
Gale Encyclopedia of Genetic Disorder / Gale Encyclopedia of Genetic Disorders, Two Volume Set - Volume 2 - M-Z - I
.pdf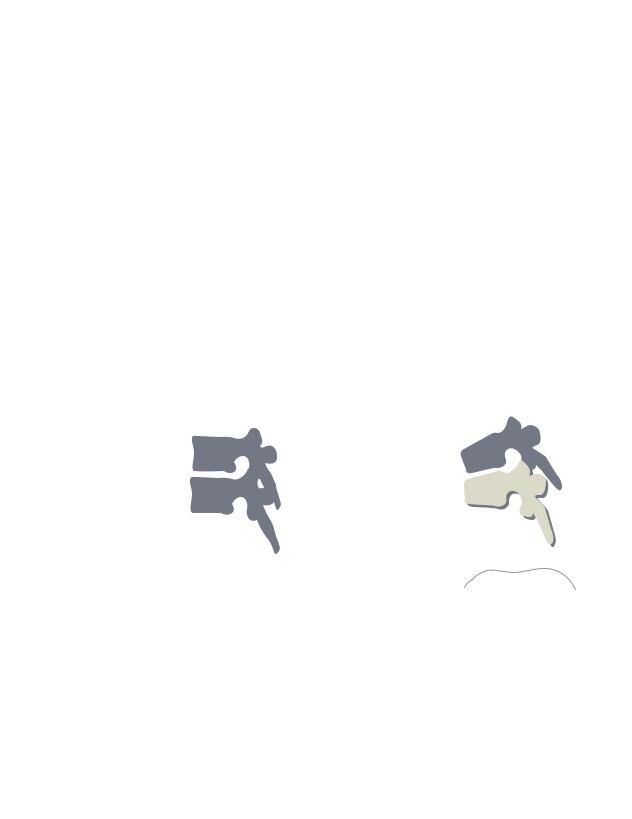
syndrome Marfan
B. Positive thumb sign
A.Pectus excavatum
|
|
|
Normal spine |
Scoliosis |
|
C. Positive elbow sign |
D. |
Scoliosis of the vertebral |
|||
|
|
|
|
|
|
|
|
|
|
|
|
E. |
Normal anatomy |
Kyphosis |
Five common clinical signs for Marfan syndrome. Pectus excavatum (A) refers to the inward curve of the chest. Positive thumb sign (B) is the apperance of the thumb tip when making a closed fist. Positive elbow sign (C) is the ability to touch one’s elbows behind their back. Scoliosis (D) is a marked side-to-side curvature of the spine, and kyphosis (E) is the hunchback form resulting from an outward curvature of the spine.
G A L E E N C Y C L O P E D I A O F G E N E T I C D I S O R D E R S |
709 |
Marfan syndrome
•Cataracts. Patients with Marfan syndrome are more likely to develop cataracts, and to develop them much earlier in life, sometimes as early as 40 years of age.
•Retinal detachment. Patients with Marfan syndrome are more vulnerable to this disorder because of the weakness of their connective tissues. Untreated retinal detachment can cause blindness. The danger of retinal detachment is an important reason for patients to avoid contact sports or other activities that could cause a blow on the head or being knocked to the ground.
•Other facial problems. Patients with Marfan sometimes develop dental problems related to crowding of the teeth caused by a high-arched palate and a narrow jaw.
Other disorders
•Striae. Striae are stretch marks in the skin caused by rapid weight gain or growth; they frequently occur in pregnant women, for example. Patients with Marfan often develop striae over the shoulders, hips, and lower back at an early age because of rapid bone growth. Although the patient may be self-conscious about the striae, they are not a danger to health.
•Obstructive sleep apnea. Obstructive sleep apnea refers to partial obstruction of the airway during sleep, causing irregular breathing and sometimes snoring. In patients with Marfan syndrome, obstructive sleep apnea is caused by the unusual flexibility of the tissues lining the patient’s airway. This disturbed breathing pattern increases the risk of aortic dissection.
Diagnosis
Presently, there is no objective diagnostic test for Marfan syndrome, in part because the disorder does not produce any measurable biochemical changes in the patient’s blood or body fluids, or cellular changes that could be detected from a tissue sample. Although researchers in molecular biology are currently investigating the FBNI gene through a process called mutational analysis, it is presently not useful as a diagnostic test because there is evidence that there can be mutations in the fibrillin gene that do not produce Marfan syndrome. Similarly, there is no reliable prenatal test, although some physicians have used ultrasound to try to determine the length of fetal limbs in at-risk pregnancies.
The diagnosis is made by taking a family history and a thorough examination of the patient’s eyes, heart, and bone structure. The examination should include an echocardiogram taken by a cardiologist, a slit-lamp eye examination by an ophthalmologist, and a work-up of the patient’s spinal column by an orthopedic specialist. In
terms of the cardiac examination, a standard electrocardiogram (EKG) is not sufficient for diagnosis; only the echocardiogram can detect possible enlargement of the aorta. The importance of the slit-lamp examination is that it allows the doctor to detect a dislocated lens, which is a significant indication of the syndrome.
