
The Nitro Group in Organic Synthesis
.pdfREFERENCES 181
95b. Kies, F. M., P. Poggendorf, S. Picasso, and V. Jager. Chem. Commun., 119 (1998). 96a. Ram, S., and R. E. Ehrenkaufer. Tetrahedron Lett., 25, 3415 (1984).
96b. Ram, S., and E. E. Ehrenkaufer. Synthesis, 133 (1986).
96c. Kotler, T., G. E. Decker, and K. Sandhoff. Tetrahedron, 90, 13425 (1994).
97.Zschiesche, R., and H. U. Reisig. Liebigs Ann., 551 (1989).
98.Barrett, A. G. M., and C. B. Spolling. Tetrahedron Lett., 29, 5733 (1988).
99.Colvin, E. W., A. K. Beck, and D. Seebach. Helv. Chim. Acta., 64, 2264 (1981).
100.Loyd, D. H., and D. E. Nichols. J. Org. Chem., 51, 4294 (1986).
101.Petrini, M., R. Ballini, and G. Rosini. Synthesis, 713 (1987).
102a. Satoh, T., S. Suzuki, Y. Suzuki, Y. Miyaji, and Z. Imai. Tetrahedron Lett., 4555 (1969). 102b. deLaszlo, S. E., S. V. Ley, and R. A. Porter. J. Chem. Soc., Chem. Commun., 344 (1986).
103.Yoon, N. M., and J. Choi. Synlett, 135 (1993).
104.Yoo, S., and S. Lee. Synlett., 419 (1990).
105.Osby, J. O., and B. Ganem. Tetrahedron Lett., 26, 6413 (1985).
106.Heinzman, S.W., and B. Ganem. J. Am. Chem. Soc., 104, 6801 (1982).
107.Chapuzet, J. M., B. Cote, M. Lavoie, E. Martel, C. Raffin, and J. Lessard. Novel Trends in Electro Organic Synthesis, ed. by S. Torii, pp 321–324, Kodansha, Tokyo (1995).
108.Fitch, R. W., and F. A. Luzzio. Tetrahedron Lett., 35, 6013 (1994).
109.Curran, T. T., G. A. Flynn, D. E. Rudisill, and P. M. Weintraub. Tetrahedron Lett., 36, 4761 (1995).
110.Harsy, S. G. Tetrahedron, 46, 7403 (1990).
111.Kende, A. S., and J. S. Mendoza. Tetrahedron Lett., 32, 1699 (1991).
112a. Nightingale, D. V., and J. R. Janes. J. Am. Chem. Soc., 66, 352 (1944). 112b. Baer, H. H., and W. Rank. Can. J. Chem., 50, 1292 (1972).
113.Wehrli, P. A., and B. Schaer. Synthesis, 649 (1977).
114.Koos, M. Tetrahedron Lett., 37, 415 (1996).
115a. Maguire, M. P., P. L. Feldman and H. Rapoport. J. Org. Chem., 55, 948 (1990). 115b. Yadav, J. S., B. V. S. Reddy, R. Srinivas, and T. Ramalingam. Synlett., 1447 (2000).
116.Shono, T., H. Hamaguch, H. Mikami, H. Nogusa, and S. Kashimura. J. Org. Chem., 48, 2103 (1983).
117.Feuer, H., R. S. Bartlett, B. F. Vincent, Jr., and R. S. Anderson. J. Org. Chem., 30, 2880 (1965).
118.Kabalka, G. W., L. H. M. Guindi, and R. S. Varma. Tetrahedron, 46, 7443 (1990).
119.Ho, T. L., and T. L. Wong. Synthesis, 196 (1974).
120a. Barton, D. H. R., I. Fernandez, C. S. Richard, and S. Z. Zard. Tetrahedron, 43, 551 (1987). 120b. Albanese, D., D. Kandini, and M. Penso. Synthesis, 333 (1990).
