
Gale Encyclopedia of Genetic Disorder / Gale Encyclopedia of Genetic Disorders, Two Volume Set - Volume 2 - M-Z - I
.pdf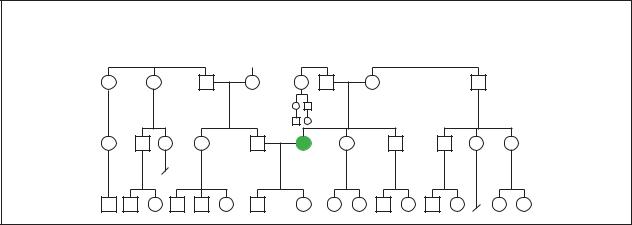
Myasthenia Gravis
Sporadic
Sporatic occurance of Myathenia gravis in a family. (Gale Group)
improvement may take months or even several years to fully develop. Thymectomy is not usually recommended for children with MG, since the thymus continues to play an important immune role throughout childhood.
Immune-suppressing drugs are used to treat MG if response to pyridostigmine and thymectomy are not adequate. Drugs include corticosteroids such as prednisone, and the non-steroids azathioprine (Imuran) and cyclosporine (Sandimmune).
Plasma exchange may be performed to treat myasthenic crisis or to improve very weak patients before thymectomy. In this procedure, blood plasma is removed and replaced with purified plasma free of autoantibodies. It can produce a temporary improvement in symptoms, but is too expensive for long-term treatment. Another blood treatment, intravenous immunoglobulin therapy, is also used for myasthenic crisis. In this procedure, large quantities of purified immune proteins (immunoglobulins) are injected. For unknown reasons, this leads to symptomatic improvement in up to 85% of patients. It is also too expensive for long-term treatment.
People with weakness of the bulbar muscles may need t3o eat softer foods that are easier to chew and swallow. In more severe cases, it may be necessary to obtain nutrition through a feeding tube placed into the stomach (gastrostomy tube).
Some drugs should be avoided by people with MG because they interfere with normal neuromuscular function. Drugs to be avoided or used with caution include:
•Many types of antibiotics, including erythromycin, streptomycin, and ampicillin
•Some cardiovascular drugs, including Verapamil, betaxolol, and propranolol
•Some drugs used in psychiatric conditions, including chlorpromazine, clozapine, and lithium.
Many other drugs may worsen symptoms as well, so patients should check with the doctor who treats their MG before taking any new medications.
A Medic-Alert card or bracelet provides an important source of information to emergency providers about the special situation of a person with MG. They are available from health care providers.
Prognosis
Most people with MG can be treated successfully enough to prevent their condition from becoming debilitating. In some cases, however, symptoms may worsen even with vigorous treatment, leading to generalized weakness and disability. MG rarely causes early death except from myasthenic crisis. There is no known way to prevent myasthenia gravis. Thymectomy improves symptoms significantly in many patients, and relieves them entirely in some. Avoiding heat can help minimize symptoms.
Resources
BOOKS
Swash, Michael, and Martin Schwarz. Neuromuscular Diseases: A Practical Approach to Diagnosis and Management. Springer, 1997.
PERIODICALS
Drachman, D. B. “Myasthenia Gravis.” New England Journal of Medicine 330 (1994): 1797-1810.
Robinson, Richard. “The Body At War with Itself.” Quest 4 no. 3 (1997): 20-24.
gravis Myasthenia
G A L E E N C Y C L O P E D I A O F G E N E T I C D I S O R D E R S |
779 |

Myopia
ORGANIZATIONS
Muscular Dystrophy Association. 3300 East Sunrise Dr.,
Tucson, AZ 85718. (520) 529-2000 or (800) 572-1717.
http://www.mdausa.org .
Myasthenia Gravis Foundation of America. 5841 Cedar Lake
Rd., Suite 204, Minneapolis, MN 55416. (800) 541-5454.
Fax: (952) 545-6073.
WEBSITES
Immune Deficiency Foundation.
http://www.primaryimmune.org .
Myasthenia Gravis Foundation of America
http://www.myasthenia.org .
National Institute of Neurological Disorders and Stroke Fact Sheet on Myasthenia Gravis. http://www.ninds.nih.gov/ health_and_medical/pubs/myasthenia_gravis.htm .
Catherine L. Tesla, MS, CGC
I Myopia
Definition
Myopia is the medical term for nearsightedness. People with myopia see objects more clearly when they are close to the eye, while distant objects appear blurred or fuzzy. Reading and close-up work may be clear, but distance vision is blurry.
