
Gale Encyclopedia of Genetic Disorder / Gale Encyclopedia of Genetic Disorders, Two Volume Set - Volume 2 - M-Z - I
.pdf
stage cancers is almost 99%. Sixty-three percent of the patients survive 10 years, and 51% survive 15 years after initial diagnosis. Studies on the prognosis of hereditary prostate cancer are ongoing.
Resources
BOOKS
Bostwick, David, et al. Prostate Cancer: What Every Man— And His Family—Needs to Know. New York: Villard Books, 1999.
Naros, Steven, et al. “Cancers of the Prostate and Testes.” In
Inherited Susceptibility to Cancer: Clinical, Predictive and Ethical Perspectives. Ed. William D. Foulkes and Shirley V. Hodgson, 246–54. Cambridge: Cambridge University Press, 1998.
ORGANIZATIONS
American Cancer Society. 1599 Clifton Rd. NE, Atlanta, GA 30329. (800) 227-2345. http://www.cancer.org .
American Foundation for Urologic Disease, Inc. 1128 North Charles St., Baltimore, MD 21201-5559. (410) 468-1808.http://www.afud.org .
National Cancer Institute. Office of Communications, 31 Center Dr. MSC 2580, Bldg. 1 Room 10A16, Bethesda, MD 20892-2580. (800) 422-6237. http://www.nci.nih
.gov .
WEBSITES
National Prostate Cancer Coalition. http://www.4npcc.org .
US TOO! International, Inc. http://www.ustoo.com .
Kristin Baker Niendorf, MS, CGC
I Proteus syndrome
Definition
Proteus syndrome is characterized by excessive growth of cells. This can result in asymmetrical growth, benign (noncancerous) tumors, and pigmented skin lesions.
Description
Proteus syndrome is a rare condition. It was first described in 1979 by Michael Cohen. Hans-Rudolf Wiedemann named the condition after the Greek god Proteus, who could assume many forms. The disorder gained wide recognition when it became publicized that Joseph (John) Merrick, the person depicted in the movie The Elephant Man, probably had Proteus syndrome.
The excess growth of tissue that characterizes Proteus syndrome is progressive. It also tends to affect some tissues and not others. This can result in asymmetrical growth in the body, such as the skull, bones, spine, hands, feet, fingers, and toes. Proteus syndrome often results in overgrowth of one side of the body and not the other. Benign tumors on the surface of the skin or inside the body may also occur. Raised brown patches on the skin and an overgrowth of tissues on the soles of the feet or the palms of the hands are common. The types of tissues and organs that are affected and the severity of the effects vary from person to person and within the course of a lifetime. Proteus syndrome is sometimes associated with mental delay.
Genetic profile
The specific cause of Proteus syndrome is unclear. Proteus syndrome appears to occur randomly, suggesting that it is not inherited. Research suggests that Proteus syndrome results from an unknown gene that is changed (mutated) in some cells, but normal in other cells of the body. This is called mosaicism.
The tissues and organs that are affected in Proteus syndrome and the severity of effects probably depend on how many cells contain the mutated gene, and what type of cells contain it. Someone with many cells containing the changed Proteus gene are more likely to have more severe effects then someone with only a few cells changed. Someone with many cells changed in a particular part of the body, such as the hand, are more likely to have excessive growth in that area. The changed Proteus gene will affect cell growth even after the baby is fully developed, since cell division continues to take place and is necessary for the growth of tissues and organs and for the replacement of damaged cells. The changed Proteus gene mainly results in excessive growth of cells and tissues from infancy to adolescence.
Demographics
Only 100 to 200 cases of Proteus syndrome have been reported around the world. Both males and females are equally likely to be affected with Proteus syndrome.
Signs and symptoms
Individuals with Proteus syndrome can have a wide range of manifestations. The effects can also range from mild to severe. The most common manifestations of Proteus syndrome include:
• Overgrowth of hands, feet, fingers, or toes (gigantism)
syndrome Proteus
G A L E E N C Y C L O P E D I A O F G E N E T I C D I S O R D E R S |
961 |

Proteus syndrome
K E Y T E R M S
Autosomal dominant—A pattern of genetic inheritance where only one abnormal gene is needed to display the trait or disease.
Benign tumor—An abnormal proliferation of cells that does not spread to other sites.
Chromosome—A microscopic thread-like structure found within each cell of the body and consists of a complex of proteins and DNA. Humans have 46 chromosomes arranged into 23 pairs. Changes in either the total number of chromosomes or their shape and size (structure) may lead to physical or mental abnormalities.
Connective tissue—A group of tissues responsible for support throughout the body; includes cartilage, bone, fat, tissue underlying skin, and tissues that support organs, blood vessels, and nerves throughout the body.
Cyst—An abnormal sac or closed cavity filled with liquid or semisolid matter.
Deoxyribonucleic acid (DNA)—The genetic material in cells that holds the inherited instructions for growth, development, and cellular functioning.