121.Hwu, J. R., W. N. Tseng, H. V. Atel, F. Wong, D. N. Horng, B. R. Liaw, and L. C. Lin. J. Org. Chem., 64, 2211 (1999).
122.Bartra, M., P. Romea, F. Urpi, and J. Vilarrasa. Tetrahedron, 46, 587 (1990).
123.Laso, N. M., B. Q. Sire, and S. Z. Zard. Tetrahedron Lett., 37, 1605 (1996).
124a. Dumestre, D., L. E. Kaim, and A. Gregoire. Chem. Commun., 775 (1999). 124b. Domling, A., and I. Ugi. Angew. Chem. Int. Ed. Engl., 39, 3168 (2000).
125. Chow, Y. L. The chemistry of amino, nitroso and nitro compounds and their derivatives, Supplement F, ed. by S. Patai, p. 127 John Wiley, New York (1982).
126a. Huu, D. P., M. Petrusova, J. N. BeMiller, and L Petrus. Synlett., 1319 (1998).
126b. Huu, D. P., M. Petrusova, J. N. BeMiller, and L. Petrus. Tetrahedron Lett., 40, 3053 (1999).
127.Aurich, H. G. In the Chemistry of amino, nitroso and nitro compounds, ed. by S. Patai, John Wiley, 1982, Part 1, p. 565–622.
128a. Chiarelli, R., M. Novak, A. Rassat, and J. L. Tholence. Nature, 363, 147 (1993). 128b. Viret, J. P., G. Rest, and A. Rassat. Tetrahedron Lett., 40, 7102 (1999).
129.Chary, K. P., S. R. Ram, and D. S. Iyengar. Synlett, 683 (2000).
130.J. I. G. Cadogan, Synthesis, 11 (1969).
131.Fisher, B., and L. Sheihet. J. Org. Chem., 63, 393 (1998).

7.1 R–Nu FROM R–NO2 185
|
BnO |
OBn |
|
BnO |
|
OBn |
OCH2OMe |
|
|
|
|
|
|||
|
|
O |
NO2 |
|
+ |
|
O |
|
|
|
|
|
|||
|
|
|
|
|
|
|
|
|
|
|
|
|
|||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