Description
To understand myopia it is necessary to have a basic knowledge of the main parts of the eye’s focusing system: the cornea, the lens, and the retina. The cornea is a tough, transparent, dome-shaped tissue that covers the front of the eye (not to be confused with the white, opaque sclera). The cornea lies in front of the iris (the colored part of the eye). The lens is a transparent, doubleconvex structure located behind the iris. The retina is a thin membrane that lines the rear of the eyeball. Lightsensitive retinal cells convert incoming light rays into electrical signals that are sent along the optic nerve to the brain, which then interprets the images.
In people with normal vision, parallel light rays enter the eye and are bent by the cornea and lens (a process called refraction) to focus precisely on the retina, providing a crisp, clear image. In the myopic eye, the focusing power of the cornea (the major refracting structure of the eye) and the lens is too great with respect to the length of the eyeball. Light rays are bent too much, and they converge in front of the retina. This inaccuracy is called a refractive error. In other words, an overfocused fuzzy image is sent to the brain.
There are many types of myopia. Some common types include:
•Physiologic
•Pathologic
•Acquired.
By far the most common form, physiologic myopia develops in children sometime between the ages of five and 10 years and gradually progresses until the eye is fully grown. Physiologic myopia may include refractive myopia (the cornea and lens-bending properties are too strong) and axial myopia (the eyeball is too long). Pathologic myopia is a far less common abnormality. This condition begins as physiologic myopia, but rather than stabilizing, the eye continues to enlarge at an abnormal rate (progressive myopia). This more advanced type of myopia may lead to degenerative changes in the eye (degenerative myopia). Acquired myopia occurs after infancy. This condition may be seen in association with uncontrolled diabetes and certain types of cataracts. Antihypertensive drugs and other medications can also affect the refractive power of the lens.
Genetic profile
Eye care professionals have debated the role of genetics in the development of myopia for many years. Some believe that a tendency toward myopia may be inherited, but the actual disorder results from a combination of environmental and genetic factors. Environmental factors include close work; work with computer monitors or other instruments that emit some light (electron microscopes, photographic equipment, lasers, etc.); emotional stress; and eye strain.
A variety of genetic patterns for inheriting myopia have been suggested, ranging from a recessive pattern with complete penetrance in people who are homozygotic for myopia to an autosomal dominant pattern; an autosomal recessive pattern; and various mixtures of these patterns. One explanation for this lack of agreement is that the genetic profile of high myopia (defined as a refractive error greater than -6 diopters) may differ from that of low myopia. Some researchers think that high myopia is determined by genetic factors to a greater extent than low myopia.
Another explanation for disagreement regarding the role of heredity in myopia is the sensitivity of the human eye to very small changes in its anatomical structure. Since even small deviations from normal structure cause significant refractive errors, it may be difficult to single out any specific genetic or environmental factor as their cause.
780 |
G A L E E N C Y C L O P E D I A O F G E N E T I C D I S O R D E R S |

K E Y T E R M S
Accommodation—The ability of the lens to change its focus from distant to near objects. It is achieved through the action of the ciliary muscles that change the shape of the lens.
Cornea—The transparent structure of the eye over the lens that is continous with the sclera in forming the outermost, protective, layer of the eye.
Diopter (D)—A unit of measure for describing refractive power.
Laser-assisted in-situ keratomileusis (LASIK)—A procedure that uses a cutting tool and a laser to modify the cornea and correct moderate to high levels of myopia.
Lens—The transparent, elastic, curved structure behind the iris (colored part of the eye) that helps focus light on the retina.
Ophthalmologist—A physician specializing in the medical and surgical treatment of eye disorders.
Optic nerve—A bundle of nerve fibers that carries visual messages from the retina in the form of electrical signals to the brain.
Optometrist—A medical professional who examines and tests the eyes for disease and treats visual disorders by prescribing corrective lenses and/or vision therapy. In many states, optometrists are licensed to use diagnostic and therapeutic drugs to treat certain ocular diseases.
Orthokeratology—A method of reshaping the cornea using a contact lens. It is not considered a permanent method to reduce myopia.
Peripheral vision—The ability to see objects that are not located directly in front of the eye. Peripheral vision allows people to see objects located on the side or edge of their field of vision.
Photorefractive keratectomy (PRK)—A procedure that uses an excimer laser to make modifications to the cornea and permanently correct myopia. As of early 1998, only two lasers have been approved by the FDA for this purpose.
