
Gale Encyclopedia of Genetic Disorder / Gale Encyclopedia of Genetic Disorders, Two Volume Set - Volume 2 - M-Z - I
.pdf
other types of genes have been identified which seem to be responsible for the abnormal processing of phenylalanine in brain tissue. These abnormalities add to the severity of PKU symptoms experienced by patients who inherit these genes. In more detail, the association of multiple types of genes with a single condition, such as PKU, is referred to as molecular heterogeneity.
Demographics
One in 50 individuals in the United States have inherited a gene for PKU. About five million Americans are PKU carriers. About one in 15,000 babies test positive for PKU in the United States. Studies indicate that the incidence of this disease in Caucasian and Native American populations is higher than in AfricanAmerican, Hispanic, and Asian populations.
Signs and symptoms
Untreated PKU patients develop a broad range of symptoms related to severely impaired cognitive function, sometimes referred to as mental retardation. Other symptoms can include extreme patterns of behavior, delayed speech development, seizures, a characteristic body odor, and light body pigmentation. The light pigmentation is due to a lack of melanin, which normally colors the hair, skin, and eyes. Melanin is made from the amino acid tyrosine, which is lacking in untreated cases of PKU. Physiologically, PKU patients show high levels of phenylalanine and low levels of tyrosine in the blood. Babies do not show any visible symptoms of the disease for the first few months of life. However, typical PKU symptoms usually do show up by a baby’s first birthday.
Diagnosis
The primary diagnostic test for PKU is the measurement of phenylalanine levels in a drop of blood taken from the heel of a newborn baby’s foot. This screening procedure is referred to as the Guthrie test (Guthrie bacterial inhibition assay). In this test, PKU is confirmed by the appearance of bacteria growing around high concentrations of phenylalanine in the blood spot. PKU testing was introduced in the early 1960s and is the largest genetic screening program in the United States. It is required by law in all 50 states. Early diagnosis is critical. It ensures the early treatment PKU babies need to develop normally and avoid the complications of PKU.
The American Academy of Pediatrics recommends that this test be performed on infants between 24 hours and seven days after birth. The preferred time for testing is after the baby’s first feeding. If the initial PKU test produces a positive result, then follow-up tests are performed to confirm the diagnosis and to determine if the
A technician is performing a test to screen for PKU.
(Custom Medical Stock Photo, Inc.)
elevated phenylalanine levels may be caused by some medical condition other than PKU. Treatment for PKU is recommended for babies that show a blood phenylalanine level of 7–10 mg/dL or higher for more than a few consecutive days. Another, more accurate test procedure for PKU measures the ratio (comparison) of the amount of phenylalanine to the amount of tyrosine in the blood.
Newer diagnostic procedures (called mutation analysis and genotype determination) can actually identify the specific types of PAH gene mutations inherited by PKU infants. Large-scale studies have helped to clarify how various mutations affect the ability of patients to process phenylalanine. This information can help doctors develop more effective customized treatment plans for each of their PKU patients.
Treatment and management
The severity of the PKU symptoms experienced by people with this disease is determined by both lifestyle
Phenylketonuria
G A L E E N C Y C L O P E D I A O F G E N E T I C D I S O R D E R S |
921 |
Phenylketonuria
and genetic factors. In the early 1950s, researchers first demonstrated that phenylalanine-restricted diets could eliminate most of the typical PKU symptoms—except for mental retardation. Today, dietary therapy (also called nutrition therapy) is the most common form of treatment for PKU patients. PKU patients who receive early and consistent dietary therapy can develop fairly normal mental capacity to within about five IQ points of their healthy peers. By comparison, untreated PKU patients generally have IQ scores below 50.
Infants with PKU should be put on a specialized diet as soon as they are diagnosed to avoid progressive brain damage and other problems caused by an accumulation of phenylalanine in the body. A PKU diet helps patients maintain very low blood levels of phenylalanine by restricting the intake of natural foods that contain this amino acid. Even breast milk is a problem for PKU babies. Special PKU dietary mixtures or formulas are usually obtained from medical clinics or pharmacies.
Phenylalanine is actually an essential amino acid. This means that it has to be obtained from food because the body cannot produce this substance on its own. Typical diets prescribed for PKU patients provide very small amounts of phenylalanine and higher quantities of other amino acids, including tyrosine. The amount of allowable phenylalanine can be increased slightly as a child becomes older.
In addition, PKU diets include all the nutrients normally required for good health and normal growth, such as carbohydrates, fats, vitamins, and minerals. High protein foods like meat, fish, chicken, eggs, nuts, beans, milk, and other dairy products are banned from PKU diets. Small amounts of moderate protein foods (such as grains and potatoes) and low protein foods (some fruits and vegetables, low protein breads and pastas) are allowed. Sugar-free foods, such as diet soda, which contain the artificial sweetener aspartame, are also prohibited foods for patients with PKU. That is because aspartame contains the amino acid phenylalanine.
