmission of a disease in this type of inheritance. This is because males pass
898 |
G A L E E N C Y C L O P E D I A O F G E N E T I C D I S O R D E R S |

their Y chromosome to each son, instead of the X chromosome with the disease gene. Each daughter of an affected male is an obligate carrier of the disease since they will always inherit his X chromosome. There is a 50% that each son of a carrier woman will be affected. There is a 50% chance that each daughter of a carrier female will be a carrier.
Resources
BOOKS
Baker, Diane. A Guide to Genetic Counseling. New York: A. Wiley and Sons, Inc. 1998.
Harper, Peter S. Practical Genetic Counseling. Oxford: Butterworth Heinmann 1998.
Rose, Peter, and Anneke Lucassen. “Taking a Family History.” In Practical Genetics for Primary Care. Oxford: Oxford University Press, 1999.
PERIODICALS
Bennett, Robin et al. “Recommendations for Standardized Human Pedigree Nomenclature.” The Journal of Genetic Counseling (December 1995): 267–79.
Sonja Rene Eubanks, MS, CGC
I Pelizaeus-Merzbacher disease
Definition
Pelizaeus-Merzbacher disease (PMD) is a neurological condition that affects myelin, the insulation surrounding the nerves in the brain and spinal cord.
Description
PMD was named for two German doctors, F. Pelizaeus and L. Merzbacher, who first described the condition in the late 1800s. The severity of characteristics in PMD can range from mild to severe. PMD primarily affects males, but occasionally females have mild or moderate symptoms. PMD is also called a leukodystrophy, meaning that it affects the myelin, sometimes called the white matter, in the brain and spinal cord. The brain and the spinal cord together are called the central nervous system.
Genetic profile
PMD is caused by a mutation or change in the proteolipid protein gene (PLP). The PLP gene has the instructions to make proteolipid protein, one of the proteins that make up myelin in the central nervous system. When
there is a mutation in the PLP gene, the myelin is not formed properly or is not made at all, resulting in PMD.
Genes are organized on structures called chromosomes. There are hundreds to thousands of genes on each chromosome. There are 46 chromosomes in each cell of the body. These are grouped into 23 pairs. The first 22 pairs are the same in both males and females. The 23rd pair is called the sex chromosomes; having one X chromosome and one Y chromosome causes a person to be male; having two X chromosomes causes a person to be female. A fetus acquires one member of each pair from the mother’s egg and one member from the father’s sperm.
The PLP gene is located on the X chromosome. Since males have only one X chromosome, they have only one copy of the PLP gene. Thus, a male with a mutation in his PLP gene will have PMD. Females have two X chromosomes and therefore have two copies of the PLP gene. If they have a mutation in one copy of their PLP genes, they may only have mild symptoms of PMD or no symptoms at all. This is because their normal copy of the PLP gene does make normal myelin. Females who have one copy of the PLP gene with a mutation and one normal copy are called carriers.
Inheritance
PMD is passed on through families by X-linked recessive inheritance. This means that affected males are related through females in the family. A male does not pass PMD on to his sons. Females pass on one of their X chromosomes to their sons or daughters. If the normal X chromosome is passed on, her son or daughter will be unaffected and cannot pass PMD onto their children. However, if the X chromosome with the PLP mutation is passed on, a daughter will be a carrier while the son would have PMD. Therefore, a female PLP mutation carrier has a 50%, or one in two chance of having a normal child (son or daughter), a 25%, or one in four chance of having a carrier daughter, and a 25%, or one in four chance of having an affected son.
Males with PMD usually do not reproduce and therefore do not pass PMD on.