Radial keratotomy (RK)—A surgical procedure involving the use of a diamond-tipped blade to make several spoke-like slits in the peripheral (nonviewing) portion of the cornea to improve the focus of the eye and correct myopia by flattening the cornea.
Refraction—The bending of light rays as they pass from one medium through another. Used to describe the action of the cornea and lens on light rays as they enter they eye. Also used to describe the determination and measurement of the eye’s focusing system by an optometrist or ophthalmologist.
Refractive eye surgery—A general term for surgical procedures that can improve or correct refractive errors by permanently changing the shape of the cornea.
Retina—The light-sensitive layer of tissue in the back of the eye that receives and transmits visual signals to the brain through the optic nerve.
Visual acuity—The ability to distinguish details and shapes of objects.
Genetic markers and gene mapping
Since 1992, genetic markers that may be associated with genes for myopia have been located on human chromosomes 1, 2, 12, and 18. There is some genetic information on the short arm of chromosome 2 in highly myopic people. Genetic information for low myopia appears to be located on the short arm of chromosome 1, but it is not known whether this information governs the structure of the eye itself or vulnerability to environmental factors.
In 1998, a team of American researchers presented evidence that a gene for familial high myopia with an autosomal dominant transmission pattern could be mapped to human chromosome 18 in eight North
American families. The same group also found a second locus for this form of myopia on human chromosome 12 in a large German/Italian family. In 1999, a group of French researchers found no linkage between chromosome 18 and 32 French families with familial high myopia. These findings have been taken to indicate that more than one gene is involved in the transmission of the disorder.
Family studies
It has been known for some years that a family history of myopia is one of the most important risk factors for developing the condition. Only 6-15% of children with myopia come from families in which neither parent is myopic. In families with one myopic parent, 23-40%
Myopia
G A L E E N C Y C L O P E D I A O F G E N E T I C D I S O R D E R S |
781 |
Myopia
of the children develop myopia. If both parents are myopic, the rate rises to 33%-60% for their children. One American study found that children with two myopic parents are six times as likely to develop myopia themselves as children with only one or no myopic parents. The precise interplay of genetic and environmental factors in these family patterns, however, is not yet known.
One multigenerational study of Chinese patients indicated that third generation family members had a higher risk of developing myopia even if their parents were not myopic. The researchers concluded that, at least in China, the genetic factors in myopia have remained constant over the past three generations while the environmental factors have intensified. The increase in the percentage of people with myopia over the last 50 years in the United States has led American researchers to the same conclusion.
Demographics
Myopia is the most common eye disorder in humans around the world. It affects between 25% and 35% of the adult population in the United States and the developed countries, but is thought to affect as much as 40% of the population in some parts of Asia. Some researchers have found slightly higher rates of myopia in women than in men.
The age distribution of myopia in the United States varies considerably. Five-year-olds have the lowest rate of myopia (less than 5%) of any age group. The prevalence of myopia rises among children and adolescents in school until it reaches the 25%-35% mark in the young adult population. It declines slightly in the over-45 age group; about 20% of 65-year-olds have myopia. The figure drops to 14% for Americans over 70.
Other factors that affect the demographic distribution of myopia are income level and education. The prevalence of myopia is higher among people with above-average incomes and educational attainments. Myopia is also more prevalent among people whose work requires a great deal of close focusing, including work with computers.
Signs and symptoms
Myopia is said to be caused by an elongation of the eyeball. This means that the oblong (as opposed to normal spherical) shape of the myopic eye causes the cornea and lens to focus at a point in front of the retina. A more precise explanation is that there is an inadequate correlation between the focusing power of the cornea and lens and the length of the eye.
People are generally born with a small amount of hyperopia (farsightedness), but as the eye grows this decreases and myopia does not become evident until later. This change is one reason why some researchers think that myopia is an acquired rather than an inherited trait.
The symptoms of myopia are blurred distance vision, eye discomfort, squinting, and eye strain.
Diagnosis
The diagnosis of myopia is typically made during the first several years of elementary school when a teacher notices a child having difficulty seeing the chalkboard, reading, or concentrating. The teacher or school nurse often recommends an eye examination by an ophthalmologist or optometrist. An ophthalmologist—M.D. or D.O. (Doctor of Osteopathy)—is a medical doctor trained in the diagnosis and treatment of eye problems. Ophthalmologists also perform eye surgery. An optometrist (O.D.) diagnoses, manages, and/or treats eye and visual disorders. In many states, optometrists are licensed to use diagnostic and therapeutic drugs.