The symptoms of Marfan syndrome in some patients resemble the symptoms of homocystinuria, which is an inherited disorder marked by extremely high levels of homocystine in the patient’s blood and urine. This possibility can be excluded by a urine test.
In other cases, the diagnosis remains uncertain because of the mildness of the patient’s symptoms, the absence of a family history of the syndrome, and other variables. These borderline conditions are sometimes referred to as marfanoid syndromes.
Treatment and management
The treatment and management of Marfan syndrome is tailored to the specific symptoms of each patient. Some patients find that the syndrome has little impact on their overall lifestyle; others have found their lives centered on the disorder.
Cardiovascular system
After a person has been diagnosed with Marfan syndrome, he or she should be monitored with an echocardiogram every six months until it is clear that the aorta is not growing larger. After that, the patient should have an echocardiogram once a year. If the echocardiogram does not allow the physician to visualize all portions of the aorta, CT (computed tomography) or MRI (magnetic resonance imaging) may be used. In cases involving a possible aortic dissection, the patient may be given a TEE (transesophageal echocardiogram).
MEDICATIONS. A patient may be given drugs called beta-blockers to slow down the rate of aortic enlargement and decrease the risk of dissection by lowering the blood pressure and decreasing the forcefulness of the heartbeat. The most commonly used beta-blockers in patients with Marfan are propranolol (Inderal) and atenolol (Tenormin). Patients who are allergic to beta-blockers may be given a calcium blocker such as verapamil.
Because patients with Marfan syndrome are at increased risk for infective endocarditis, they must take a prophylactic dose of an antibiotic before having dental work or minor surgery, as these procedures may allow bacteria to enter the bloodstream. Penicillin and amoxicillin are the antibiotics most often used.
710 |
GALE ENCYCLOPEDIA OF GENETIC DISORDERS |
SURGICAL TREATMENT. Surgery may be necessary if the width of the patient’s aorta increases rapidly or reaches a critical size (about 2 in, 5 cm). As of 2000, the most common surgical treatment involves replacing the patient’s aortic valve and several inches of the aorta itself with a composite graft, which is a prosthetic heart valve sewn into one end of a Dacron tube. This surgery has been performed widely since about 1985; most patients who have had a composite graft have not needed additional surgery.
Patients who have had a valve replaced must take an anticoagulant medication, usually warfarin (Coumadin), in order to minimize the possibility of a clot forming on the prosthetic valve.
Musculoskeletal system
Children diagnosed with Marfan syndrome should be checked for scoliosis by their pediatricians at each annual physical examination. The doctor simply asks the child to bend forward while the back is examined for changes in the curvature. In addition, the child’s spine should be x rayed in order to measure the extent of scoliosis or kyphosis. The curve is measured in degrees by the angle between the vertebrae as seen on the x ray. Curves of 20° or less are not likely to become worse. Curves between 20° and 40° are likely to increase in children or adolescents. Curves of 40° or more are highly likely to worsen, even in an adult, because the spine is so badly imbalanced that the force of gravity will increase the curvature.
Scoliosis between 20° and 40° in children is usually treated with a back brace. The child must wear this appliance about 23 hours a day until growth is complete. If the spinal curvature increases to 40° or 50°, the patient may require surgery in order to prevent lung problems, back pain, and further deformity. Surgical treatment of scoliosis involves straightening the spine with metal rods and fusing the vertebrae in the straightened position.
Spondylolisthesis is treated with a brace in mild cases. If the slippage is more than 30°, the slipped vertebra may require surgical realignment.
Dural ectasia can be distinguished from other causes of back pain on an MRI. Mild cases are usually not treated. Medication or spinal shunting to remove some of the spinal fluid are used to treat severe cases.
Pectus excavatum and pectus carinatum can be treated by surgery. In pectus excavatum, the deformed breastbone and ribs are raised and straightened by a metal bar. After four to six months, the bar is removed in an outpatient procedure.
Protrusio acetabulae may require surgery in adult life to provide the patient with an artificial hip joint, if the arthritic pains are severe.
Pain in the feet or limbs is usually treated with a mild analgesic such as acetaminophen. Patients with Marfan syndrome should consider wearing shoes with low heels, special cushions, or orthotic inserts. Foot surgery is rarely necessary.
Visual and dental concerns
Patients with Marfan syndrome should have a thorough eye examination, including a slit-lamp examination, to test for dislocation of the lens as well as nearsightedness. Dislocation can be treated by a combination of special glasses and daily use of 1% atropine sulfate ophthalmic drops, or by surgery.
Because patients with Marfan syndrome are at increased risk of glaucoma, they should have the fluid pressure inside the eye measured every year as part of an eye examination. Glaucoma can be treated with medications or with surgery.