Ideally, school-age children with PKU should be taught to assume responsibility for managing their diet, recording food intake, and for performing simple blood tests to monitor their phenylalanine levels. Blood tests should be done in the early morning when phenylalanine levels are highest. Infants and young children require more frequent blood tests than older children and adults. The amount of natural foods allowed in a diet could be adjusted to ensure that the level of phenylalanine in the blood is kept within a safe range—two to 6 mg/dL before 12 years of age and 2–15 mg/dL for PKU patients over 12 years old.
A specialized PKU diet can cause abnormal fluctuations in tyrosine levels throughout the day. Thus, some
health professionals recommend adding time released tyrosine that can provide a more constant supply of this amino acid to the body. It should be noted that some PKU patients show signs of learning disabilities even with a special diet containing extra tyrosine. Research studies suggest that these patients may not be able to process tyrosine normally.
For PKU caregivers, providing a diet that is appealing as well as healthy and nutritious is a constant challenge. Many patients with PKU, especially teenagers, find it difficult to stick to the relatively bland PKU diet for extended periods of time. Some older patients decide to go off their diet plan simply because they feel healthy. However, many patients who abandon careful nutritional management develop cognitive problems, such as difficulties remembering, maintaining focus, and paying attention. Many PKU health professionals contend that all patients with PKU should adhere to a strictly controlled diet for life.
One promising line of PKU research involves the synthesis (manufacturing) of a new type of enzyme that can break down phenylalanine in food consumed by the patient. This medication would be taken orally and could prevent the absorption of digested phenylalanine into the patient’s bloodstream.
In general, medical researchers express concern about the great variation in treatment programs currently available to PKU patients around the world. They have highlighted the urgent need for new, consistent international standards for proper management of PKU patients, which should emphasize comprehensive psychological as well as physiological monitoring and assessment.
PKU and Pregnancy
Women with PKU must be especially careful with their diets if they want to have children. They should ensure that phenylalanine blood levels are under control before conception and throughout pregnancy. Mothers with elevated (higher than normal) phenylalanine levels are high risk for having babies with significant birth disorders, such as microencephaly (smaller than normal head size), and congenital heart disease (abnormal heart structure and function), stunted growth, mental impairment, and psychomotor (coordination) difficulties. This condition is referred to as maternal PKU and can even affect babies who do not have the PKU disease.
Prognosis
Early newborn screening, careful monitoring, and a life-long strict dietary management can help PKU patients to live normal, healthy, and long lives.
922 |
G A L E E N C Y C L O P E D I A O F G E N E T I C D I S O R D E R S |

Resources
BOOKS
Brust, John C. M. The Practice Of Neural Science: From
Synapses To Symptoms. New York: McGraw-Hill, 2000.
Gilroy, John. Basic Neurology. 3rd ed. New York: McGrawHill, 2000.
Koch, Jean Holt. Robert Guthrie—The PKU Story: Crusade
Against Mental Retardation. Pasadena, CA: Hope Publishing House, 1997.
Ratey, John J. A User’s Guide To The Brain: Perception, Attention, And The Four Theaters Of The Brain. 1st ed. New York: Pantheon Books, 2001.
Schuett, Virginia E. Low Protein Cookery For Phenylketonuria.
3rd ed. Madison: University of Wisconsin Press, 1997.
Walker, John M. Genetics and You. Totowa, N.J.: Humana Press, 1996.
Weiner, William J., Christopher G. Goetz, eds. Neurology For The Non-Neurologist. 4th ed. Philadelphia: Lippincott, Williams & Wilkins, 1999.
PERIODICALS
Burgard, P. “Development of intelligence in early treated phenylketonuria.” European Journal of Pediatrics 159, Suppl. 2 (October 2000): S74–9.
Chang, Pi-Nian, Robert M. Gray, and Lisa Lehn O’Brien. “Review: Patterns of academic achievement among patients treated early with phenylketonuria.” European Journal of Pediatrics 159, no.14 (2000): S96–9.
Eastman, J.W., et al. “Use of the phenylalanine:tyrosine ratio to test newborns for phenylketonuria in a large public health screening programme.” Journal of Medical Screening 7, no. 3 (2000): 131–5.
MacDonald, A. “Diet and compliance in phenylketonuria.”
European Journal of Pediatrics 159, Suppl. 2 (Oct. 2000): S136–41.
Smith, Isabel, and Julie Knowles. “Behaviour in early treated phenylketonuria: a systematic review.” European Journal of Pediatrics 159, no. 14 (2000): S89–93.
Stemerdink, B.A., et al. “Behaviour and school achievement in patients with early and continuously treated phenylketonuria.” Journal of Inherited Metabolic Disorders 23, no. 6 (2000): 548–62.
van Spronsen, F.J.F., et al. “Phenylketonuria: tyrosine supplementation in phenylalanine-restricted diets.”