A patient’s distance vision is tested by reading letters or numbers on a chart posted a set distance away (usually 20 ft). The doctor asks the patient to view images through a variety of lenses to obtain the best correction. The doctor also examines the inside of the eye and the retina. An instrument called a slit lamp is used to examine the cornea and lens. The eyeglass prescription is written in terms of diopters (D), which measure the degree of refractive error. Mild to moderate myopia usually falls between -1.00D and -6.00D. Normal vision is commonly referred to as 20/20 to describe the eye’s focusing ability at a distance of 20 ft from an object. For example, 20/50 means that a myopic person must stand 20 ft away from an eye chart to see what a normal person can see at 50 ft. The larger the bottom number, the greater the myopia.
Treatment and management
People with myopia have three main options for treatment: eyeglasses, contact lenses, and for those who meet certain criteria, refractive eye surgery.
Eyeglasses
Eyeglasses are the most common method used to correct myopia. Concave glass or plastic lenses are placed in frames in front of the eyes. The lenses are ground to the thickness and curvature specified in the eyeglass prescription. The lenses cause the light rays to diverge so that they focus further back, directly on the retina, producing clear distance vision.
782 |
G A L E E N C Y C L O P E D I A O F G E N E T I C D I S O R D E R S |
Contact lenses
Contact lenses are a second option for treatment. Contact lenses are extremely thin round discs of plastic that are worn on the eye in front of the cornea. Although there may be some initial discomfort, most people quickly grow accustomed to contact lenses. Hard contact lenses, made from a material called PMMA, are virtually obsolete. Rigid gas permeable lenses (RGP) are made of plastic that holds its shape but allows the passage of some oxygen into the eye. Some believe that RGP lenses may halt or slow the progression of myopia because they maintain a constant, gentle pressure that flattens the cornea. As of 2001, the National Eye Institute is conducting an ongoing study of RGP lenses called the Contact Lens and Myopia Progression (CLAMP) Study, with results to be published in 2003. A procedure called orthokeratology acts on this principle of “corneal molding;” however, when contact lenses are discontinued for a period of time, the cornea will generally go back to its original shape.
Soft contact lenses are made of flexible plastic and can be up to 80% water. Soft lenses offer increased comfort and the advantage of extended wear; some can be worn continuously for up to one week. While oxygen passes freely through soft lenses, bacterial contamination and other problems can occur, requiring replacement of lenses on a regular basis. It is very important to follow the cleaning and disinfecting regimens prescribed because protein and lipid buildup can occur on the lenses, causing discomfort or increasing the risk of infection. Contact lenses offer several benefits over glasses, including: better vision, less distortion, clear peripheral vision, and cosmetic appeal. In addition, contacts will not fog up from perspiration or changes in temperature.
Refractive eye surgery
For people who find glasses and contact lenses inconvenient or uncomfortable, and who meet selection criteria regarding age, degree of myopia, general health, etc., refractive eye surgery is a third treatment alternative. There are three types of corrective surgeries available as of 2001: 1) radial keratotomy (RK), 2) photorefractive keratectomy (PRK), and 3) laser-assisted in-situ keratomileusis (LASIK), which is still under clinical evaluation by the Food and Drug Administration (FDA). Refractive eye surgery improves myopic vision by permanently changing the shape of the cornea so that light rays focus properly on the retina. These procedures are performed on an outpatient basis and generally take 1030 minutes.
GALE ENCYCLOPEDIA OF GENETIC DISORDERS
|
Retina |
Myopia |
Cornea |
|
|
|
|
|
Light |
|
|
Lens |
|
|
|
Normal eye |
|
Light |
|
|
|
Nearsightedness (myopia) |
|
This illustration compares the difference between a normal eye shape and light refraction versus a myopic eye. (Gale Group)
RADIAL KERATOTOMY Radial keratotomy (RK), the first of these procedures made available, has a high associated risk. It was first developed in Japan and the Soviet Union, and was introduced into the United States in 1978. The surgeon uses a delicate diamond-tipped blade, a microscope, and microscopic instruments to make several spoke-like “radial” incisions in the non-viewing (peripheral) portion of the cornea. As the incisions heal, the slits alter the curve of the cornea, making it more flat, which may improve the focus of images onto the retina.
PHOTOREFRACTIVE KERATECTOMY Photorefractive keratectomy (PRK) involves the use of a computer to measure the shape of the cornea. Using these measurements, the surgeon applies a computer-controlled laser to make modifications to the cornea. The PRK procedure flattens the cornea by vaporizing small amounts of tissue from the cornea’s surface. As of early 2001, only two excimer lasers are approved by the FDA for PRK, although other lasers have been used. It is important to make sure
783
Myopia
the laser being used is FDA approved. Photorefractive keratectomy can treat mild to moderate forms of myopia. The cost is approximately $2,000 per eye.