Cataracts are treated with increasing success by implant surgery. It is important, however, to seek treatment at medical centers with eye surgeons familiar with the possible complications of cataract surgery in patients with Marfan syndrome.
All persons with Marfan syndrome should be taught to recognize the signs of retinal detachment (sudden blurring of vision in one eye becoming progressively worse without pain or redness) and to seek professional help immediately.
Children with Marfan should be evaluated by their dentist at each checkup for crowding of the teeth and possible misalignment, and referred to an orthodontist if necessary.
People with Marfan syndrome should avoid sports or occupations that require heavy weight lifting, rough physical contact, or rapid changes in atmospheric pressure (e.g., scuba diving). Weight lifting increases blood pressure, which in turn may enlarge the aorta. Rough physical contact may cause retinal detachment. Sudden changes in air pressure may produce pneumothorax. Regular noncompetitive physical exercise, however, is beneficial for patients. Good choices include brisk walking, shooting baskets, and slow-paced tennis.
Social and lifestyle issues
Smoking is particularly harmful for patients with Marfan because it increases their risk of emphysema.
Until very recently, women with Marfan syndrome were advised to avoid pregnancy because of the risk of
syndrome Marfan
GALE ENCYCLOPEDIA OF GENETIC DISORDERS |
711 |

Marshall syndrome
aortic enlargement or dissection. The development of beta-blockers and echocardiograms, however, allows doctors now to monitor patients throughout pregnancy. It is recommended that patients have an echocardiogram during each of the three trimesters of pregnancy. Normal, vaginal delivery is not necessarily more stressful than a Caesarian section, but patients in prolonged labor may have a Caesarian birth to reduce strain on the heart. A pregnant woman with Marfan syndrome should also receive genetic counseling regarding the 50% risk of having a child with the syndrome.
Children and adolescents with Marfan syndrome may benefit from supportive counseling regarding appearance, particularly if their symptoms are severe and causing them to withdraw from social activities. In addition, families may wish to seek counseling regarding the effects of the syndrome on relationships within the family. Many people respond with guilt, fear, or blame when a genetic disorder is diagnosed in the family, or they may overprotect the affected member. Support groups are often good sources of information about Marfan syndrome; they can offer helpful suggestions about living with it as well as emotional support.
Prognosis
The prognosis for patient’s with Marfan syndrome has improved markedly in recent years. As of 1995, the life expectancy of people with the syndrome had increased to 72 years; up from 48 years in 1972. This dramatic improvement is attributed to new surgical techniques, improved diagnosis, and new techniques of medical treatment.
The most important single factor in improving the patient’s prognosis is early diagnosis. The earlier that a patient can benefit from the new techniques and lifestyle modifications, the more likely he or she is to have a longer life expectancy.
Resources
BOOKS
Beers, Mark H., and Robert Berkow, eds. Pediatrics Whitehouse Station, NJ: Merck Research Laboratories, 1999.
Pyeritz, Reed E., and Cheryll Gasner. The Marfan Syndrome. New York: National Marfan Syndrome, 1999.
Thoene, Jess G. “Marfan Syndrome.” In Physician’s Guide to Rare Diseases. 2nd ed. Montvale, NJ: Dowden Publishing Company, Inc., 1995.
Wynbrandt, James, and Mark D. Ludman. “Marfan Syndrome.” In The Encyclopedia of Genetic Disorders and Birth
Defects. New York and Oxford: Facts on File, 1991.
PERIODICALS
DePaepe, A., et al. “Revised diagnostic criteria for the Marfan syndrome.” American Journal of Medical Genetics 62 (1996): 417–26.
Shores, J., et al. “Chronic (-adrenergic blockade protects the aorta in the Marfan syndrome: a prospective, randomized trial of propranolol.” New England Journal of Medicine
330 (1994): 1335–41.
Silverman, D., et al. “Life expectancy in the Marfan syndrome.”
American Journal of Cardiology 75 (1995): 157–60.
ORGANIZATION
Alliance of Genetic Support Groups, 4301 Connecticut Avenue, Washington, DC, 20008. (202). 652-5553. http:www
.geneticalliance.org .
National Marfan Foundation, 382 Main Street, Port Washington, NY, 11050. (516). 883-8712. http:www.marfan.org .
Rebecca J. Frey, PhD
Marie-Strumpell spondylitis bechterew syndrome see Ankylosing spondylitis
Maroteaux-Lamy syndrome (MPS VI) see
Mucopolysaccharidosis (MPS)
I Marshall syndrome
Definition
Marshall syndrome is a very rare genetic disorder with an autosomal dominant pattern that equally affects males and females. It is caused by an abnormality in collagen, which is a key part of connective tissue.
Description
Marshall syndrome was first described by Dr. D. Marshall in 1958 and it has been studied periodically by researchers since then. The disease is most apparent in the facial features of those affected, which include an upturned nose, eyes spaced widely apart, making them appear larger than normal, and a flat nasal bridge. This facial formation gives subjects a childlike appearance. The upper part of the skull is unusually thick, and deposits of calcium may appear in the cranium. Patients may also have palate abnormalities. In addition, they may experience early osteoarthritis, particularly in the knees.
