The Nitro Group in Organic Synthesis
.pdf
|
|
|
|
7.2 R–H FROM R–NO2 211 |
O |
|
|
|
HO |
H |
|
HO |
Bu3SnH, AIBN |
|
|
|
|
|
|
amberlyst A-21 |
|
NO2 |
benzene |
|
NO2 |
|
|
|
|
|
|
62 % |
|
|
|
|
OH |
|
|
|
|
|
1) CrO3 |
H |
|
|
H |
2) MeLi |
|
|
|
|
||
|
|
|
3) SOCl2 |
Cedrene |
|
|
52% |
|
|
|
|
|
80% (3 steps) |
|
|
|
|
|
|
|
|
Scheme 7.19. |
|
|
|
|
|
H |
MgBr |
|
Me |
O |
|
|
|
|
|
||
|
CHO |
|
|
|
O2N |
|
|
|
|
|
DBU |
|
|
|
SPh |
|
O2N |
SPh |
|
|
|
|||
|
|
|
||
|
|
|
66% |
|
HO |
|
Bu3SnH, AIBN |
|
|
|
|
|
|
|
|
|
|
|
HO |
O2N |
|
SPh |
|
55% |
|
60% |
|
|
|
Scheme 7.20.
7.2.2 Ionic Denitration
Denitration is generally carried out via a radical process using tin hydride, but some nitro groups are replaced by hydrogen via an ionic process. Rosini and coworkers have developed an indirect denitration method of α-nitroketones by the treatment of the corresponding tosylhydrazones with LiAlH4, in which 1,4-elimination of HNO2 and the reduction of tosylazoalkanes to tosylhydrazones occur (Eq. 7.87).134
|
O |
|
|
|
|
|
H |
|
|
|
|
|
|
||||||
|
|
|
|
N |
N |
|
|
|
|
|
|
||||||||
R1 |
|
|
|
R2 |
|
TsNHNH2 |
|
Ts |
|
|
|
|
LiAlH4 |
|
|||||
|
|
|
|
|
|
R2 |
|
|
|
|
|||||||||
|
|
|
|
|
|
|
|
|
R1 |
|
|
|
|
|
0–10 ºC |
||||
|
|
|
NO2 |
|
|
|
|
|
|
|
|
|
|||||||
|
|
|
|
|
|
|
|
NO2 |
|
|
|
|
|
|
|||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||
|
|
|
|
|
|
|
H |
|
|
|
|
O |
|||||||
|
|
|
|
N |
N |
|
|
|
|
||||||||||
|
|
|
|
|
Ts |
+ |
|
|
|
|
|
|
|
|
|
||||
|
|
|
|
|
|
|
|
R2 |
|
H3O |
|
R1 |
|
|
|
|
R2 |
||
|
|
|
|
|
|
|
|
|
|
|
|
||||||||
|
|
|
|
R1 |
|
|
|
|
|
|
|
|
|
(7.87) |
|||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||
|
|
|
|
|
|
|
H |
|
|
|
|
|
|
|
H |
||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||

212 SUBSTITUTION AND ELIMINATION OF NO2 IN R–NO2
Although this method is not a general procedure, being specific for α-nitroketones, it has several merits to avoid the use of toxic reagents such as organotin compounds. Functionalized ketones have been prepared by this denitration reaction, in which functionalized nitroalkanes are used as alkyl anion synthons. For example, 3-nitropropanal ethylene acetal can be used as
synthon of the 3-oxo-propyl anion and 1,4-dicarbonyl compounds are prepared, as shown in Eq. 7.88.135
O O |
NO2 |
|
O O NO2 |
|
|
NO |
O |
|
TsNHNH2 |
|
O |
O |
2 |
|
|
H |
|
H |
|
H |
|||
|
|
H |
|
|
|||
|
|
NNHTs |
|
|
|||
|
|
|
|
|
|
||
|
O |
|
|
|
|
|
|
|
|
93% |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
O O |
|
O |
|
|
|
LiAlH4 |
1) TsOH |
|
|
|
|
||
H |
H |
|
|
|
|||
|
|
2) BF3 |
|
|
(7.88) |
||
|
|
NNHTs |
|
O |
|
||
|
|
|
|
|
|
||
|
|
94% |
|
|
85% |
|
|
(Z)-1-Nitro-3-nonene is converted into a pheromone, (Z)-5-undecen-2-one, via nitro-aldol reaction (see Section 3.2.3), followed by oxidation, and denitration, as shown in Eq. 7.89.136
|
|
NO2 |
|
|
|
|
|
|
|
|
NO2 |
|||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
79% |
O |
|||||||||
|
|
|
|
|
|
|
||||||||
1) TsNHNH2 |
|
|
|
|
|
|
|
|
|
|
|
|||
|
|
|
|
|
|
|
|
|
|
|
||||
|
|
|
|
|
|
|
|
|
|
|
||||
2) LiAlH4 |
|
|
|
|
|
|
|
|
(7.89) |
|||||
3) H O+ |
|
|
|
O |
|
|
|
|||||||
3 |
|
|
|
70–80% |
|
|
|
|||||||
The simultaneous denitration-deoxygenation of α-nitroketones is performed on treatment with TsNHNH2 and NaBH4 at 80 °C to give alkanes (Eq. 7.90).137
O
|
|
1) TsNHNH2 |
|
|
|
(7.90) |
|
|
|
2) NaBH4, 80 ºC |
|
|
|
||
NO2 |
71% |
||||||
|
|
||||||