LASER-ASSISTED IN-SITU KERATOMILEUSIS Laserassisted in-situ keratomileusis (LASIK) is the newest of these procedures. It is recommended for moderate to severe cases of myopia. A variation on the PRK method, LASIK uses lasers and a cutting tool called a microkeratome to cut a circular flap on the cornea. The flap is flipped back to expose the inner layers of the cornea. The cornea is treated with a laser to change the shape and focusing properties, then the flap is replaced.
Risks
All of these surgical procedures carry risks, the most serious being corneal scarring, corneal rupture, infection, cataracts, and loss of vision. In addition, a study published in March 2001 warns that mountain climbers who have had LASIK surgery should be aware of possible changes in their vision at high altitudes. The lack of oxygen at high altitudes causes temporary changes in the thickness of the cornea.
Since refractive eye surgery does not guarantee 20/20 vision, it is important to have realistic expectations before choosing this treatment. In a 10-year study conducted by the National Eye Institute between 1983 and 1993, over 50% of people with radial keratotomy gained 20/20 vision, and 85% passed a driving test (requiring 20/40 vision) after surgery, without glasses or contact lenses. Even if the patient gains near-perfect vision, however, there are potentially irritating side effects, such as postoperative pain, poor night vision, variation in visual acuity, light sensitivity and glare, and optical distortion. Refractive eye surgeries are considered elective procedures and are rarely covered by insurance plans.
Myopia treatments under research include corneal implants and permanent surgically placed contact lenses.
Alternative treatments
Some eye care professionals recommend treatments to help improve circulation, reduce eye strain, and relax the eye muscles. It is possible that by combining exercises with changes in behavior, the progression of myopia may be slowed or prevented. Alternative treatments include: visual therapy (also referred to as vision training or eye exercises); discontinuing close work; reducing eye strain (taking a rest break during periods of prolonged near vision tasks); and wearing bifocals to decrease the need to accommodate when doing close-up work.
Prognosis
Glasses and contact lenses can (but not always) correct the patient’s vision to 20/20. Refractive surgery can make permanent improvements for the right candidates.
While the genetic factors that influence the transmission and severity of myopia cannot be changed, some environmental factors can be modified. They include reducing close work; reading and working in good light; taking frequent breaks when working at a computer or microscope for long periods of time; maintaining good nutrition; and practicing visual therapy (when recommended).
Eye strain can be prevented by using sufficient light for reading and close work, and by wearing corrective lenses as prescribed. Everyone should have regular eye examinations to see if their prescription has changed or if any other problems have developed. This is particularly important for people with high (degenerative) myopia who are at a greater risk of developing retinal detachment, retinal degeneration, glaucoma, or other problems.
Resources
BOOKS
Birnbaum, Martin H. Optometric Management of Nearpoint
Vision Disorders. Boston: Butterworth-Heinemann, 1993. Curtin, Brian J. The Myopias: Basic Science and Clinical
Management. Philadelphia: Harper & Row, 1985. Rosanes-Berrett, Marilyn B. Do You Really Need Eyeglasses?
Barrytown, NY: Station Hill Press, 1990.
Zinn, Walter J., and Herbert Solomon. Complete Guide to Eyecare, Eyeglasses, and Contact Lenses. Hollywood, FL: Lifetime Books, 1996.
PERIODICALS
Edwards, M.H. “Effect of parental myopia on the development of myopia in Hong Kong Chinese.” Ophthalmic Physiologic Optometry 18 (November 1998): 477-483.
Naiglin, L. et al. “Familial high myopia: evidence of an autosomal dominant mode of inheritance and genetic heterogeneity.” Annals of Genetics 42 (3) (1999): 140-146.
“Nine Ways to Look Better: If You Want to Improve Your Vision—Or Just Protect What You Have—Try These Eye Opening Moves.” Men’s Health 13 (Jan.-Feb. 1998): 50.
Pacella, R. et al. “Role of genetic factors in the etiology of juve- nile-onset myopia based on a longitudinal study of refractive error.” Optometry and Visual Science 76 (June 1999): 381-386.
Saw, S.M., et al. “Myopia: gene-environment interaction.”
Annals of the Academy of Medicine of Singapore 29 (May 2000): 290-297.