Gene—A building block of inheritance, which contains the instructions for the production of a particular protein, and is made up of a molecular sequence found on a section of DNA. Each gene is found on a precise location on a chromosome.
Mosaicism—A genetic condition resulting from a
mutation, crossing over, or nondisjunction of chromosomes during cell division, causing a variation in the number of chromosomes in the cells.
Nevi—Plural of nevus.
Nevus—Any anomaly of the skin present at birth, including moles and various types of birthmarks.
Protein—Important building blocks of the body, composed of amino acids, involved in the formation of body structures and controlling the basic functions of the human body.
Spleen—Organ located in the upper abdominal cavity that filters out old red blood cells and helps fight bacterial infections. Responsible for breaking down spherocytes at a rapid rate.
Spontaneous—Occurring by chance.
Thymus gland—An endocrine gland located in the front of the neck that houses and transports T cells, which help to fight infection.
Tissue—Group of similar cells that work together to perform a particular function. The four basic types of tissue include muscle, nerve, epithelial, and connective tissues.
Vascular malformation—Abnormality of the blood vessels that often appears as a red or pink patch on the surface of the skin.
Vertebra—One of the 23 bones which comprise the spine. Vertebrae is the plural form.
•overgrowth of one side of the limbs, face, or body (hemihypertrophy)
•overgrowth of the connective tissue on the soles of the feet or palms of the hand or, less commonly, in the abdomen or nose (connective tissue nevi)
•darkened, discolored, and often rough and raised patches of skin (skin surface nevi)
•benign tumors on the skin surface and under the skin
•benign tumors of the fat cells (lipoma) or areas of significantly decreased or increased body fat
•abnormalities of the skull resulting in a large or asymmetrical head
•benign bony growths projecting outward from the end of the bones (exostosis)
People with Proteus syndrome can have curvature of the spine. They may also have an enlarged spleen or
thymus. Approximately 12–13% of people with the disorder have cystic abnormalities of the lungs, which can interfere with the normal functioning of the lungs. Abnormalities in the blood vessels called vascular malformations, which appear as pink or red patches on the surface of the skin, are common. About one third of people with Proteus syndrome are mentally retarded; skull abnormalities are often seen in those with impairment. People with Proteus syndrome can have a distinctive facial appearance, with a long and narrow face, down-slanting eyes, wide and forward-tipping nostrils, a low nose bridge, and a mouth that remains open when at rest. Many effects can result from the presence of tumors and bony growths that affect other organs and tissues.
Sometimes mild or moderate effects of Proteus syndrome, such as benign tumors, are present at birth. As a person grows and develops, the tissue overgrowth pro-
962 |
G A L E E N C Y C L O P E D I A O F G E N E T I C D I S O R D E R S |

gresses and changes. This progression is often irregular; it is characterized by periods of major overgrowth and other periods of absent overgrowth. The effects therefore change over the course of a lifetime. However, most changes occur before adolescence, since tissue overgrowth tends to plateau at that time.
Diagnosis
There is no blood test available to diagnose Proteus syndrome. A diagnosis can be made only by careful observation of the individual, perhaps over a period of time, and through imaging studies. These may include x ray evaluations of the skull and skeletal system; magnetic resonance imaging (MRI) of the limbs, nervous system, and abdomen; and computed tomography (CT) scans of the chest.
The great variability of Proteus syndrome from person to person makes it hard to diagnose. There are no definitive and universal diagnostic guidelines. Some tentative guidelines were established, however, at the First National Conference on Proteus Syndrome Diagnostic Criteria.
Treatment and management
There is no cure for Proteus syndrome. Treatment largely involves the management of effects of the disorder, such as the removal of tumors or bony overgrowths. Removal of tumors is not recommended, though, unless they are causing major problems, since these tumors usually grow back. Surgery to remove an overgrown portion of the bone should be performed only if the bony overgrowth is affecting normal functioning. Bony overgrowths in the ear, for example, may need to be removed if they are interfering with hearing. This type of surgery, however, can sometimes increase the growth of the remaining bone. Psychological counseling to help children with Proteus syndrome deal with the disorder should be considered. In order for counseling to be effective, it is preferable that it begins at a young age.
Prognosis
The long-term prognosis of Proteus syndrome is not known. The life expectancy is likely to vary greatly from person to person. Those with tumors and bony overgrowths affecting critical organs are likely to have a poorer prognosis.
Resources
PERIODICALS
Barona-Mazuera, Maria, et al. “Proteus Syndrome: New Findings in Seven Patients.” Pediatric Dermatology 14, no. 1 (1997): 1–5.
Biesecker, Leslie, et al. “Proteus Syndrome: Diagnostic Criteria, Differential Diagnosis, and Patient Evaluation.”
American Journal of Medical Genetics 84, no. 5 (1999): 389–95.
Cavero, Juan, Evelyn Castro, and Luz Junco. “Proteus Syndrome.” International Journal of Dermatology 39 (2000): 698–709.