American Journal of Clinical Nutrition 73, no. 2 (2001): 153–7.
Wappner, Rebecca, et al. “Management of Phenylketonuria for Optimal Outcome: A Review of Guidelines for Phenylketonuria Management and a Report of Surveys of Parents, Patients, and Clinic Directors.” Pediatrics 104, no. 6 (December 1999): e68.
ORGANIZATIONS
Allergy and Asthma Network. Mothers of Asthmatics, Inc. 2751 Prosperity Ave., Suite 150, Fairfax, VA 22031. (800) 878-4403. Fax: (703)573-7794.
American Academy of Allergy, Asthma & Immunology. 611 E. Wells St, Milwaukee, WI 53202. (414) 272-6071. Fax: (414) 272-6070. http://www.aaaai.org/default.stm .
Centers for Disease Control. GDP Office, 4770 Buford Highway NE, Atlanta, GA 30341-3724. (770) 488-3235.http://www.cdc.gov/genetics .
Children’s PKU Network. 1520 State St., Suite 111, San Diego, CA 92101-2930. (619) 233-3202. Fax: (619) 233 0838. pkunetwork@aol.com.
March of Dimes Birth Defects Foundation. 1275 Mamaroneck Ave., White Plains, NY 10605. (888) 663-4637. resourcecenter@modimes.org. http://www.modimes
.org .
National PKU News. Virginia Schuett, editor/dietician. 6869 Woodlawn Avenue NE #116, Seattle, WA 98115-5469. (206) 525-8140. Fax: (206) 525-5023. http://www
.pkunews.org .
University of Washington PKU Clinic. CHDD, Box 357920, University of Washington, Seattle, WA. (206) 685-3015. Within Washington State: (877) 685-3015. Clinic Coordinator: vam@u.washington.edu. http://depts
.washington.edu/pku/contact.html. .
WEBSITES
Consensus Development Conference on Phenylketonuria (PKU): Screening and Management, October 16–18, 2000. http://odp.od.nih.gov/consensus/news/upcoming/ pku/pku_info.htm#overview .
Genetics and Public Health in the 21st Century. Using Genetic
Information to Improve Health and Prevent Disease.
http://www.cdc.gov/genetics/_archive/publications/
Table .
Marshall G. Letcher, MA
Phocomelia see Roberts SC phocomelia
Phytanic acid oxidase deficiency see
Refsum disease
Phytanic acid storage disease see Infantile refsum disease
I Pierre-Robin sequence
Definition
Pierre-Robin Sequence consists of the micrognathia, (small lower jaw), or retrognathia (lower jaw displaced to
sequence Robin-Pierre
G A L E E N C Y C L O P E D I A O F G E N E T I C D I S O R D E R S |
923 |

Pierre-Robin sequence
K E Y T E R M S
Endoscopy—A slender, tubular optical instrument used as a viewing system for examining an inner part of the body and, with an attached instrument, for biopsy or surgery.
Fibroid—A non-cancerous tumor of connective tissue made of elongated, threadlike structures, or fibers, which usually grow slowly and are contained within an irregular shape. Fibroids are firm in consistency but may become painful if they start to break down or apply pressure to areas within the body. They frequently occur in the uterus and are generally left alone unless growing rapidly or causing other problems. Surgery is needed to remove fibroids.
Uterus—A muscular, hollow organ of the female reproductive tract. The uterus contains and nourishes the embryo and fetus from the time the fertilized egg is implanted until birth.
the back), glossoptosis (displacement of the tongue into the throat) and obstruction of the airway. It is usually accompanied by a cleft palate (an opening in the roof of the mouth). The term sequence is used to describe the pattern of multiple anomalies derived from a single known prior anomaly or mechanical factor.
Description
Children born with Pierre-Robin sequence are found to have small mandibles (lower jaws), or mandibles that are displaced back, tongues that are pushed back into the throat, and difficulty breathing of varying degrees. They also have difficulty feeding. Pierre-Robin sequence is usually accompanied by a cleft palate. It is also known as Pierre-Robin syndrome.
Genetic profile
Pierre-Robin sequence can occur in association with other syndromes; isolated (not associated with other malformations); or in associations with other developmental disorders that do not represent a specific syndrome. Heredity has not been proven to be a factor in the cause of isolated Pierre-Robin sequence. Pierre-Robin sequence found in association with numerous syndromes may have a mode of inheritance that is related to the syndrome itself. The mode of inheritance includes single gene as well as chromosomal abnormalities.