Wu, M.M. and M.H. Edwards. “The effect of having myopic parents: an analysis of myopia in three generations.”
Optometry and Visual Science 76 (June 1999): 341-342.
784 |
GALE ENCYCLOPEDIA OF GENETIC DISORDERS |

Young, T.L., et al. “Evidence that a locus for familial high myopia maps to chromosome 18p.” American Journal of Human Genetics 63 (July 1998): 109-119.
Young, T.L., et al. “A second locus for familial high myopia maps to chromosome 12q.” American Journal of Human
Genetics 63 (November 1998): 1419-1424.
ORGANIZATIONS
American Academy of Ophthalmology. PO Box 7424, San Francisco, CA 94120-7424. (415) 561-8500. http://www
.eyenet.org .
American Optometric Association. 243 North Lindbergh Blvd., St. Louis, MO 63141. (314) 991-4100. http://www
.aoanet.org .
International Myopia Prevention Association. RD No. 5, Box 171, Ligonier, PA 15658. (412) 238-2101.
Myopia International Research Foundation. 1265 Broadway, Room 608, New York, NY 10001. (212) 684-2777.
National Eye Institute. Bldg. 31 Rm 6A32, 31 Center Dr., MSC 2510, Bethesda, MD 20892-2510. (301) 496-5248. 2020@nei.nih.gov. http://www.nei.nih.gov .
Rebecca J. Frey, PhD
Risa Palley Flynn
Myotonia atrophica see Myotonic dystrophy
I Myotonic dystrophy
Definition
Myotonic dystrophy is a progressive disease in which the muscles are weak and are slow to relax after contraction.
Description
Myotonic dystrophy (DM), also called dystrophia myotonica, myotonia atrophica, or Steinert disease, is a common form of muscular dystrophy. DM is an inherited disease, affecting both males and females. About 30,000 people in the United States are affected. Symptoms may appear at any time from infancy to adulthood. DM causes general weakness, usually beginning in the muscles of the hands, feet, neck, or face. It slowly progresses to involve other muscle groups, including the heart. DM affects a wide variety of other organ systems as well.
A severe form of DM, congenital myotonic dystrophy, may appear in newborns of mothers who have DM. Congenital means that the condition is present from birth.
K E Y T E R M S
Electrocardiogram (ECG, EKG)—A test that uses electrodes attached to the chest with an adhesive gel to transmit the electrical impulses of the heart muscle to a recording device.
Electromyography (EMG)—A test that uses electrodes to record the electrical activity of muscle. The information gathered is used to diagnose neuromuscular disorders.
Muscular dystrophy—A group of inherited diseases characterized by progressive wasting of the muscles.
Sleep apnea—Temporary cessation of breathing while sleeping.
Trinucleotide repeat expansion—A sequence of three nucleotides that is repeated too many times in a section of a gene.
Genetic profile
The most common type of DM is called DM1 and is caused by a mutation in a gene called myotonic dystrophy protein kinase (DMPK). The DMPK gene is located on chromosome 19. When there is a mutation in this gene, a person develops DM1. The specific mutation that causes DM1 is called a trinucleotide repeat expansion.
Some families with symptoms of DM do not have a mutation in the DMPK gene. As of early 2001, scientists have found that the DM in many of these families is caused by a mutation in a gene on chromosome 3. However the specific gene and mutation have not yet been identified. These families are said to have DM2.
Trinucleotide repeats
In the DMPK gene, there is a section of the genetic code where the three letters CTG are repeated a certain number of times. In people who have DM1, this word is repeated too many times—more than the normal number of 37 times—and thus this section of the gene is too big. This enlarged section of the gene is called a trinucleotide repeat expansion.
People who have repeat numbers in the normal range will not develop DM1 and cannot pass it to their children. Having more than 50 repeats causes DM1. People who have 38–49 repeats have a premutation and will not develop DM1, but can pass DM1 onto their children. Having repeats numbers greater than 1,000 causes congenital myotonic dystrophy.
dystrophy Myotonic
G A L E E N C Y C L O P E D I A O F G E N E T I C D I S O R D E R S |
785 |

Myotonic dystrophy
TABLE 1
Relationship between phenotype and CTG repeat length in myotonic dystrophy
Phenotype |
Clinical signs |
CTG repeat size |
Age of onset (Years) |
Average age of death (Years) |
Premutation |
None |
38 to 49 |
Normal |
Normal |
Mild |
Cataracts mild myotonia |
50 to 150 |
20–70 |
60–normal |
Classical |
Weakness myotonia |
|
|
|
|
Cataracts |
|
|
|
|
Balding |
|
|
|
|
Cardiac arrhythmia |
100 to 1000–1500 |
|
|
|
Others |
10–30 |
48–55 |
|
Congenital |
Infantile hypotonia |
|
|
|
|
Respiratory deficits |
1000 to 2000 |
|
|
|
Mental retardation |
Birth to 10 |
45 |
In general, the more repeats in the affected range that someone has, the earlier the age of onset of symptoms and the more severe the symptoms. However, this is a general rule. It is not possible to look at a person’s repeat number and predict at what age they will begin to have symptoms or how their condition will progress.