BnO |
|
OCH2OMe |
BnO |
NO2 |
|
|
|
|
|
|
|
|
||
|
|
|
|
|
|
|
|
|
|
|
|||||
|
|
|
|
BnO |
OBn |
|
|
|
BnO |
OBn |
|
|
|||
|
|
|
|
|
|
|
OCH2OMe |
|
|
|
|||||
|
|
|
|
|
O |
|
1) MeONa |
|
OCH2OMe |
||||||
|
|
|
|
|
|
|
|
|
O |
||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
BnO |
|
NO2 |
|
|
2) O3 |
|
CHO |
|
|
|
|
+ |
– |
|
|
|
|
|
|
BnO |
|
|
||||
|
|
|
49% |
|
|
|
|
|
|
|
|||||
|
NaCH2NO2 |
|
|
|
|
|
|
|
|
|
|||||
|
|
|
|
|
|
|
|
|
|
|
|||||
|
|
DMSO |
|
|
|
+ |
|
|
|
|
|
|
|
|
|
|
|
|
|
BnO |
OBn |
|
|
|
BnO |
|
|
|
|
||
|
|
|
|
|
|
|
|
OBn |
|
|
|||||
|
|
|
|
|
O |
NO2 |
|
1) MeONa |
O |
CHO |
|
||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
OCH2OMe |
|
2) O3 |
|
|
OCH2OMe |
|||
|
|
|
|
|
BnO |
|
|
|
BnO |
|
|||||
|
|
|
|
|
|
17% |
|
|
|
|
|
|
|
||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||
Ph |
O |
OMOM |
+ |
– |
Ph |
O |
O |
OMOM |
1) MeONa |
Ph |
O |
O |
OMOM |
||
|
O |
O |
|
|
O |
|
|
2) O3 |
|
O |
|
||||
MOMO |
|
O |
NaCH2NO2 |
MOMO |
|
|
O |
MOMO |
|
O |
|||||
|
|
|
|
|
|
AcHN |
|
|
|
|
AcHN R |
||||
|
AcHN NO2 O |
|
DMSO |
|
|
|
|
O |
|
|
|
O |
|||
|
|
|
|
|
|
|
|
NO2 |
|
|
R = CHO, CO2Me |
||||
|
|
|
|
|
|
|
|
94% |
|
|
|
|
|||
|
|
|
|
|
|
|
|
|
|
|
|
88% |
|
||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
NaBH4 |
|
Ph |
O |
OMOM |
|
|
Ph |
O |
O |
OMOM |
|
Ph |
O |
O |
OMOM |
||
|
O |
O |
|
|
|
|
O |
|
|
|
|
O |
|
||
MOMO |
|
O |
|
Bu SnH |
MOMO |