De Becker, Inge. “Ocular Manifestations in Proteus Syndrome.”
American Journal of Medical Genetics 92 (2000): 350–52. Gilbert-Barness, Enid, Michael Cohen, and John Opitz.
“Multiple Meningiomas, Craniofacial Hyperostosis and Retinal Abnormalities in Proteus Syndrome.” American Journal of Medical Genetics 93 (2000): 234–40.
ORGANIZATIONS
Proteus Syndrome Foundation. 6235 Whetstone Dr., Colorado Springs, CO 80918. (719)264-8445. abscit@aol.com.http://www.kumc.edu/gec/support/proteus.html .
WEBSITES
McKusick, Victor A. “Proteus Syndrome.” Online Mendelian Inheritance in Man. http://www3.ncbi.nlm.nih.gov/ htbin-post/Omim/dispmim?176920 . (January 2001).
Proteus Syndrome Foundation. Proteus Syndrome Newsletter.
http://www.kumc.edu/gec/support/newslet2.html .
Lisa Maria Andres, MS, CGC
I Prune-belly syndrome
Definition
Prune-belly syndrome is characterized by the following three findings: lack of abdominal muscles, undescended testes, and abnormal development of the urinary tract. Also known as Eagle-Barrett syndrome, this rare disorder was first described in 1839.
Description
Prune-belly syndrome displays a wide range of severity. Affected individuals will have little to no muscle in their abdominal wall. The abdomen will appear wrinkled, like a prune. In male infants, the testicles, although present, are usually not seen. They remain inside the infant’s abdomen. They fail to move to the normal position during development of the fetus. Undescended testes
syndrome belly-Prune
G A L E E N C Y C L O P E D I A O F G E N E T I C D I S O R D E R S |
963 |

Prune-belly syndrome
K E Y T E R M S
Creatinine—A waste product of the body found in the urine. It is useful in determining the overall kidney function.
Pyelonephritis—Inflammation of the kidney commonly caused by bacterial infections.
Ultrasound—An imaging technique that uses sound waves to help visualize internal structures in the body.
are a risk factor for infertility and testicular cancer later in the infant’s life.
There are a variety of urinary tract abnormalities that occur in this syndrome. The kidneys may not form fully, and the level of development of the kidneys varies. The ureters, the tubes that connect the kidneys to the bladder, may be very large. In the portions that are very large, the urine may not be able to flow as well as normal. The bladder, the organ that holds the urine, may also be very large. A connection between the umbilicus and bladder may be present as well. The urethra may have areas that are very dilated and others that are very narrow. The narrowing may not allow the urine to flow out well. This blockage causes the bladder to become very large. The drainage of the fetus’ bladder is what makes up the amniotic fluid during pregnancy. If the bladder cannot be drained, then not enough amniotic fluid will be present. The lack of amniotic fluid, or oligohydramnios, can cause poor formation of the fetus’ lungs. The bladder in these patients may become so large that a mass can be seen and felt on the baby.
Ten percent of cases may have various abnormalities of the heart or large blood vessels. A percentage of cases will have abnormalities of their musculoskeletal system such as: dislocation of the hips, abnormal indentation of their chest, malformed feet or fingers, and a spine that is not aligned properly.
Genetic profile
A specific genetic defect is unknown. Multiple cases in families are rare but have been reported. The risk of recurrence in future pregnancies is unknown but is thought to be low.
Demographics
Despite the lack of a specific genetic defect or pattern of inheritance, over 95% of affected individuals are
male. The incidence of this syndrome is estimated at one in 40,000 births.
Signs and symptoms
There are many symptoms that infants may experience in the newborn period. Most of these depend on the extent of damage that exists in the lungs and urinary tract. Infants who have poorly developed lungs, may be unable to breathe on their own at birth. They may also develop a collapsed lung or pneumothorax. If the infant does not have a normal rib cage then their ability to move air into and out of their lungs is impaired. This can lead to infections in the lung.
Since infants may not be able to eliminate of all their urine, they are at risk of having repeated urinary tract infections.
Diagnosis
At birth, the syndrome is easily diagnosed based on the three findings that have been described. There is no specific prenatal or genetic test that can diagnose prunebelly syndrome. The diagnosis of prune-belly syndrome can be made in the prenatal period by ultrasound. Ultrasound can show some of the findings in this syndrome such as: distended bladder and ureters, oligohydramnios, and cryptorchidism. An enlarged bladder can be seen in other syndromes besides prune-belly, however, these findings on ultrasound should alert a physician to prune-belly as a possible cause.
Treatment and management
The potential treatments for prune-belly syndrome depend upon whether the diagnosis is made at birth or in utero. It also varies depending upon how severe the abnormalities are. Over the past two decades, different surgical procedures have been performed on fetuses in an attempt to correct the urinary tract obstructions that occur. One of these procedures is the vesicoamniotic shunt. This procedure relieves bladder obstructions by placing a tube in the fetal bladder allowing amniotic fluid to be produced as usual. The production of amniotic fluid allows for normal development of the lungs. There is not much information regarding the long-term outcomes for persons who receive these shunts. In infants who survive but have renal failure, kidney transplantation has been attempted with some success.