Exactly how the trinucleotide repeat expansion causes myotonia, the inability to relax muscles, is not yet understood. The disease somehow blocks the flow of electrical impulses across the muscle cell membrane. Without proper flow of charged particles, the muscle cannot return to its relaxed state after it has contracted.
Anticipation
Sometimes when a person who has repeat numbers in the affected or premutation range has children, the expansion grows larger. This is called anticipation. A larger expansion can result in an earlier age of onset in children than in their affected parent. Anticipation happens more often when a mother passes DM1 onto her children then when it is passed from the father. Occasionally repeat sizes stay the same or even get smaller when they are passed to a person’s children.
Inheritance
DM is inherited through autosomal dominant inheritance. This means that equal numbers of males and females are affected. It also means that only one gene in the pair needs to have the mutation in order for a person to be affected. Since a person only passes one copy of each gene onto their children, there is a 50% or one in two chance that a person who has DM will pass it onto each of their children. This percentage is not changed by results of other pregnancies. A person with a premutation also has a 50%, or one in two, chance of passing the altered gene on to each of their children. However, whether or not their children will develop DM1 depends
on whether the trinucleotide repeat becomes further expanded. A person who has repeat numbers in the normal range cannot pass DM1 onto their children.
Demographics
DM occurs in about one of 20,000 people and has been described in people from all over the world.
Signs and symptoms
There is a range in the severity of symptoms in DM and not everyone will have all of the symptoms listed here.
Myotonic dystrophy causes weakness and delayed muscle relaxation called myotonia. Symptoms of DM include facial weakness and a slack jaw, drooping eyelids called ptosis, and muscle wasting in the forearms and calves. A person with DM has difficulty relaxing his or her grasp, especially in the cold. DM affects the heart muscle, causing irregularities in the heartbeat. It also affects the muscles of the digestive system, causing constipation and other digestive problems. DM may cause cataracts, retinal degeneration, low IQ, frontal balding, skin disorders, atrophy of the testicles, and diabetes. It can also cause sleep apnea—a condition in which normal breathing is interrupted during sleep. DM increases the need for sleep and decreases motivation. Severe disabilities do not set in until about 20 years after symptoms begin. Most people with myotonic dystrophy maintain the ability to walk, even late in life.
A severe form of DM, congenital myotonic dystrophy, may appear in newborns of mothers who have DM1. Congenital myotonic dystrophy is marked by severe weakness, poor sucking and swallowing responses, respiratory difficulty, delayed motor development, and mental retardation. Death in infancy is common in this type.
786 |
G A L E E N C Y C L O P E D I A O F G E N E T I C D I S O R D E R S |
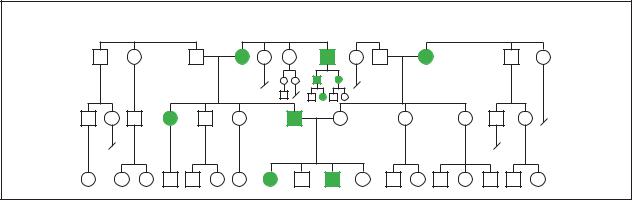
Myotonic Dystrophy
dystrophy Myotonic
(Gale Group)
Some people who have a trinucleotide repeat expansion in their DMPK gene do not have symptoms or have very mild symptoms that go unnoticed. It is not unusual for a woman to be diagnosed with DM after she has an infant with congenital myotonic dystrophy.
Predictive testing
It is possible to test someone who is at risk for developing DM1 before they are showing symptoms to see whether they inherited an expanded trinucleotide repeat. This is called predictive testing. Predictive testing cannot determine the age of onset that someone will begin to have symptoms, or the course of the disease.