Mutations
Different types of mutations or changes in the PLP gene cause PMD. Everyone in a family who has the condition or is a carrier has the exact same PLP mutation. The most common type of mutation is a duplication (doubling) of the PLP gene. This means that two copies of the PLP gene are present on one X chromosome. Having this extra copy causes the myelin to be abnormal and leads to PMD. About 50–75% of people with PMD have a PLP
disease Merzbacher-Pelizaeus
G A L E E N C Y C L O P E D I A O F G E N E T I C D I S O R D E R S |
899 |
Pelizaeus-Merzbacher disease
duplication. The duplication usually causes a severe form of PMD. Another 15–20% of people with PMD have point mutations within their PLP gene. A point mutation is like a typo in the gene. This typo changes the message of the gene and also causes the myelin to be abnormal. A few patients with PMD have a deletion of the PLP gene as their cause of PMD. This means that they have no copies of the PLP gene if they are male or one copy if they are female. Another 5–20% of patients have characteristics of PMD, but no mutation has been found in their PLP gene. Scientists are working to determine the cause of disease in these people.
Demographics
PMD has been described in people from all over the world and from many different ethnic backgrounds. The condition is rare and estimated to affect approximately one in 300,000 individuals in the United States.
Signs and symptoms
There is a range in the severity of symptoms of PMD. Rough categories have been set up based on the age of onset and severity of symptoms. However, many patients do not fall neatly into one of these categories and instead fall somewhere in between. Patients with different severities have been seen in the same family.
In the most severe form of PMD, symptoms are first noticed shortly after birth or in infancy. This is called connatal PMD. One of the first signs usually noticed is nystagmus, a side-to-side jerking of the eyes. This does not usually cause problems with vision. Patients can have significant mental retardation and never learn to walk, talk, or care for themselves. They may have noisy breathing called stridor and difficulty sucking. Seizures may be present in these children. They are often small for their age and have trouble gaining weight. Early on, they have floppy muscles called hypotonia, but later develop spasticity, which is stiffness or tightness in the muscles and joints.
Those patients who have classical PMD, which is less severe than the connatal type, usually have nystagmus. Nystagmus develops within the first few months of life. Other symptoms typically develop within the first few years. These children also have hypotonia that turns into spasticity. Sometimes these patients will learn to walk. However, they may need a wheelchair as their spasticity increases. Shaking of the head and neck called titubation may occur. Although these children often have moderate mental retardation, they often learn to talk and often understand more than is evident by their speech.
A less severe type of PMD is called the PLP null syndrome. Those affected do not usually have nystagmus
and their spasticity may be mild. Symptoms develop in early childhood. This group of patients may also have a peripheral neuropathy, which is a problem with the nerves that run from the spinal cord through the body. This can cause weakness and problems with sensation (telling if something is hot or cold, for example). These patients usually talk and walk. They may have mild to moderate mental retardation.
There are some people who have PLP mutations who are very mildly affected. They have spasticity and sometimes have other problems such as a spastic bladder. Intelligence is normal or mildly impaired. Although these individuals have mutations in the PLP gene, their condition is given a different name, spastic paraplegia 2 (SPG2).
Diagnosis
When problems are first noticed in an infant or a child, they will usually be referred to a pediatric neurologist who is specially trained in diseases of the nerves and muscles in children. At the initial evaluation, the neurologist will perform a clinical examination to evaluate the child’s development and how well the nerves and muscles work. At this time, a thorough family history should be taken to determine if there are others in the family that are affected and if so, how they are related.
One of the initial tests that may be ordered is magnetic resonance imaging (MRI). In this test, pictures of the brain are taken and the amount of white matter in the brain is measured. In people with PMD, the amount of white matter is usually significantly reduced compared to normal. However, a decrease in white matter is seen in other neurological conditions and is not specific to PMD. Therefore, an MRI can be helpful in making the diagnosis of PMD, but if changes are seen on MRI, it does not confirm the diagnosis of PMD. Changes in the white matter may only be seen after one to two years of age when the brain has matured.
If no one else in the family is known to be affected, testing may be performed to rule out conditions other than PMD. Often PMD may not initially be suspected when no one else is affected in the family. It is not uncommon for people to be misdiagnosed initially. Sometimes the diagnosis of PMD is made only after a second affected child is born into a family.
Genetic testing
The only way to be absolutely sure that someone has PMD is by genetic testing, usually done by a blood test. First, the genetic material is evaluated to see if a PLP gene duplication is present. If this test is negative, additional testing can be done to look for other mutations in
900 |
G A L E E N C Y C L O P E D I A O F G E N E T I C D I S O R D E R S |