Prognosis
Approximately 20% of patients with this syndrome are stillborn. Thirty percent of infants do not survive past
964 |
G A L E E N C Y C L O P E D I A O F G E N E T I C D I S O R D E R S |

The distinctive “prune-like” appearance of the abdominal area is evident in this infant. (Custom Medical Stock Photo, Inc.)
two years due to renal failure or infection. The remaining 50% of the infants will have a variety of urinary tract problems. A recent study looked at what factors may predict which children with prune-belly syndrome will develop renal failure. In this study, 35 patients with prune-belly syndrome between 1960 and 1997 were examined. Developing pyelonephritis (infection and inflammation of the kidney) at some point in time, having an elevated baseline creatinine, and having both kidneys look abnormal on an ultrasound were predictive for developing renal failure.
Resources
BOOKS
Behrman, Richard, et al. “Prune-Belly Syndrome.” In Nelson Textbook of Pediatrics. Philadelphia: W.B. Saunders Company, 2000.
PERIODICALS
Freedman, A.L., et al. “Long-term outcome in children after antenatal intervention for obstructive uropathies.” The Lancet 354 (July 1999): 374–7.
Leeners, B., et al. “Prune-Belly Syndrome: Therapeutic Options Including In Utero Placement of a Vesicoamniotic Shunt.” Journal of Clinical Ultrasound 28, no. 9 (November/December 2000): 500–7.
ORGANIZATIONS
National Organization for Rare Disorders (NORD). PO Box 8923, New Fairfield, CT 06812-8923. (203) 746-6518 or (800) 999-6673. Fax: (203) 746-6481. http://www
.rarediseases.org .
WEBSITES
Online Mendelian Inheritance in Man (OMIM). http://www
.ncbi.nlm.nih.gov/htbin-post/Omim/dispmim?100100 .
David Elihu Greenberg, MD
Pseudo-Hurler disease see GM1 gangliosidosis
Pseudothalidomide syndrome see Roberts
SC phocomelia
I Pseudoxanthoma elasticum
Definition
Pseudoxanthoma elascticum (PXE) is an inherited connective tissue disorder in which the elastic fibers present in the skin, eyes, and cardiovascular system gradually become calcified and inelastic.
Description
PXE was first reported in 1881 by Rigal, but the problem with elastic fibers was described in 1986 by Darier who gave the condition its name. PXE is also known as Grönblad-Strandberg-Touraine syndrome and systemic elastorrhexis.
The course of PXE varies greatly between individuals. Typically, it is first noticed during adolescence as yel- low-orange bumps on the side of the neck. Similar bumps may appear at other places where the skin bends a lot, like the backs of the knees and the insides of the elbows. The skin in these areas tends to get thick, leathery, inelastic, and acquire extra folds. These skin problems have no serious consequences, and for some people, the disease progresses no further.
Bruch’s membrane, a layer of elastic fibers in front of the retina, becomes calcified in some people with PXE. Calcification causes cracks in Bruch’s membrane, which can be seen through an ophthalmoscope as red, brown, or gray streaks called angioid streaks. The cracks can eventually (e.g., in 10–20 years) cause bleeding, and the usual resultant scarring leads to central vision deterioration. However, peripheral vision is unaffected.
Arterial walls and heart valves contain elastic fibers that can become calcified. This leads to a greater susceptibility to the conditions that are associated with hardening of the arteries in the normal aging population—high blood pressure, heart attack, stroke, and arterial obstruc- tion—and, similarly, mitral valve prolapse. Heart disease and hypertension associated with PXE have been reported in children as young as four to 13 years of age. Although often appearing at a younger age, the overall incidence of these conditions is only slightly higher for people with PXE than it is in the general population.
elasticum Pseudoxanthoma
G A L E E N C Y C L O P E D I A O F G E N E T I C D I S O R D E R S |
965 |

Pseudoxanthoma elasticum
K E Y T E R M S
Angioid streaks—Gray, orange, or red wavy branching lines in Bruch’s membrane.
Bruch’s membrane—A membrane in the eye between the choroid membrane and the retina.
Carrier—A person who possesses a gene for an abnormal trait without showing signs of the disorder. The person may pass the abnormal gene on to offspring.
Claudication—Pain in the lower legs after exercise caused by insufficient blood supply.
Connective tissue—A group of tissues responsible for support throughout the body; includes cartilage, bone, fat, tissue underlying skin, and tissues that support organs, blood vessels, and nerves throughout the body.
Deletion—The absence of genetic material that is normally found in a chromosome. Often, the genetic material is missing due to an error in replication of an egg or sperm cell.