Diagnosis
Diagnosis of DM is not difficult once the disease is considered. However, the true problem may be masked because symptoms can begin at any age, can be mild or severe, and can occur with a wide variety of associated complaints. Diagnosis of DM begins with a careful medical history and a thorough physical exam to determine the distribution of symptoms and to rule out other causes. A family history of DM or unexplained weakness helps to establish the diagnosis.
A definitive diagnosis of DM1 is done by genetic testing, usually by taking a small amount of blood. The DNA in the blood cells is examined and the number of repeats in the DMPK gene is determined. Various other tests may be done to help establish the diagnosis, but only rarely would other testing be needed. An electromyogram (EMG) is a test used to examine the response of the muscles to stimulation. Characteristic changes are seen in DM that helps distinguish it from other muscle diseases. Removing a small piece of muscle tissue for microscopic examination is called a muscle biopsy. DM is marked by characteristic changes in the structure of muscle cells that
can be seen on a muscle biopsy. An electrocardiogram could be performed to detect characteristic abnormalities in heart rhythm associated with DM. These symptoms often appear later in the course of the disease.
Prenatal testing
Testing a pregnancy to determine whether an unborn child is affected is possible if genetic testing in a family has identified a DMPK mutation. This can be done at 10–12 weeks gestation by a procedure called chorionic villus sampling (CVS), which involves removing a tiny piece of the placenta and analyzing DNA from its cells. It can also be done by amniocentesis after 16 weeks gestation by removing a small amount of the amniotic fluid surrounding the baby and analyzing the cells in the fluid. Each of these procedures has a small risk of miscarriage associated with it and those who are interested in learning more should check with their doctor or genetic counselor.
Another procedure, called preimplantation diagnosis allows a couple to have a child that is unaffected with the genetic condition in their family. This procedure is experimental and not widely available. Those interested in learning more about this procedure should check with their doctor or genetic counselor.
Treatment and management
Myotonic dystrophy cannot be cured, and no treatment can delay its progression. However, many of the symptoms it causes can be treated. Physical therapy can help preserve or increase strength and flexibility in muscles. Ankle and wrist braces can be used to support weakened limbs. Occupational therapy is used to develop tools and techniques to compensate for loss of strength and dexterity. A speech-language pathologist can provide retraining for weakness in the muscles controlling speech and swallowing.
G A L E E N C Y C L O P E D I A O F G E N E T I C D I S O R D E R S |
787 |
Myotonic dystrophy
Irregularities in the heartbeat may be treated with medication or a pacemaker. A yearly electrocardiogram is usually recommended to monitor the heartbeat. Diabetes mellitus in DM is treated in the same way that it is in the general population. A high-fiber diet can help prevent constipation. Sleep apnea may be treated with surgical procedures to open the airways or with nighttime ventilation. Treatment of sleep apnea may reduce drowsiness. Lens replacement surgery is available when cataracts develop. Pregnant woman should be followed by an obstetrician familiar with the particular problems of DM because complications can occur during pregnancy, labor, and delivery.
Wearing a medical bracelet is advisable. Some emergency medications may have dangerous effects on the heart rhythm in a person with DM. Adverse reactions to general anesthesia may also occur.
Prognosis
The course of myotonic dystrophy varies. When symptoms appear earlier in life, disability tends to become more severe. Occasionally people with DM may require a wheelchair later in life. Children with congenital DM usually require special educational programs and physical and occupational therapy. For both types of DM,
respiratory infections pose a danger when weakness becomes severe.
Resources
PERIODICALS
The International Myotonic Dystrophy Consortium (IDMC). “New nomenclature and DNA testing guidelines for myotonic dystrophy type 1 (DM1).” Neurology 54 (2000): 1218–1221.
Meola, Giovanni. “Myotonic Dystrophies.” Current Opinion in Neurology 13 (2000): 519–525.
ORGANIZATIONS
Muscular Dystrophy Association. 3300 East Sunrise Dr., Tucson, AZ 85718. (520) 529-2000 or (800) 572-1717.http://www.mdausa.org .
WEBSITES
Myotonic Dystrophy Website.http://www.umd.necker.fr/myotonic_dystrophy.html .
Smith, Corrine O’Sullivan. “Myotonic Dystrophy: Making an Informed Choice About Genetic Testing.” University of Washington. http://www.depts.washington.edu/neurogen/Myotonic.pdf .
NCBI Genes and Disease Web Page. http://www.ncbi.nlm.nih
.gov/disease/Myotonic.html .
Gene Clinics. http://www.geneclinics.org .
Karen M. Krajewski, MS, CGC
788 |
G A L E E N C Y C L O P E D I A O F G E N E T I C D I S O R D E R S |