712 |
G A L E E N C Y C L O P E D I A O F G E N E T I C D I S O R D E R S |

Myopia (nearsightedness), cataracts, and glaucoma are common in Marshall syndrome. Moderate to severe hearing loss is often preceded by many incidents of otitis media (middle ear infection) and can occur in children as young as age three. Some patients also have osteoarthritis, particularly of the knees.
In the years following Dr. Marshall’s discovery, some physicians have argued that Marshall syndrome is actually a subset of Stickler syndrome, a more common genetic disorder. Individuals with both syndromes have similar facial features and symptoms. However, other experts have argued against this view, stating that Marshall syndrome is a distinct disorder on its own. For example, most patients with Stickler syndrome have cataracts, while this problem is less common among those with Marshall syndrome. In addition, most subjects with Marshall syndrome have moderate to severe hearing loss, which rarely occurs among those with Stickler syndrome, who have normal hearing.
Genetic research performed in 1998 and 1999 revealed that both sides were right. There are clear genetic differences between the two syndromes. There are also patients who have apparent overlaps of both syndromes.
In 1998, a study used genetic testing to establish that a collagen genetic mutation on COL11A1 caused Marshall syndrome and that a change on COL2A1 caused Stickler syndrome. It also found that other types of mutations could cause overlaps of both syndromes.
A study in 1999 described a genetic study of 30 patients from Europe and the United States, all of whom were suspected to have either Marshall or Stickler syndrome. These genetic findings confirmed those of the previous (1998) study. Twenty-three novel mutations of COL11A1 and COL2A1 were found among the subjects. Some patients had genetic overlaps of both Marshall and Stickler syndromes.
Physical differences were also noted between the two syndromes. For example, all the patients with Marshall syndrome had moderate to severe hearing loss, while none of the patients with Stickler syndrome had hearing loss. About half the patients with overlapping disorders of both diseases had hearing loss. All the patients with Marshall syndrome had short noses, compared to about 75% of the patients with Stickler syndrome. Palate abnormalities occur in all patients with Stickler syndrome, compared to only about 80% of those with Marshall syndrome. Also, about a third of the Stickler patients had dental abnormalities, compared to 11% of the patients with Marshall syndrome. Those with Stickler (71%) had a higher percentage of cataracts than those with Marshall syndrome (40%). Patients with
K E Y T E R M S
Cataract—A clouding of the eye lens or its surrounding membrane that obstructs the passage of light resulting in blurry vision. Surgery may be performed to remove the cataract.
Collagen—The main supportive protein of cartilage, connective tissue, tendon, skin, and bone.
Glaucoma—An increase in the fluid eye pressure, eventually leading to damage of the optic nerve and ongoing visual loss.
Myopia—Nearsightedness. Difficulty seeing objects that are far away.
Osteoarthritis—A degenerative joint disease that causes pain and stiffness.
Saddle nose—A sunken nasal bridge.
Marshall syndrome were much more likely to have short stature than those with Stickler syndrome.
Genetic profile
The gene name for Marshall syndrome is Collagen, Type XI, alpha 1. The gene symbol is COL11A1. The chromosomal location is 1p21. Marshall syndrome is an autosomal dominant genetic trait and the risk of an affected parent transmitting the gene to the child is 50%. Human traits are the product of the interaction of two genes from that condition, one received from the father and one from the mother. In dominant disorders, a single copy of the abnormal gene (received from either parent) dominates the normal gene and results in the appearance of the disease. The risk of transmitting the disorder from affected parent to offspring is 50% for each pregnancy regardless of the sex of the resulting child.
Demographics
Because of the rarity of this disease, very little demographic data is available. Less than 100 cases of individuals with this syndrome have been reported worldwide in medical literature. Some cases are probably undiagnosed because of the high expense of genetic testing. It is known that Marshall syndrome presents in infancy or early childhood and severe symptoms such as hearing loss and cataracts manifest before the age of 10 years. Adults with the syndrome retain the facial traits that are characteristic of this disease, such as flat nose, large nasal bridge and widely spaced eyes. Among those with
syndrome Marshall
G A L E E N C Y C L O P E D I A O F G E N E T I C D I S O R D E R S |
713 |

Marshall-Smith syndrome
Stickler syndrome, in contrast, these distinctive facial characteristics diminish in adulthood.
Signs and symptoms
Characteristic features of this disease are short upturned nose with a flat nasal bridge. Some patients also have glaucoma, crossed eyes, detached retinas, and protruding upper teeth. Patients often have short stature compared to other family members without the disease.
Diagnosis
Individuals are diagnosed by their features as well as by the very early onset of serious eye and ear disease. Because Marshall syndrome is an autosomal dominant hereditary disease, physicians can also note the characteristic appearance of the biological parent of the child. Genetic testing is costly, thus, it is not ordered for most people. As a result, people may be diagnosed as possible Marshall syndrome or possible Stickler syndrome, based on their symptoms and appearance.