Dominant trait—A genetic trait where one copy of the gene is sufficient to yield an outward display of the trait; dominant genes mask the presence of recessive genes; dominant traits can be inherited from a single parent.
Elastic fiber—Fibrous, stretchable connective tissue made primarily from proteins, elastin, collagen, and fibrillin.
Gene—A building block of inheritance, which contains the instructions for the production of a particular protein, and is made up of a molecular sequence found on a section of DNA. Each gene is found on a precise location on a chromosome.
Mitral valve—The heart valve that prevents blood from flowing backwards from the left ventricle into the left atrium. Also known as bicuspid valve.
Mutation—A permanent change in the genetic material that may alter a trait or characteristic of an individual, or manifest as disease, and can be transmitted to offspring.
Recessive trait—An inherited trait or characteristic that is outwardly obvious only when two copies of the gene for that trait are present.
Arterial inelasticity can lead to bleeding from the gastrointestinal tract and, rarely, acute vomiting of blood.
Genetic profile
PXE is caused by changes in the genetic material, called mutations, that are inherited in either a dominant or recessive mode. A person with the recessive form of the disease (which is most common) must possess two copies of the PXE gene to be affected, and, therefore, must have received one from each parent. In the dominant form, one copy of the abnormal gene is sufficient to cause the disease. In some cases, a person with the dominant form inherits the abnormal gene from a parent with PXE. More commonly, the mutation arises as a spontaneous change in the genetic material of the affected person. These cases are called “sporadic” and do not affect parents or siblings, although each child of a person with sporadic PXE has a 50% risk to inherit the condition.
Both males and females can develop PXE, although the skin findings seem to be somewhat more common in females.
The actual genetic causes of this condition were not discovered until 2000. The recessive, dominant, and sporadic forms of PXE all appear to be caused by different mutations or deletions in a single gene called ABCC6 (also known as MRP6), located on chromosome 16. Although the responsible gene has been identified, how it causes PXE is still unknown.
Genetic researchers have since identified mutations in a number of persons with PXE, most of whom have been found to have the recessive type. Affected individuals in these families had mutations in both copies of the gene and parents, who are obligate carriers, had a mutation in only one copy. Contrary to the usual lack of symptoms in carriers of recessive genes, some carriers of recessive PXE have been found to have cardiovascular symptoms typical of PXE.
Although the recessive type is the most common, there are also familial and sporadic cases that have been found to be caused by dominant mutations in the ABCC6 gene.
Demographics
PXE is rare and occurs in about one in every 160,000 people in the general population. It is likely, though, that PXE is underdiagnosed because of the presence of mild symptoms in some affected persons and the lack of awareness of the condition among primary care physicians.
966 |
G A L E E N C Y C L O P E D I A O F G E N E T I C D I S O R D E R S |
Signs and symptoms
A wide range in the type and severity of symptoms exists between people with PXE. The age of onset also varies, although most people notice initial symptoms during adolescence or early adulthood. Often, the first symptoms to appear are thickened skin with yellow bumps in localized areas such as the folds of the groin, arms, knees, and armpits. These changes can also occur in the mucous membranes, most often in the inner portion of the lower lip. The appearance of the skin in PXE has been likened to a plucked chicken or Moroccan leather.
Angioid streaks in front of the retina are present in most people with PXE and an ophthalmologic examination can be used as an initial screen for the condition. Persons with PXE often complain of sensitivity to light. Because of the progressive breakdown of Bruch’s membrane, affected persons are at increased risk for bleeding and scarring of the retina, which can lead to decreased central vision but does not usually cause complete blindness.
Calcium deposits in the artery walls contribute to early-onset atherosclerosis, and another condition called claudication, inadequate blood flow that results in pain in the legs after exertion. Abnormal bleeding, caused by calcification of the inner layer of the arteries, can occur in the brain, retina, uterus, bladder, and joints but is most common in the gastrointestinal tract.
Diagnosis
The presence of calcium in elastic fibers, as revealed by microscopic examination of biopsied skin, unequivocally establishes the diagnosis of PXE.
Treatment and management
PXE cannot be cured, but plastic surgery can treat PXE skin lesions, and laser surgery is used to prevent or slow the progression of vision loss. Excessive blood loss due to bleeding into the gastrointestinal tract or other organ systems may be treated by transfusion. Mitral valve prolapse (protrusion of one or both cusps of the mitral heart valve back into the atrium during heart beating) can be corrected by surgery, if necessary.
Measures should be taken to prevent or lessen cardiovascular complications. People with PXE should control their cholesterol and blood pressure, and maintain normal weight. They should exercise for cardiovascular health and to prevent or reduce claudication later in life. They should also avoid the use of tobacco, thiazide antihypertensive drugs, blood thinners like coumadin, and non-steroidal anti-inflammatory drugs like aspirin and
ibuprofen. In addition, they should avoid strain, heavy lifting, and contact sports, since these activities could trigger retinal and gastrointestinal bleeding.