The cause of the abnormal lower jaw in Pierre-Robin syndrome may be mechanical, genetic, teratogenic, or
multi-factorial. Mechanical factors such as fibroids inside the uterus may constrict the lower jaw preventing it from growing. Single gene or chromosomal abnormalities produce syndromes that have Pierre-Robin sequence. Teratogenic (anything that affects development of the embryo) causes include maternal use of alcohol. Multi-factorial inheritance means that the cause is a combination of environmental and hereditary factors.
Demographics
The incidence of Pierre-Robin sequence is reported to be one out of 8,500 live births. Other reports show that the range is one out of 2,000 to one out of 50,000 live births. This wide range is due to different diagnostic criteria and the presence or absence of associated syndromes. Fewer than 20% of newborns born with PierreRobin sequence have the isolated type.
Signs and symptoms
In Pierre-Robin sequence, the lower jaw of the fetus displaces the tongue backwards into the throat. The tongue located in this abnormal position blocks the embryonic structures from joining in the midline in order to form the palate, the roof of the mouth. The result is a cleft palate, which is an opening in the roof of the mouth. The size of the cleft palate varies as well as its position. It is not present in all patients.
Babies born with Pierre-Robin have difficulty feeding and breathing because the tongue—pushed backwards by the lower jaw—obstructs the throat. Feeding and breathing difficulties may be very mild or very severe.
Affected persons may also develop hearing problems due to fluid collecting in the ears.
Diagnosis
Prenatal ultrasonic examination may show findings to indicate the possibility of Pierre-Robin sequence alerting the physician to be prepared at birth for the possibility of the baby having breathing and feeding problems.
Treatment and management
The type of treatment varies according to the severity of the symptoms and their duration. Babies may not require any therapy if they have no symptoms of breathing difficulties and no feeding difficulties
If breathing difficulties are mild, the easiest management is keeping the baby in the prone position. This position causes the tongue to fall forward, relieving the obstruction. A thorough evaluation of these patients must
924 |
G A L E E N C Y C L O P E D I A O F G E N E T I C D I S O R D E R S |
be conducted, which includes endoscopy of the airways and upper digestive tract. Close monitoring must be maintained because the prone position may obscure breathing difficulties.
If positioning the patient prone fails, a nasopharyngeal airway may be used but only for a short time. The airway is a tube passed through the nose into the upper airway, which the baby can breath through.
If the above methods fail or are required for a prolonged length of time, then some type of surgical intervention will be required. Surgical procedures include glossopexy, in which the tongue is sutured to the lower lip in order to prevent it from moving back into the throat causing obstruction. Subperiosteal release of the floor of the mouth muscles on the lower jaw is an operation in which the tongue can no longer move back into the throat because muscles are released from their insertions. Tracheotomy is performed by surgically cutting an opening in the trachea (windpipe). This opening bypasses the obstruction. The choice of surgical intervention varies according to the duration and severity of respiratory obstruction; other causes of respiratory obstruction that may be present; and the experience of the surgeon. Glossopexy and tracheotomy are temporary and reversed when the baby can breath adequately on its own.
The treatment of feeding difficulty varies according to the degree of difficulty. It has been found that the severity of feeding difficulty is proportional to the severity of airway obstruction. Feeding is usually accomplished with specialized cleft palate nipples and bottles or nasogastric tubes (a feeding tube passed through the nose and into the stomach). Sometimes a gastrotomy tube is needed for feeding. This is a tube passed through a surgical opening made in the abdominal wall and stomach.
Children with Pierre-Robin sequence are prone to hearing loss due to fluid collecting behind the tympanic membrane (ear drum), and may require drainage tubes placed into the ear.
If the child has a cleft palate, it is usually surgically repaired between the ages of nine and 18 months.
Prognosis
The prognosis for individuals with Pierre-Robin sequence varies with the severity of symptoms and if it is associated with other congenital abnormalities. The more severe the symptoms and associated congenital abnormalities, the greater the risk of complications.
The rate at which the lower jaw starts to catch up in growth depends on the cause of Pierre-Robin
sequence. The majority of children with the isolated type will achieve near normal jaw size within a few years of birth. If Pierre-Robin sequence is part of a syndrome that has a small jaw, the jaw may remain small throughout life.
Resources
PERIODICALS
Bath, A. P. “Management of the upper airway obstruction in Pierre Robin Sequence.” Journal of Laryngology and
Otology 111, no. 12 (December 1997): 1155–7.
Cohen, N. M. Jr. “Pierre Robin Sequences and complexes: causal heterogeneity and pathogenetic/phenotypic variability.” (Editorial). American Journal of Medical Genetics
84, no. 4 (June 4, 1999): 311–5.
Cruz, M. “Pierre Robin Sequences: secondary respiratory difficulties and intrinsic feeding abnormalities.” Layrngoscope 109, no. 10 (October 1999): 1632–6.
Hsieh, Y. Y. “The Prenatal Diagnosis of Pierre Robin Sequence” Prenatal Diagnosis 19, no. 6 (June 1999): 567–9.