Treatment and management
Marshall syndrome cannot be cured; however, the symptoms caused by the disease should be treated. Children with Marshall syndrome should have annual eye and ear checkups because of the risk for cataracts and hearing loss. Cataract surgery will be needed if cataracts develop. At present, the only treatment for the progressive hearing loss is a hearing aid. The flat “saddle nose” can be altered with cosmetic surgery. If a child with Marshall syndrome has osteoarthritis, doctors may advise against contact sports.
Prognosis
As they age, vision and hearing problems will generally worsen for patients with Marshall syndrome. Many will also develop osteoarthritis at an earlier age than for patients without Marshall syndrome, such as in the teens or twenties. Because there are so few identified cases, it is unknown what the life expectancy is of afflicted individuals.
Resources
PERIODICALS
Annunen, Susanna, et al. “Splicing mutations of 54-bp exons in the COL11A1 gene cause Marshall syndrome, but other mutations cause overlapping Marshall/Stickler phenotypes.” American Journal of Human Genetics 64 (1999).
Griffith, Andrew J., et al. “Marshall syndrome associated with a splicing defect at the COL11A1 Locus.” American
Journal of Human Genetics 62, no. 4 (1998).
ORGANIZATIONS
National Organization for Rare Disorders (NORD). PO Box 8923, New Fairfield, CT 06812-8923. (203) 746-6518 or (800) 999-6673. Fax: (203) 746-6481. http://www
.rarediseases.org .
Stickler Involved People. 15 Angelina, Augusta, KS 67010. (316) 775-2993. http://www.sticklers.org/sip .
WEBSITES
Annunen, Susanna. “From rare syndromes to a common disease: Mutations in minor cartilage collagen genes cause Marshall and Stickler syndromes and intervertebral disc disease.” Academic dissertation, Oulu University Library, Oulu, Finland. http:/herkules.oulu.fi/ isbn9514254139/ . (1999).
“Entry 120280: Collagen, Type XI, Alpha-1; COL11A1.”
OMIM—Online Mendelian Inheritance in Man.
http://www3.ncbi.nlm.nih.gov/htbin-post/Omim/ dispmim?120280 .
Christine Adamec
I Marshall-Smith syndrome
Definition
Marshall-Smith syndrome is a childhood condition involving specific facial characteristics, bone maturation that is advanced for the individual’s age, failure to grow and gain weight appropriate for the individual’s age, and severe respiratory (breathing) problems.
Description
Marshall-Smith syndrome (MSS) was first described in two males seen in 1971 by Drs. Marshall, Graham, Scott, and Smith. They noticed changes in the skeletal system of these patients. Bones normally mature through several stages, naturally progressing through these stages with time. Specifically, a young child’s bones have more cartilage and less calcium deposits than an adult’s bones. A child’s bones appear less “dense” on an x ray than an adult’s bones. A constant feature of MSS is skeletal maturation that is advanced for age. For example, in 1993 a newborn child with MSS was found to have the “bone age” of a three year-old child.
Specific facial features in MSS include a wide and prominent forehead, protruding and widely spaced eyes, a very small chin, and a small, upturned nose. Because individuals may not gain weight or grow well, they are often smaller than other children of the same age. There are often problems with structures in the respiratory tract (such as the larynx and trachea) and this can lead to dif-
714 |
G A L E E N C Y C L O P E D I A O F G E N E T I C D I S O R D E R S |

ficulty with breathing. Pneumonia, or a lung infection, is common because of this; these can occur several times.
Significant mental and physical delays are almost always expected in MSS. Since children with MSS are often hospitalized for long periods of time to help treat respiratory problems, they may also be slower to do physical things like crawling or walking.
No two patients with MSS have the exact same symptoms, as there is some variability with the condition. There are no alternate names for Marshall-Smith syndrome, though it is sometimes incorrectly referred to as Weaver syndrome, a separate condition with similar symptoms.
Families with MSS can be put under a great deal of stress, because long-term hospitalizations in the intensive care unit are common for children with MSS.
Genetic profile
The vast majority of people with MSS are unique in their family; there is usually no family history of the condition. Because of this, MSS is thought to be a random, sporadic event when it occurs. As of 2001, no specific gene has been associated with MSS, and other genetic background is still largely unknown. Standard genetic testing, such as chromosome analysis and metabolic studies, typically are normal for patients with MSS.
In 1999, a group in Saudi Arabia reported a young girl with features of MSS who had a chromosome abnormality. She was found to have some duplication of the material on a region of chromosome 2. This has led researchers to believe that the gene for MSS may actually be on chromosome 2. As of 2001, this is the only individual with MSS found to have a chromosome abnormality. Current research is under way to determine the exact genetic cause for MSS.
Demographics
Marshall-Smith syndrome is very rare in the general population. In fact, no statistical rates are available for the condition. It appears to be present across the world, affecting males and females equally.