|
|
O |
1) CS2, NaOH MOMO |
|
O |
||||
|
AcHNMe |
O |
|
3 |
|
AcHN |
O |
O |
|
|
|
AcHN |
O |
||
|
|
AIBN |
|
|
|
|
|
|
|||||||
|
|
86% |
|
|
|
|
|
|
2) Me2 SO4 |
|
|
70% |
OH |
||
|
|
|
|
|
|
|
|
|
|
|
|
|
|||
|
|
|
|
|
|
|
S |
|
SMe |
DMSO |
|
|
|
||
|
|
|
|
|
|
|
|
|
|
|
|
|
|||
|
|
Na/NH3 |
|
|
|
|
89% |
|
|
|
MeOCH2Cl |
||||
|
|
|
|
|
|
|
|
|
|
|
|
Et(i-Pr2)N |
|||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||
|
HO |
OMOM |
|
|
|
HO |
|
OMOM |
|
Ph |
O |
|
OMOM |
||
|
O |
|
|
|
|
O |
|
|
|
O |
|
||||
|
HO |
|
|
|
|
HO |
|
|
Na/NH3 |
|
O |
|
|||
MOMO |
|
O |
|
|
MOMO |
|
|
O |
MOMO |
|
O |
||||
|
AcHNMe |
|
|
|
AcHN |
|
|
|
AcHN |
||||||
|
O |
|
|
|
|
O |
|
|
O |
||||||
|
|
88% |
|
|
|
|
|
|
OMOM |
|
|
|
OMOM |
||
|
|
|
|
|
|
|
|
|
87% |
|
|
|
91% |
||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||
Scheme 7.2.
7.1.2 Ionic Process
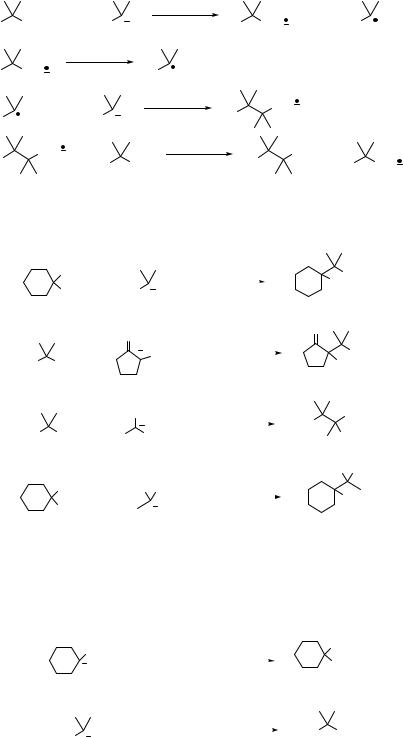
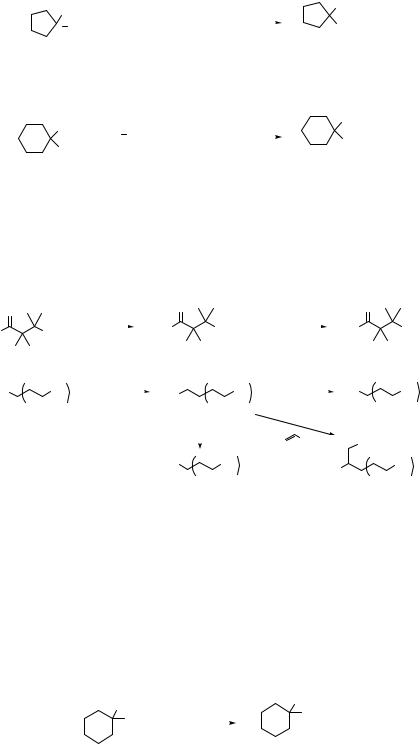
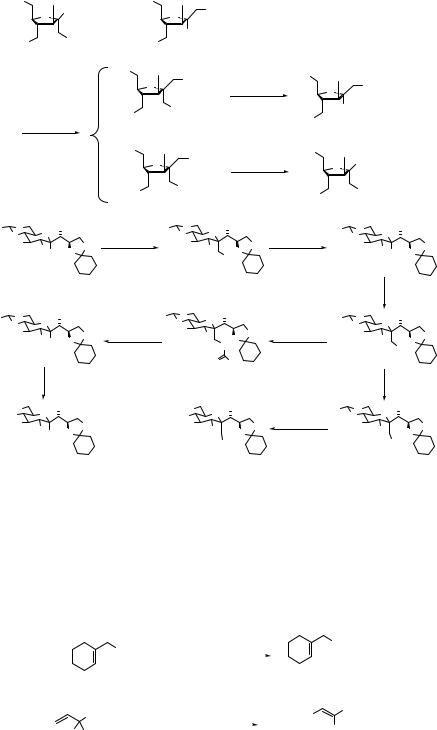
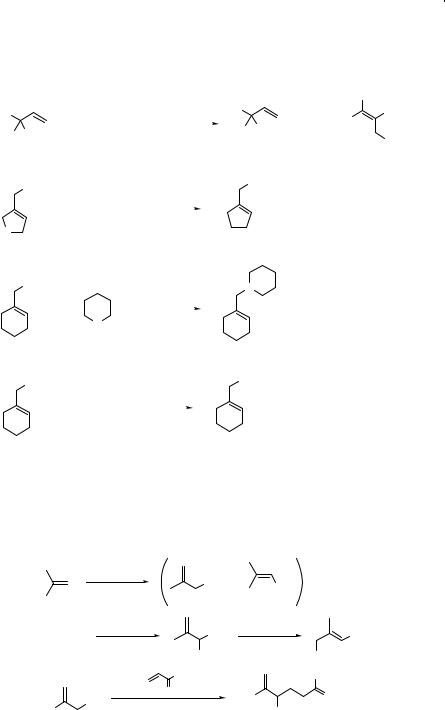
Simple nitroalkanes such as nitroethane, 1-nitropropane, or 2-nitropropane are generally bad electrophiles for the SN2 reactions.14 In contrast, nitro groups at allylic positions are readily displaced by thiolate ions (Eq. 7.13)15 or lithium dialkylcuprates (Eq. 7.14).16
NO2 |
|
|
HMPA |
SPh |
(7.13) |
|
+ PhSNa |
|
|||||
|
50 ºC |
|
|
|||
|
|
|
|
|
||
|
|
|
|
|
62% |
|
Me |
|
|
|
|
CO2Me |
|
|
|
ether |