Marques, I. L. “Etiopathogenesis of isolated Robin Sequence.”
Cleft Palate-Craniofacial Journal 35, no. 6 (November 1998): 517–25.
Myer, C. M. “Airway management in Pierre Robin Sequence.”
Otolaryngology–Head and Neck Surgery 118, no. 5 (May 1998): 630–5.
Prows, C. A. “Beyond Pierre Robin Syndrome.” Neonatal Network 18, no. 5 (August 1999): 13–9.
Van Der Haven. “The Jaw Index: New guide defining Micrognatia in newborns.” Cleft Palate-Craniofacial
Journal 34, no. 3 (May 1997): 240–1.
Vester, F. “Pierre Robin Syndrome: Mandibular growth during the first year of life.” Annals of Plastic Surgery 42, no. 2 (February 1999): 154–7.
ORGANIZATION
Let’s Face It. Box 29972, Bellingham, WA 98228-1972. (360) 676-7325. letsfaceit@faceit.org. http://www.faceit
.org// .
WEBSITES
About Face International. http://aboutfaceinternational.org/ .
Pierre Robin Network. http://www.pierrerobin.org .
Farris F. Gulli, MD
Pierre-Robin syndrome see Pierre-Robin sequence
Pierre-Robin syndrome with fetal chrondrodysplasia see Weissenbacher-
Zweymuller syndrome
sequence Robin-Pierre
G A L E E N C Y C L O P E D I A O F G E N E T I C D I S O R D E R S |
925 |
Pituitary dwarfism
I Pituitary dwarfism
Definition
Dwarfism is a condition in which the growth of the individual is very slow or delayed. There are many forms of dwarfism. The word pituitary is in reference to the pituitary gland in the body. This gland regulates certain chemicals (hormones) in the body. Therefore, pituitary dwarfism is decreased bodily growth due to hormonal problems. The end result is a proportionate little person, because the height as well as the growth of all other structures of the individual are decreased.
Description
Pituitary dwarfism is caused by problems arising in the pituitary gland. The pituitary gland is also called the hypophysis. The pituitary gland is divided into two halves: the anterior (front) and posterior (back) halves. The anterior half produces six hormones: growth hormone, adrenocorticotropin (corticotropin), thyroid stimulating homone (thyrotropin), prolactin, follicle stimulating hormone, and lutenizing hormone. The posterior pituitary gland only produces two hormones. It produces antidiuretic hormone (vasopressin) and oxytocin.
Most forms of dwarfism are a result of decreased production of hormones from the anterior half of the pituitary gland. The most common form is due to decreases of growth hormone which will be discussed here. These decreases during childhood cause the individual’s arms, legs, and other structures to develop normal proportions for their bodies, but at a decreased rate.
When all of the hormones of the anterior pituitary gland are not produced, this is called panhypopituitarism. Another type of dwarfism occurs when only the growth hormone is decreased. Dwarfism can also result from a lack of somatomedin C (also called insulin like growth factor, IGF-1) production. Somatomedin C is a hormone produced in the liver that increases bone growth when growth hormone is present. The African pygmy and the Levi-Lorain dwarfs lack the ability to produce somatomedin C in response to growth hormone. All causes of dwarfism lead to a proportionate little person.
Growth is the body’s response to different hormones. The forebrain contains a small organ called the hypothalamus, which is responsible for releasing hormones in response to the body’s needs for purposes of regulation. Growth hormone is produced in the anterior pituitary gland when growth hormone-releasing hormone (GHRH), is released by the hypothalamus. Growth hormone is then released and stimulates the liver to produce
K E Y T E R M S
Adrenocorticotropin (corticotrophin)—A hormone that acts on cells of the adrenal cortex, causing them to produce male sex hormones and hormones that control water and mineral balance in the body.
Antidiuretic hormone (vasopressin)—A hormone that acts on the kidneys to regulate water balance.
Craniopharyngioma—A tumor near the pituitary gland in the craniopharyngeal canal that often results in intracranial pressure.
Deprivational dwarfism—A condition where emotional disturbances are associated with growth failure and abnormalities of pituitary function.
Follicle-stimulating hormone (FSH)—A hormone that in females stimulates estrogen and in males stimulates sperm production.
Growth hormone—A hormone that eventually stimulates growth. Also called somatotropin.
Hormone—A chemical messenger produced by the body that is involved in regulating specific bodily functions such as growth, development, and reproduction.
Lutenizing hormone—A hormone secreted by the pituitary gland that regulates the menstrual cycle and triggers ovulation in females. In males it stimulates the testes to produce testosterone.
Oxytocin—A hormone that stimulates the uterus to contract during child birth and the breasts to release milk.
Panhypopituitarism—Generalized decrease of all of the anterior pituitary hormones.