Signs and symptoms
The most medically serious complication in MSS is the associated respiratory problems. Structures in the respiratory system, such as the larynx and trachea, may not function properly because they can be “floppy,” soft, and less muscular than usual. Because of this, airways can become plugged or clogged, since air does not move through to clear them like usual. Mucus may start col-
K E Y T E R M S
Cartilage—Supportive connective tissue which cushions bone at the joints or which connects muscle to bone.
Corpus callosum—A thick bundle of nerve fibers deep in the center of the forebrain that provides communications between the right and left cerebral hemispheres.
Gastrostomy—The construction of an artificial opening from the stomach through the abdominal wall to permit the intake of food.
Hirsuitism—The presence of coarse hair on the face, chest, upper back, or abdomen in a female as a result of excessive androgen production.
Larynx—The voice box, or organ that contains the vocal cords.
Phalanges—Long bones of the fingers and toes, divided by cartilage around the knuckles.
Trachea—Long tube connecting from the larynx down into the lungs, responsible for passing air.
Tracheostomy—An opening surgically created in the trachea (windpipe) through the neck to improve breathing.
Umbilical hernia—Protrusion of the bowels through the abdominal wall, underneath the navel.
lecting, causing an increased amount of bacteria that can lead to pneumonia. Ear infections are common, because the bacteria can spread to the ears as well. Internal nasal passages may be narrower in people with MSS, which can also pose difficulty with breathing.
Children with MSS may have problems with eating, due to similar reasons that they may have difficulty breathing. Additionally, they may have a weak “suck” and “swallowing” reflex, normally controlled by muscular movements. As mentioned earlier, another feature of MSS is lack of proper growth and weight gain. This can be in part due to the difficulty in feeding for these individuals, though they are often very small even at birth.
Advanced bone age is present in all people with MSS. In particular, the bones of someone with MSS appear more dense on an x ray than they should, according to their age. While x rays of their hands and wrists often determine a person’s “bone age,” people with MSS often have a generalized advanced bone age within their
syndrome Smith-Marshall
G A L E E N C Y C L O P E D I A O F G E N E T I C D I S O R D E R S |
715 |
Marshall-Smith syndrome
entire skeleton. They may also have broad middle phalanges of the hand, which can be seen on an x ray.
Facial characteristics of people with MSS include those mentioned earlier, but other features may also occasionally be present. These can be blue-tinged sclerae (the white sections of the eyes), a large head circumference (measurement around the head), and a small, triangleshaped face (with the point of the triangle being at the chin).
Occasionally, creases in the hands are “deeper” than usual in people with MSS. The first (“big”) toe can also be longer and bigger than usual. Additional features include hirsuitism and an umbilical hernia. Hearing loss can sometimes occur. Ears may be larger, have a “crumpled” appearance, or be lower on the head than usual.
Changes in the brain can occur in MSS. An individual was reported in 1997 to have a smaller optic nerve (the nerve the connects the eyes to the brain) than usual, and had some vision problems as a result. Some children may be missing the corpus callosum, a structure in the brain. Mental and physical delays are commonly present in MSS, and are usually quite significant. These may in part be due to the brain abnormalities that are sometimes seen. There may be partial to complete lack of speech for individuals with MSS, another sign of the mental delays.
Diagnosis
Because there is no genetic testing available for Marshall-Smith syndrome, all individuals have been diagnosed through a careful physical examination and study of their medical history.
Advanced skeletal age can be seen on x rays of the patient’s hands and wrists, since this is the typical way to assess bone age. A full x ray survey of the body is a good way to assess age of other bones as well. Advanced bone age is always seen in Marshall-Smith syndrome, but it may also be present in other genetic syndromes. Sotos syndrome involves similar skeletal findings, but individuals are generally larger than usual and can have mental delays. Weaver syndrome includes advanced skeletal maturation, but individuals are often larger than usual and have other specific facial characteristics (such as very narrow, small eyes). These and other conditions can be ruled out if the respiratory complications and facial characteristics seen in MSS are not present.
Treatment and management
As mentioned earlier, long hospitalizations are common for people with MSS. Most of these involve treating severe respiratory complications of MSS. These types of complications often necessitate placing a tracheotomy to assist with breathing. Manual removal of the mucus
buildup by suctioning near the tracheotomy is common. Frequent pneumonia is common, and intravenous antibiotics are often the treatment, as in people without MSS. There is no specific treatment for the advanced bone age.
Because feeding can be difficult for children with MSS, a gastrostomy is often needed, and feeding is done directly through the gastrostomy tube. It is a challenge to make sure children with MSS maintain proper growth, and sometimes a gastrostomy is the only way to achieve this.
Prognosis
Marshall-Smith syndrome is considered a childhood condition because affected individuals do not typically survive past childhood. There is no long-term research on the disease due to it being rare and not typically present in adults.