People with PXE should have regular eye examinations by an ophthalmologist and report any eye problems immediately. Regular check-ups with a physician are also recommended, including periodic blood pressure readings.
Some people have advocated a calcium-restricted diet, but it is not yet known whether this aids the problems brought about by PXE. It is known, however, that calcium-restriction can lead to bone disorders.
Prognosis
The prognosis is for a normal life span with an increased chance of cardiovascular and circulatory problems, hypertension, gastrointestinal bleeding, and impaired vision. However, now that the gene for PXE has been identified, the groundwork for research to provide effective treatment has been laid. Studying the role of the ABCC6 protein in elastic fibers may lead to drugs that will improve or prevent the problems caused by PXE.
Genetic tests are now available that can provide knowledge needed to both diagnose PXE in symptomatic persons and predict it prior to the onset of symptoms in persons at risk. Prenatal diagnosis of PXE, by testing fetal cells for mutations in the ABCC6 gene, can be done in early pregnancy by procedures such as amniocentesis or chorionic villus sampling. For most people, PXE is compatible with a reasonably normal life, and prenatal diagnosis is not likely to be highly desired.
Genetic testing to predict whether an at-risk child will develop PXE may be helpful for medical management. A child who is found to carry a mutation can be monitored more closely for eye problems and bleeding, and can begin the appropriate lifestyle changes to prevent cardiovascular problems.
Resources
BOOKS
Pope, F. Michael. “Pseudoxanthoma Elasticum, Cutis Laxa, and Other Disorders of Elastic Tissue.” In Emery and Rimoin’s Principles and Practice of Medical Genetics. 3rd ed. Ed. David L. Rimoin, J. Michael Connor, and Reed E. Pyeritz. New York: Churchill Livingstone, 1997.
PERIODICALS
Ringpfeil, F., et al. “Pseudoxanthoma Elasticum: Mutations in the MRP6 Gene Encoding a Transmembrane AFP-binding Cassette (ABC) Transporter.” Proceedings of the National Academy of Sciences 97 (May 2000): 6001–6.
Sherer, D.W., et al. “Pseudoxanthoma Elasticum: An Update.” Dermatology 199 (1999):3–7.
elasticum Pseudoxanthoma
G A L E E N C Y C L O P E D I A O F G E N E T I C D I S O R D E R S |
967 |

Pyloric stenosis
ORGANIZATIONS
National Association for Pseudoxanthoma Elasticum. 3500 East 12th Avenue, Denver, CO 80206. (303) 355-3866. Fax: (303) 355-3859. Pxenape@estreet.com.http://www.napxe.org .
PXE International, Inc. 23 Mountain Street, Sharon, MA 02067. (781) 784-3817. Fax: (781) 784-6672. PXEInter @aol.com. http://www.pxe.org/ .
Barbara J. Pettersen, MS, CGC
I Pyloric stenosis
Definition
Pyloric stenosis is a disorder that occurs when the pyloric sphincter muscle, which is found at the outlet of the stomach, thickens and becomes enlarged causing the cavity (lumen) of the pylorus to narrow and lengthen. This blocks the passage of food from the stomach to the small intestine (the portion of bowel that continues digestion after food leaves the stomach).
Description
Pyloric stenosis occurs due to enlargement of the walls of the pyloric sphincter. The pyloric sphincter is a circular smooth muscle at the outlet of the stomach that controls the flow of food from the stomach to the small intestine. The muscle cells become enlarged (hypertrophied) causing a narrowing (stenosis) of the pyloric lumen. This causes food to be pushed back into the stomach. Symptoms of pyloric stenosis typically appear two to six weeks after birth. In rare cases it occurs in older adults, not of genetic cause but due to an ulcer (inflammatory lesion of the mucous-like tissue in the stomach) or hardening of the tissue (fibrosis) at the outlet of the stomach. Alternate names associated with the disorder are Hypertrophic pyloric stenosis and Infantile hypertrophic pyloric stenosis.
Genetic profile
The exact cause of pyloric stenosis is unknown. It generally occurs in one in 300 births. The incidence of pyloric stenosis may be higher if a parent or sibling had the condition. It is also more common in the first-born child. Family correlation studies have shown that there is higher expression (concordance) of pyloric stenosis in identical twins (monozygotic) than in fraternal twins (dizygotic). The risk for first-degree relatives (brothers, sisters) of females is higher than those of males. This is also true of second-degree relatives (cousins).
K E Y T E R M S
Pyloric sphincter—Circular smooth muscle found at the outlet of the stomach.
Stenosis—The constricting or narrowing of an opening or passageway.
It has been suggested that motilin receptors, which are responsible for motility, might have an involvement in pyloric stenosis. The development of functional motilin receptors occurs around the age of onset for most cases of pyloric stenosis. Studies have found that the use of an antibiotic, called erythromycin for pertussis (a contagious respiratory disease also known as whooping cough) prophylaxis may increase the risk for pyloric stenosis. Erythromycin is a motilin agonist (acts on something to produce a predictable response) and high doses can cause an increase in non-propagated contractions and motility. The lack of neuronal nitric oxide synthase in pyloric tissue may cause a spasm (a twitching or involuntary contraction) in the pyloric muscle in individuals with pyloric stenosis. Neuronal nitric oxide synthase is needed for the synthesis of nitric oxide, which opposes the contraction force in active muscle.