Prolactin—A hormone that helps the breast prepare for milk production during pregnancy.
Puberty—Point in development when the gonads begin to function and secondary sexual characteristics begin to appear.
Thyroid stimulating hormone (thyrotropin)—A hormone that stimulates the thyroid gland to produce hormones that regulate metabolism.
IGF-1. In return, IGF-1 stimulates the long bones to grow in length. Thus, growth can be slowed down or stopped if there is a problem making any of these hormones or if there is a problem with the cells receiving these hormones.
926 |
G A L E E N C Y C L O P E D I A O F G E N E T I C D I S O R D E R S |
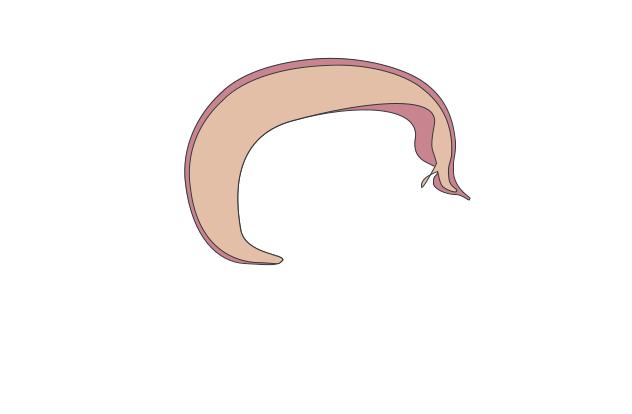
Cerebrum
Pineal gland
Cerebellum
Pituitary gland
Cerebral peduncle
Spinal cord
dwarfism Pituitary
(Gale Group)
Genetic profile
Pituitary dwarfism has been shown to run in families. New investigations are underway to determine the specific cause and location of the gene responsible for dwarfism. The human cell contains 46 chromosomes arranged in 23 pairs. Most of the genes in the two chromosomes of each pair are identical or almost identical with each other. However, with dwarfism, there appears to be disruption on different areas of chromosome 3 and 7. Some studies have isolated defects for the production of pituitary hormones to the short arm (the “p” end) of chromosome 3 at a specific location of 3p11. Other studies have found changes on the short arm of chromosome 7.
Demographics
Some estimates show that there are between 10,000 and 15,000 children in the United States who have growth problems due to a deficiency of growth hormone.
Signs and symptoms
A child with a growth hormone deficiency is often small with an immature face and chubby body build. The
child’s growth will slow down and not follow the normal growth curve patterns. In cases of tumor, most commonly craniopharyngioma (a tumor near the pituitary gland), children and adolescents may present with neurological symptoms such as headaches, vomiting, and problems with vision. The patient may also have symptoms of double vision. Symptoms such as truly bizarre and excessive drinking behaviors (polydipsia) and sleep disturbances may be common.
Diagnosis
The primary symptom of pituitary dwarfism is lack of height. Therefore, a change in the individual’s growth habits will help lead to a diagnosis. Another diagnostic technique uses an x ray of the child’s hand to determine the child’s bone age by comparing this to the child’s actual chronological age. The bone age in affected children is usually two years or more behind the chronological age. This means that if a child is ten years old, his or her bones will look like they are those of an eight-year- old child. The levels of growth hormone and somatomedin C must also be measured with blood tests.
Hypopituitarism may be gained or acquired following birth for several reasons. It could be due to trauma to
G A L E E N C Y C L O P E D I A O F G E N E T I C D I S O R D E R S |
927 |
Pituitary dwarfism
Pituitary Dwarfism Syndrome
Very short (dwarfism) "Baby doll" face Often hypoglycemic
(Gale Group)
the pituitary gland such as a fall or following surgery to the brain for removal of a tumor. It may also be due to the child’s environment (deprivational dwarfism).
On examination by the doctor there may be optic nerve atrophy, if the dwarfism is due to a type of tumor. X rays of the area where the pituitary gland is located (sella turcica) or more advanced imaging such as magnetic resonance imaging (MRI) or computed tomography (CT) may show changes of the pituitary gland itself. Computed tomography is an advanced form of x ray that will help determine the integrity of the bone and how much calcification the tumor is producing. Magnetic resonance imaging, will also help in the diagnosis. MRI is a type of imaging device that can visualize soft tissues such as muscle and fat.
If the dwarfism is due to environmental and emotional problems, the individual may be hospitalized to monitor hormone levels. Following a few days of hospitalization, hormone levels may become normal due to avoidance of the original environment.
Treatment and management
The main course of therapy is growth hormone replacement therapy when there is lack of growth hormone in the body. A pediatric endocrinologist, a doctor specializing in the hormones of children, usually administers this type of therapy before a child’s growth plates have fused or joined together. Once the growth plates have fused, GH replacement therapy is rarely effective.