n-C5H11 |
|
||
+ |
(n-Bu)2CuLi |
|
|
|
Me |
(7.14) |
|
–30 ºC |
|||||
O2N CO2Me |
|
|
70% (E/Z = 96/4) |
|
||
|
|
|
|
|
|
|
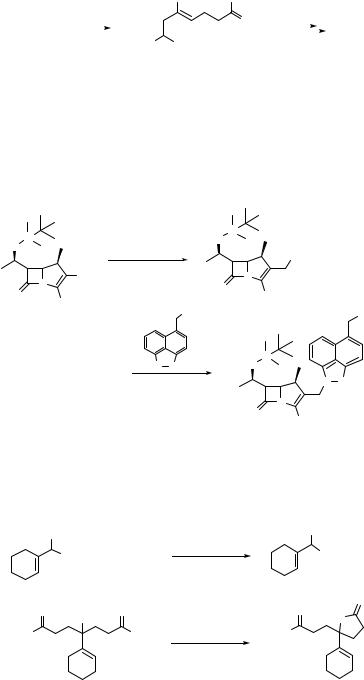
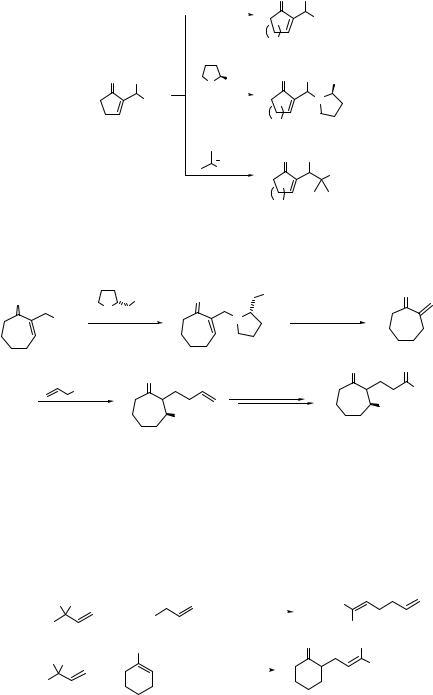
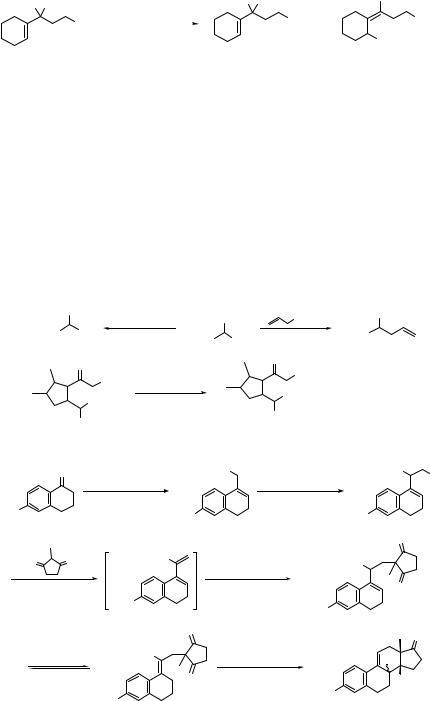
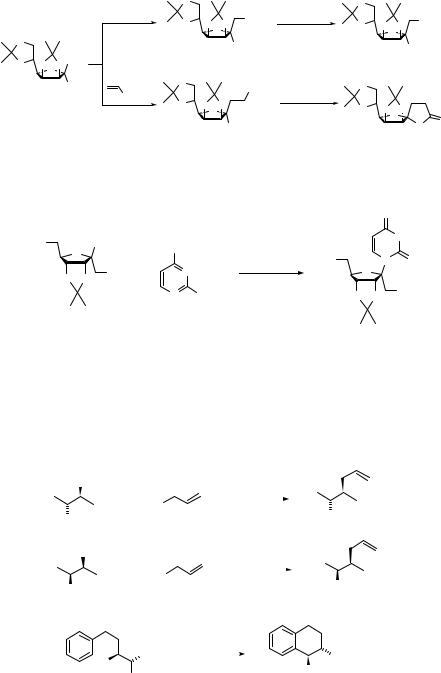
190 SUBSTITUTION AND ELIMINATION OF NO2 IN R–NO2
α-Nitro ethers effect a similar SN1-type substitution under solvolytic conditions; tertiary nitro compounds derived from 1-deoxy-1-nitroaldose and formaldehyde or methyl acrylate undergo denitro-hydroxylation or intramolecular lactonization, respectively (Eq. 7.29).33
|
|
O |
|
|
|
|
O |
|
|
|
|
|
HCHO |
|
OH |
|
NaHCO3 |
|
|
OH |
|
O |
|
O |
O O O |
|
H2O-dioxane |
O |
O O O |
|
|
|
|
|
|
NO2 |
|
70 ºC |
|
76%OH |
|
||
|
|
|
|
|
|
|
||||
|
|
O H |
|
|
|
|
|
|||
O O |
O |
|
|
|
|
|
|
(7.29) |
||
|
|
|
|
|
|
|
|
|||
|
|
O |
|
CO2Me |
|
O |
|
|
|
|
|
|
NO2 |
|
NaHCO3 |
|
|
|
|||
|
|
CO2Me |
|
|
|
|
|
|
|
|
|
|
O |
O O O |
|
|
H2O-dioxane |
O |
O O O |
|
O |
|
|
|
NO2 |
|
70 ºC |
|
|
O |
||
|
|
|
|
|
69% |
|
||||
|
|
|
|
|
|
|
|
|||
This type of substitution reaction is useful for the synthesis of biologically active nucleosides. 1-Deoxy-1-nitroribose reacts with 2,4-bis(trimethylsilyloxy)pyrimidine in the presence of FeCl3 in MeCN to give the nucleoside in 77% yield (Eq. 7.30).34
|
|
|
|
|
|
|
O |
RO |
|
NO2 |
OSiMe3 |
|
|
NH |
|
|
|
|
|
||||
O |
|
|
RO |
|
N O |
||
|
|
|
|
|
|
||
|
|