Demographics
Pyloric stenosis affects males three to four times more than females and appears to have an increased incidence in caucasians.
Signs and symptoms
Symptoms include:
•Regurgitation and non-bilious vomiting. Infants may bring food back up during or after feeding. Vomiting may become projectile (expelled with force) and vomit may have a “coffee ground” color. Vomit should not contain stomach bile, which is acidic and a brownishgreen color. This would be contraindicative of pyloric stenosis.
•Olive-sized abdominal mass. A mass about the size of an olive may be felt in the upper abdomen. The mass should be hard, mobile, and non-tender.
•Pylorospasm. A spasm of the pyloric muscle may occur due to increased motility.
•Additional abnormalities. These include hunger, irritability, lethargy (prolonged sleepiness or sluggishness), weight loss, decreased urine output, constipation, and gastric (stomach) peristalsis (rhythmic contraction of smooth muscle) from the left to right.
968 |
G A L E E N C Y C L O P E D I A O F G E N E T I C D I S O R D E R S |
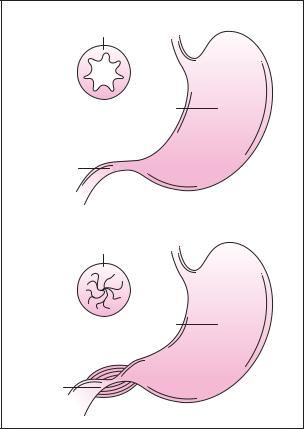
Diagnosis
An individual’s medical history and physical assessment by a doctor are necessary for a diagnosis of pyloric stenosis. A palpable mass, the size of an olive, in the upper abdominal area usually confirms a diagnosis of pyloric stenosis. When physical findings are inconclusive, an abdominal ultrasound or barium study may be performed to confirm diagnosis. An ultrasound, the preferred method of confirmation, is a non-invasive study that uses high frequency sound waves to distinguish the image of internal structures of the body. A barium study involves the ingestion of a radiographic dye. The movement of the dye through the gastrointestinal (GI) tract can be followed by fluoroscopy or x ray studies. It has been suggested that the Lipper GI series may be an effective step in confirming pyloric stenosis. This test consists of aspirating (withdrawal of fluid) and measuring gastric contents. The amount of aspirated contents is indicative of pyloric stenosis and studies have demonstrated this method to be a reliable diagnostic tool.
In adults with symptoms of pyloric stenosis, a barium swallow study is used to diagnose the disorder. X rays are taken of the abdominal structures after the ingestion of the barium radioisotope (a radioactive form of a chemical element).
Treatment and management
As of 2000, the only treatment for pyloric stenosis is surgical pyloromyotomy. Making an incision into the pyloric muscle and spreading the walls of the muscle apart completes the surgery. This allows gastric mucosa to push up through the incision and relieve the blockage.
Blood analysis should be performed before surgery and intravenous (going into the vein) fluids should be given to correct electrolyte (sodium, potassium, calcium etc.) imbalances and rehydrate infants. Following surgery the infant should start on an oral electrolyte (elements necessary for cell functioning) solution (pedialyte). Feedings will be gradually increased until the infant is tolerating 2-3 ounces of breast-milk or formula without complications. The stomach needs time to heal; therefore vomiting due to increased feedings is common. Infants are usually discharged 24–48 hours following surgery. It has been suggested that rapid advancement of the strength and volume of feedings is effective and may allow for quicker discharge from the hospital.
Adults being treated for pyloric stenosis usually have a stomach tube inserted into the muscle that remains in place after sugery.
Recurrence of pyloric stenosis after surgery is rare. As of 2000, there has been no occurrence of conditions
Cross section of pylorus
Stomach
Pylorus
Cross section of pylorus
Stomach
Pylorus
These diagrams show the cross section of a normal pylorus in relation to a stomach with pyloris stenosis where the pylorus has become extremely narrowed. Constriction of the pyloris results from enlargement of the muscle surrounding it. (Gale Group)
later in life related to the occurrence of pyloric stenosis during infancy.
Prognosis
The prognosis of pyloric stenosis is very good for those that are diagnosed early and treated with surgery. Life expectancy of infants diagnosed with pyloric stenosis is the same as that of the average individual. Parents should contact a doctor if pyloric stenosis is suspected.
Resources
BOOKS
Bowden, V. R., S. B. Dickey, and C. Smith-Greenberg. Children and their families: The continuum of care. Philadelphia: W.B. Saunders Company, 1991.