Most children with MSS die in early infancy, often by three years of age, due to severe respiratory complications and infections that may result from them. There have been reports of children surviving until age seven or eight, but these children did not have severe respiratory problems. These children give hope that the condition is variable, and not every person diagnosed with the condition will have a severely shortened lifespan.
Resources
ORGANIZATIONS
Arc (a National Organization on Mental Retardation). 1010 Wayne Ave., Suite 650, Silver Spring, MD 20910. (800) 433-5255. Fax: (301) 565-5342, Info@thearc.org,http://www.thearclink.org .
Human Growth Foundation. 997 Glen Cove Ave., Glen Head, NY 11545. (800) 451-6434 or (516) 671-4041. Fax: (516) 671-4055. hgfound@erols.com. http://www. hgf1 @hgfound.org .
Little People of America, Inc. National Headquarters, PO Box 745, Lubbock, TX 79408, Phone: (806) 737-8186 or (888) LPA-2001. Fax: (806) 797-8830, lpadatabase@juno.com,http://www.lpaonline.org .
Little People’s Research Fund, Inc. 80 Sister Pierre Dr., Towson, MD 21204-7534. (800) 232-5773 or (410) 4940055, Fax: (410) 494-0062. http://pixelscapes.com/ lprf .
MAGIC Foundation for Children’s Growth. 1327 N. Harlem Ave., Oak Park, IL 60302. (800) 362-4423 or (708) 3830808. Fax: (708) 383-0899. mary@magicfoundation.org.http://www.magicfoundation.org .
WEBSITES
“Marshall-Smith syndrome.” Health Library. http://hvlib
.integris-health.com/Library/HealthGuide/Illness Conditions .
Deepti Babu, MS
716 |
G A L E E N C Y C L O P E D I A O F G E N E T I C D I S O R D E R S |

Martin-Bell syndrome see Fragile X syndrome
MASA syndrome see X-linked hydrocephaly
I MCAD deficiency
Definition
Medium chain acyl-CoA dehydrogenase (MCAD) deficiency is a rare genetic disorder characterized by a deficiency of the MCAD enzyme. This enzyme is responsible for the breakdown of certain fatty acids into chemical forms that are useable by the human body. MCAD deficiency accounts for approximately one to three of every 100 cases of sudden infant death syndrome (SIDS). MCAD deficiency is transmitted through a non-sex linked (autosomal) recessive trait. The first recognized cases of MCAD deficiency were reported in 1982.
Description
Medium chain acyl-CoA dehydrogenase (MCAD) is one of four enzymes in the mitochondria of the cells that is responsible for the breakdown of medium chain fatty acids into acetyl-CoA. Medium chain fatty acids are defined as fatty acids containing between four and 14 carbon atoms. Acetyl-CoA, the desired product of the breakdown of these fatty acids, is a two-carbon molecule. MCAD is the enzyme responsible for the breakdown of straight-chain fatty acids with four to 14 carbons. There are two other enzymes that are responsible for the breakdown of short straight-chain chain (less than four carbon) fatty acids, and long straight-chain (more than 14 carbon) fatty acids. These other two enzymes are not able to take over the function of MCAD when MCAD is deficient.
Individuals affected with MCAD deficiency produce a form of the MCAD enzyme that is not nearly as efficient as the normal form of MCAD. This lack of efficiency results in a greatly diminished, but still functional, capability to break down medium chain fatty acids.
Genetic profile
The gene that is responsible for the production of MCAD is located on chromosome 1 at 1p31. Twenty-six different mutations of this gene have been identified as causing MCAD deficiency; however, 95–98% of all cases are the result of a single point mutation. In this mutation, an adenosine is substituted for a guanine in base 985
K E Y T E R M S
Apnea—An irregular breathing pattern characterized by abnormally long periods of the complete cessation of breathing.
Carnitine—An amino acid necessary for metabolism of the long-chain fatty acid portion of lipids. Also called vitamin B7.
Enzyme efficiency—The rate at which an enzyme can perform the chemical transformation it is expected to accomplish. This is also called turnover rate.
Founder effect—Increased frequency of a gene mutation in a population that was founded by a small ancestral group of people, at least one of whom was a carrier of the gene mutation.
Hepatomegaly—An abnormally large liver.
Hyperammonemia—An excess of ammonia in the blood.
Hypoglycemia—An abnormally low glucose (blood sugar) concentration in the blood.
Medium chain acyl-CoA dehydrogenase—
Abbreviated MCAD, this is the enzyme responsible for the breakdown of medium chain fatty acids in humans. People affected with MCAD deficiency produce a form of MCAD that is not as efficient as the normal form of MCAD.
Medium chain fatty acids—Fatty acids containing between four and 14 carbon atoms.
(G985A), which causes a substitution of lysine (AAA) by glutamic acid (GAA) in residue 329 of the MCAD protein.
MCAD deficiency is a recessive disorder. This means that in order for a person to be affected with MCAD deficiency, he or she must carry two abnormal copies of the MCAD gene. In a population of individuals known to be affected with the G985A mutation, 81% were found to be homozygous for this mutation (two chromosomes, each with the same mutation). The remaining 19% were found to be heterozygous for the G985A mutation (only one chromosome carried the G985A mutation), but their other chromosomes carried one of the other MCAD gene mutations.