Growth hormone used to be collected from recently deceased humans. However, frequent disease complications resulting from human growth hormone collected from deceased bodies lead to the banning of this method.
In the mid-1980s, techniques were discovered that could produce growth hormones in the lab. Now, the only growth hormone used for treatment is that made in a laboratory.
A careful balancing of all of the hormones produced by the pituitary gland is necessary for patients with panhypopituitarism. This form of dwarfism is very difficult to manage.
Prognosis
The prognosis for each type of dwarfism varies. A panhypopituitarism dwarf does not pass through the initial onset of adult sexual development (puberty) and never produces enough gonadotropic hormones to develop adult sexual function. These individuals also have a great deal of other medical conditions. Dwarfism due to only growth hormone deficiency has a different prognosis. These individuals do pass through puberty and mature sexually, however, they remain proportionately small in stature.
If the individual is lacking only growth hormone then growth hormone replacement therapy can be administered. The success of treatment with growth hormone varies however. An increase in height of 4–6 in (10–15 cm) can occur in the first year of treatment. Following this first year, the response to the hormone is not as successful. Therefore the amount of growth hormone administered must be tripled to maintain this rate. Long-term use is considered successful if the individual grows at least 0.75 in (2 cm) per year more than they would without the hormone. However, if the growth hormone treatment is not administered before the long bones—such as the legs and arms—fuse, then the individual will never grow. This fusion is completed by adult age.
928 |
G A L E E N C Y C L O P E D I A O F G E N E T I C D I S O R D E R S |

Improvement for individuals with dwarfism due to other causes such as a tumor, varies greatly. If the dwarfism is due to deprevational causes, then removing that child from that environment should help to alleviate the problem.
Resources
BOOKS
Guyton, Arthur C., and John E. Hall. “The Pituitary Hormones and Their Control by the Hypothalamus.” Textbook of Medical Physiology, 9th ed. Philadelphia: W.B. Saunders Company, 1996.
“Pituitary Dwarfism.” In Merck Manual. Ed. Mark H. Beers, Robert Berkow, and Mark Burs, 2378–80. Rahway, NJ: Merck & Co., Inc., 1999.
Rogol, Alan D. “Hypothalamic and Pituitary Disorders in Infancy and Childhood.” In Principles and Practice of
Endocrinology and Metabolism, 2nd ed. Edited by Kenneth L. Becker et. al., 180–88. Philadelphia: J.B. Lippencott Company, 1995.
PERIODICALS
Maheshwari, H. G., et. al. “Phenotype and Genetic Analysis of a Syndrome Caused by an Inactivating Mutation in the Growth Hormone-Releasing Hormone Receptor: Dwarfism of Sindh.” Journal of Clinical Endocrinology and Metabolism 83, no. 11 (1998): 4065–81.
Nagel, B. H. P. “Magnetic resonance images of 91 children with different causes of short stature: Pituitary size reflects growth hormone secretion.” European Journal of Pediatrics 156 (1997): 758–63.
Raskin, S., et. al. “Genetic mapping of the human pituitary-spe- cific transcriptional factor gene and its analysis in familial panhypopituitary dwarfism.” Human Genetics 98 (1996): 703–5.
ORGANIZATIONS
Human Growth Foundation. 997 Glen Cove Ave., Glen Head, NY 11545. (800) 451-6434. Fax: (516) 671-4055.http://www.hgf1@hgfound.org .
Little People of America, Inc. National Headquarters, PO Box 745, Lubbock, TX 79408. (806) 737-8186 or (888) LPA2001. lpadatabase@juno.com. http://www.lpaonline
.org .
MAGIC Foundation for Children’s Growth. 1327 N. Harlem Ave., Oak Park, IL 60302. (708) 383-0808 or (800) 3624423. Fax: (708) 383-0899. mary@magicfoundation.org.http://www.magicfoundation.org/ghd.html .
WEBSITES
“Clinical Growth Charts by the National Center for Health Statistics.” Center for Disease Control. http://www.cdc
.gov/nchs/about/major/nhanes/growthcharts/clinical_ charts.htm .
“Entry 312000: Panhypopituitarism; PHP.” OMIM—Online Mendelian Inhericance in Man. National Institutes of Health. http://www.ncbi.nlm.nih.gov/htbin-post/Omim/ dispmim?312000 .
Hill, Mark. “Development of the Endocrine System— Pituitary.” The University of New South Wales, Sydney,
Australia—Department of Embryology http://anatomy
.med.unsw.edu.au/CBL/Embryo/OMIMfind/endocrine/ pitlist.htm .