OAc + |
|
N |
FeCl3 |
O |
|
O |
O |
|
MeCN |
|
|||
|
|
|
|
OAc |
|||
|
|
|
N |
OSiMe3 |
O |
O |
|
|
|
|
|
||||
(7.30)
77%
Furthermore, a neighboring group participation of a phenylthio function is observed in the Lewis acid-catalyzed nucleophilic substitution reaction of various β-nitrosulfides. Because the β-nitrosulfides are readily available, by the Michael addition of thiols to nitroalkenes (see Michael addition Chapter 4), this reaction is very useful. The β-nitrosulfides are prepared stereoselectively, and the reaction proceeds in a stereo-specific way (retention of configuration) as shown in Eqs. 31–34.35
|
|
|
NO2 |
|
|
|
TiCl4, CH2Cl2 |
|
|
|
|
|
||||
|
|
|
|
|
|
|
|
|
||||||||
|
|
|
+ |
Me3Si |
|
|
|
|
|
|
(7.31) |
|||||
|
|
|
|
|
|
RT, 1 h |
||||||||||
|
|
|
|
|
|
|
|
|||||||||
SPh |
|
|
|
|
|
|
|
|
SPh |
|||||||
|
|
|
|
|
|
|
|
65% (100% anti) |
||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
||||
|
|
NO2 |
|
|
|
TiCl4, CH2Cl2 |
|
|
|
|
|
|||||
|
|
|
|
|
|
|
|
|
|
|||||||
|
|
|
|
+ |
Me3Si |
|
|
|
|
(7.32) |
||||||
|
|
|
|
|
|
|
|
|
||||||||
|
|
|
|
|
|
|
|
RT, 1 h |
|
|
||||||
SPh |
|
|
|
|
|
|
|
|
SPh |
|||||||
|
|
|
|
|
|
|
65% (anti/syn = 95/5) |
|||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|||||
|
|
|
|
|
NO2 |
SnCl4, CH2Cl2 |
(7.33) |
|||||||||
|
|
|
|
PhS |
RT, 6 h |
|
|
SPh |
||||||||
|
|
|
|
|
||||||||||||
|
|
|
|
|
|
|
|
|
|
|||||||
Me |
Me |
|
76% (100% trans) |
||
|

 Me
Me