Demographics
MCAD deficiency is estimated to occur in approximately one out of every 13,000 to 20,000 live births. This estimate is confounded to a certain degree by the fact that
deficiency MCAD
G A L E E N C Y C L O P E D I A O F G E N E T I C D I S O R D E R S |
717 |
MCAD deficiency
up to 25% of all individuals affected with MCAD deficiency die the first time they exhibit any symptoms of the disease. Many of these children are often misdiagnosed with either sudden infant death syndrome (SIDS) or Reye syndrome. Unless an autopsy is performed, MCAD generally goes undetected in these individuals; and, even then, unless the physician performing the autopsy is familiar with MCAD deficiency, the cause of death may still be misreported.
MCAD deficiency is seen almost exclusively in Caucasians of Northern European descent (this includes people from every European country not bordering the Mediterranean Sea). Approximately 80% of the Caucasian population of the United States can be considered a part of this subpopulation. In this subpopulation, it is estimated that one in every 40 to 100 people is a carrier of the G985A mutation, and one in every 6,500 to 20,000 people is homozygous in this mutation. Homozygous individuals (carriers of two sets of the G985A mutation) should be affected with MCAD deficiency; however, the incidence rate of MCAD deficiency is lower than that predicted from the carrier populations. There are two possible reasons for the lower number of observed cases of MCAD deficiency than the carrier data suggests should occur. First, many individuals with MCAD deficiency may be misdiagnosed. Secondly, there may be a significant number of homozygous people who for unknown reasons remain unaffected (asymptomatic).
As a comparison, one in every 29 Caucasians is a carrier for cystic fibrosis, but only one in every 3,300 people in this subpopulation develop the disease.
The high frequency of a single mutation leading to MCAD deficiency, combined with the extreme similarity of the other known mutations to this mutation, and the high concentration of MCAD deficiency within a single subpopulation, suggests a founder effect from a single person in a Germanic tribe.
Because MCAD deficiency is a recessive disease, both parents must be carriers of this trait in order for their children to be affected. If both parents carry a copy of the mutated gene, there is a 25% likelihood that their child will be homozygous for MCAD deficiency. Genetically, the probability that an affected person will have a sibling who is also affected is also 25%. In population studies of known MCAD deficient individuals, it has been observed that an average of 32% of these individuals have at least one sibling either known to be affected with MCAD deficiency or to have died with a misdiagnosis of SIDS.
Signs and symptoms
There is no classic set of symptoms that characterize MCAD deficiency. The severity of symptoms observed in
individuals affected with MCAD deficiency ranges from no symptoms at all (asymptomatic) to the occurrence of death upon the first onset of symptoms. The first symptoms of MCAD deficiency generally occur within the first three years of life. The average age of onset of the first symptoms is one year of age. Some individuals become symptomatic prior to birth. The onset of symptoms in adults is extremely rare.
Lethargy and persistent vomiting are the most typical symptoms of MCAD deficiency. The first episode of symptoms is generally preceded by a 12 to 16 hour period of stress. Most affected individuals show intermittent periods of low blood sugar (hypoglycemia) and higher than normal amounts of ammonia in the blood (hyperammonemia). An abnormally large liver (hepatomegaly) is also associated with MCAD deficiency.
Approximately half of all individuals showing symptoms of MCAD deficiency for the first time experience respiratory arrest, cardiac arrest, and/or sudden infant death. Between 20% and 25% of all MCAD deficiency affected infants die during their first episodes of symptoms.
Some individuals affected with MCAD deficiency also are affected with a degenerative disease of the brain and central nervous system (encephalopathy). Seizures, coma, and periods of halted breathing (apnea) have also been seen in people with MCAD deficiencies.
Long-term symptoms of MCAD deficiency may include: attention deficit disorder (ADD), cerebral palsy, mental retardation, and/or developmental delays.
The severity of the symptoms associated this MCAD deficiency is linked to the age of the person when the symptoms first happen. The risk of dying from an onset of the disease is slightly higher in individuals who show the first symptoms after the age of one year. The highest risk ages are the ages of 15 to 26 months. Seizures and encephalopathy are most frequently seen in affected individuals between the ages of 12 and 18 months. Seizures at these ages are often associated with future death during a symptomatic episode, recurrent seizures throughout life, the development of cerebral palsy, and/or the development of speech disabilities.
Diagnosis
The Departments of Health in Massachusetts and North Carolina require mandatory newborn screening for MCAD deficiency. California has a voluntary newborn screening policy. Additionally, Neo Gen Screening offers voluntary newborn screening at birthing centers throughout the Northeastern United States. In September 2000, Iowa also began a pilot program to screen all newborns in
718 |
G A L E E N C Y C L O P E D I A O F G E N E T I C D I S O R D E R S |