Connor, J.M., and M. A. Fergusen-Smith. Essential Medical Genetics. 4th ed. Oxford: Blackwell Scientific Publications, 1993.
stenosis Pyloric
G A L E E N C Y C L O P E D I A O F G E N E T I C D I S O R D E R S |
969 |

Pyruvate carboxylase deficiency
“Congenital Pyloric Stenosis.” In Principles and Practice of Medical Genetics. Vol. 2. Ed. Alan E.H. Emery and David L. Rimoin. New York: Churchill Livingstone, 1983.
PERIODICALS
Gollin, G., et al. “Rapid advancement of feedings after pyloromyotomy for Pyloric Stenosis.” Clinical Pediatrics 39 (2000): 187–90.
Honein, M.A., et al. “Infantile hypertrophic pyloric stenosis after pertussis prophylxis with erythromycin: a case review and cohort study.” The Lancet 354 (1999): 2101–5.
Huffman, G.B. “Evaluating infants with possible pyloric stenosis.” American Family Physician 60 (1999): 2108–9.
Patterson, L., et al. “Hypertrophy pyloric stenosis in infants following pertussis prophylaxis with erythromycin— Knoxville, Tennessee, 1999.” Morbidity and Mortality Weekly Report 48 (1999): 1117–20.
WEBSITES
Healthgate. http://www.healthgate.com .
McKusick, V.A., ed. “Entry 179010: Pyloric Stenosis, Infantile.” (June 15, 1998). Online Mendelian Inheritance in Man (OMIM). http://www.ncbi.nlm.nih.gov/htbinpost/Omim/dispmim?179010 .
McKusick, V.A., ed. “Entry 163731: Nitric Oxide Synthase 1; NOS1.” (January 23, 2001). Online Mendelian Inheritance in Man (OMIM). http://www.ncbi.nlm.nih.gov/ htbin-post/Omim/dispmim?163731 .
Yale University Department of Surgery.http://www.yalesurgery.med.yale.edu .
Laith Farid Gulli, MD
Tanya Bivins, BS
I Pyruvate carboxylase deficiency
Definition
Pyruvate carboxylase deficiency (PCD) is a rare non-sex linked (autosomal) disorder that results from an insufficient amount of the enzyme pyruvate carboxylase. This disorder is inherited as a recessive trait and it is known to be caused by more than one different mutation in the same gene (allelic variants).
Description
There are two recognized types of pyruvate carboxylase deficiency, neonatal PCD (type B) and infantile onset PCD (type A). Neonatal PCD is associated with a complete, or near complete, inability to produce pyruvate carboxylase. Infantile onset PCD is associated with a chemical change in the pyruvate carboxylase enzyme that
prevents this slightly different chemical from functioning as efficiently as the normal pyruvate carboxylase enzyme.
In order for the cells of the body to function properly, they must have energy. This energy comes in the form of the chemical ATP. ATP is primarily produced by breaking down carbohydrates and blood sugar (glucose) molecules. To begin the process of converting glucose and carbohydrates into usable energy, these molecules are first converted into pyruvate molecules. Once pyruvate molecules have been formed, one of two things will happen: if more energy is required by the cell, the molecules will be further broken down into ATP; or, if no additional energy is needed by the cell, the pyruvate molecules will be put back together to reform a glucose molecule.
These transformations of pyruvate are accomplished primarily by two enzymes: pyruvate dehydrogenase (PDH), an enzyme that begins the breakdown of the pyruvate into ATP, and pyruvate carboxylase, an enzyme that begins the chemical process to reform glucose molecules. The reformation of glucose from pyruvate is a vital step in cellular metabolism. It allows carbohydrate molecules to be converted into a more readily usable form (glucose). Glucose is not only easier to breakdown into the energy required by the cells, but it is also more able to be transported through the bloodstream than most other fuel sources. This is particularly important because certain cells (primarily those of the brain and nervous system) cannot breakdown larger molecules; they must get their energy directly from glucose.
Pyruvate carboxylase is, in effect, part of the “off switch” for the production of ATP from pyruvate. After a cell has received the amount of ATP it requires, it is the job of pyruvate carboxylase to re-convert the excess pyruvate molecules in that cell back into glucose molecules for storage or transport to another part of the body where they may be needed. Any molecules that are not put back together will degrade into lactic acid. This lactic acid will either be released into the bloodstream or it will buildup in the tissues. The buildup of lactic acid in the muscle tissues and red blood cells is normal during strenuous exercise. However, the accumulation of lactic acid in other tissues without exercise or without oxygen deprivation is symptomatic of an underlying problem in the normal metabolism of the cells.
People with PCD have either a complete inability or a severely limited ability to produce pyruvate carboxylase. Since these individuals cannot produce the amounts of this enzyme required to form glucose from pyruvate, this pyruvate is converted instead into lactic acid, which builds up in the cells. Additionally, since glucose cannot be adequately formed within the body of a pyruvate car-
970 |
G A L E E N C Y C L O P E D I A O F G E N E T I C D I S O R D E R S |