Jason S. Schliesser, DC
PK deficiency see Pyruvate kinase deficiency
PKD see Polycystic kidney disease
PKU see Phenylketonuria
I Poland anomaly
Definition
Poland anomaly is a rare pattern of malformations present at birth that includes unilateral changes in the chest and shoulder girdle muscles, forearm bones, and fingers. Although there are other associated features, the most recognized characteristics are abnormalities of the major chest muscles (pectoralis) and the presence of syndactyly or webbing that joins the fingers of the hand. Treatment of this anomaly is mainly through reconstructive surgery.
Description
Poland anomaly (also known as Poland syndactyly, Poland syndrome, Poland sequence, or Pectoral dyspla- sia-dysdactyly) was first described in 1841 by Alfred Poland, who was a medical student at Guy’s Hospital in London when he noted malformations in the body of a deceased convict named George Elt. Today, the diagnosis of Poland anomaly may encompass various combinations of the following abnormalities:
•Absence of major chest muscles: pectoralis major, pectoralis minor.
•Hand anomalies: syndactyly (webbed or fused fingers), shortened fingers.
•Underdeveloped forearm bones: ulna, radius.
•Underdeveloped or absence of the nipple and, in females, the breast.
•Absence of groups of rib cartilage.
anomaly Poland
G A L E E N C Y C L O P E D I A O F G E N E T I C D I S O R D E R S |
929 |

Poland anomaly
K E Y T E R M S
Autosomal dominant—A pattern of genetic inheritance where only one abnormal gene is needed to display the trait or disease.
Dextrocardia—Defect in which the position of the heart is the mirror image of its normal position.
Pectoralis muscles—Major muscles of the chest wall.
Renal agenesis—Absence or failure of one or both kidneys to develop normally.
Sporadic—Isolated or appearing occasionally with no apparent pattern.
Syndactyly—Webbing or fusion between the fingers or toes.
•Absence of shoulder girdle muscles: latissimus dorsi, serratus anterior.
•Underdeveloped skin and underlying tissue of the chest.
•Abnormal curvature of the spine.
•Patchy hair growth under the arm.
•Rare associations with abnormalities in the heart, kidney, or development of certain cancers.
In most cases, physical abnormalities are confined to one side of the body and tend to favor the right side by almost two to one. The manifestations of Poland anomaly are extremely variable and rarely are all the features recognized in one individual. Involvement of the pectoralis muscle and fingers is the most consistent feature.
The exact cause of Poland anomaly is not known, but may result from the interruption of fetal growth at about the 46th day of pregnancy, when the fetal fingers and pectoralis muscle are developing. Several researchers have suggested that there may be too little blood flow through the fetal subclavian artery that goes to the chest and arm; the more severe the blood flow disruption, the more numerous and severe the resulting malformations. However, the final proof for this idea has not been found.
Genetic profile
Most occurrences of Poland anomaly appear to be sporadic (i.e., random, and not associated with a inherited disorder) and are not passed on from parent to child. However, there have been rare reports of Poland anomaly that appear in multiple members of the same family. In at least one case, this familial occurrence of Poland anomaly appears to be inherited in an autosomal dominant pat-
tern. The fact that other organs systems (kidney, heart) and increased risks of certain cancers are associated with this condition supports the hypothesis that there may be some genetic abnormality. However, if there is some sort of genetic or inherited cause in some patients with Poland anomaly, it has not been identified. For purposes of genetic counseling, the Poland anomaly can be regarded as a sporadic condition with an extremely low risk of being transmitted from parent to child.
Demographics
Poland anomaly is not common. It affects one child in about 20,000 to 30,000. Geographically, estimates of the frequency range from one in 17,213 in Japanese school children, to an average of one in 32,000 live births in British Columbia, with a low incidence of one in 52,530, in Hungary. For reasons that are unclear, Poland anomaly is three times more frequent in boys than girls.
Signs and symptoms
The manifestations of Poland anomaly are most often limited to the physical manifestations described above. The degree to which this condition is disabling depends on which manifestations are present and their individual severity, but most often relate to disabilities in the affected arm and hand. Upon rare occasions, the Poland anomaly is associated with dextrocardia (in which the position of the heart is the mirror image of its normal position), renal agenesis (maldevelopment of the kidney) or the association with cancers such as leukemia, leiomyosarcoma, and non-Hodgkin lymphoma. Intelligence is not impaired by Poland anomaly.
Diagnosis
The diagnosis of Poland anomaly relies on physical exam and radiographic evaluation, such as the use of x rays or other imaging techniques to define abnormal or missing structures that are consistent with the criteria for Poland anomaly, as described above. There is no laboratory blood or genetic test that can be used to identify people with Poland anomaly.
Treatment and management
During early development and progressing through until young adulthood, children with Poland anomaly should be educated and trained in behavioral and mechanical methods to adapt to their disabilities. This program is usually initiated and overseen by a team of health care professionals including a pediatrician, physical therapist, and occupational therapist. A counselor
930 |
G A L E E N C Y C L O P E D I A O F G E N E T I C D I S O R D E R S |