The nitro groups in Eqs. 7.88–7.90 are readily replaced by hydrogen with tin hydride under radical conditions as discussed already. However, the nitro groups in the α-nitrosulfides or β-nitrosulfides are not replaced by hydrogen on treatment with tin hydride but the reaction affords desulfonated products (Eq. 7.51) and alkenes (Eq. 7.97) such radical elimination reactions are discussed in Section 7.3.1. (see Eqs. 7.91 and 7.92).138
 SPh Bu3SnH
SPh Bu3SnH  NO2
NO2
NO2 |
AIBN |
H |
(7.91) |
|
80% |
|
|
SEt |
|
|
|
NO2 |
Bu3SnH |
OH |
(7.92) |
|
AIBN |
||
|
93% |
|
|
OH |
|
|
|
|
|
|
7.2 R–H FROM R–NO2 213
The nitro groups of α- or β-nitrosulfides are cleanly replaced by hydrogen via ionic hydrogenation to give sulfides, as shown in Eqs. 7.93–7.95. The attack of hydride takes place at the more substituted carbon.139
|
|
|
SPh |
|
Et3SiH |
|
|
|
|
|
|
|
SPh |
|
|
|
|
|
|
|
|
|
|
|
|
(7.93) |
|
|
|
|
|
|
|
|
|
|
|
|
|||
|
|
|
|
|
SnCl4 |
|
|
|
|
|
|
|
|
|
|
NO2 |
|
94% |
H |
||||||||
|
|
SPh |
|
Et3SiH |
|
|
|
SPh |
|
|
|||
|
|
|
|
|
|
|
|
|
|
|
(7.94) |
||
|
|
|
|
|
|
|
|
|
|
|
|
||
|
NO2 |
|
AlCl3 |
89% |
|
|
|
||||||
|
|
|
|
||||||||||
|
|
|
|
|
|
||||||||
O2N |
|
Et3SiH |
|
|
|
|
|
SPh |
|
|
|||
|
|
|
|
|
|
|
|
|
|
|
(7.95) |
||
|
|
|
|
|
|
|
|
|
|
|
|||
|
|
|
SPh |
|
AlCl3 |
70% |
|
||||||
|
|
|
|
|
|
|
|||||||
|
|
|
|
|
|
|
|
||||||
The difficulty in controlling the regiochemistry during radical-denitration of allylic nitro compounds is well known. The migration of the double bond is a serious problem, as shown in Eq. 7.96. This problem is overcome by a hydride transfer reaction in the presence of a palladium catalyst (Eq. 7.97).140
|
|
|
CO2Me |
Bu SnH |
|
|
CO2Me |
|
CO2Me |
|
|||||
|
|
|
|
|
3 |
|
|
|
|
+ |
|
|
|
(7.96) |
|
|
NO2 |
|
|
AIBN |
|
|
|
|
|
|
|||||
|
|
|
70% (15:85) |
|
|
|
|||||||||
|
|
|
|
|
|
|
|
|
|
|
|
||||
|
R |
|
|
|
|
|
|
R |
|
|
|||||
|
Pd(0) |
|
|
|
|
|
|
H– |
|
|
|||||
|
|
|
|
|
|
|
|
|
|
|
|
|
R |
or |
R |
|
|
|
- NO2– |
|
|
|
|
|
|
|
|
||||
|
NO |
|
|
Pd |
|
|
H–: HCO2NH4, |
|
|
||||||
2 |
|
|
|
|
|
|
|
|
|
NaBH3CN, NaBH4 |
|
(7.97) |
|||
|
|
|
|
|
|
|
|
|
|
|
|
||||
The regiochemical control of Pd-catalyzed hydride transfer reaction is much more effective than that of the radical denitration, as shown in Eq. 7.98. The base-catalyzed reaction of nitroolefins with aldehydes followed by denitration provides a new synthetic method of homoallyl alcohols (Eq. 7.99).140 Exomethylene compounds are obtained by denitration of cyclic allylic nitro compounds with Pd(0), HCO2H and Et3N (Eq. 7.100).140b
|
|
|
|
|
|
|
Pd(PPh3)4 |
|
|
|
|
|
|
|
|
||||
|
|
|
|
|
|
|
|
H– |
Yield (A/B) |
||||||||||
|
|
|
|
CO2Me |
|
H– |
|
|
|
|
|||||||||
NO2 |
|
|
|
|
|
||||||||||||||
|
|
|
+ |
|
|
|
|
|
|
NaBH4 |
70% (3/97) |
(7.98) |
|||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||
|
|
|
|
|
CO2Me |
|
|
|
|
CO2Me |
|
HCO2NH4 |
|
|
|||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||
|
|
|
A |
|
|
|
|
|
|
B |
|
60% (90/10) |
|
|
|||||
|
|
|
|
|
|
|
|
|
|
Me |
|
|
|
|
|
|
|
||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||
R |
|
35% HCHO |
R |
|
OH |
NaBH4 |
|
Me |
|
|
|||||||||
|
|
|
|
||||||||||||||||
|
|
|
|
|
|
|
|
|
R |
OH |
|||||||||
|
|
|
|
|
Et3N |
|
|
|
NO2 |
|
Pd(0) |
|
|||||||
|
|
|
NO2 |
|
|
|
|
|
|
|
(7.99) |
||||||||
|
|
|
|
|
|
|
|
|
80–90% |
|
|
|
86% |
||||||
214 SUBSTITUTION AND ELIMINATION OF NO2 IN R–NO2
 NO2
NO2
HCO2H, Et3N, THF |
|
|
|
+ |
|
|
|
(7.100) |
|
|
|
|
|
|
|
||||
Pd(acac)2, Bu3P |
|
|
|
||||||
|
|
|
|
|
|
|
|
||
|
|
|
|
|
|
|
|
|
|
45 ºC, 16 h |
|
|
|
|
|
|
|
|
|
89% (86:14) |
|
|
|
|
|||||
|
|
|
|
|
|||||
This denitration of allylic nitro compounds catalyzed by Pd(0) is applied to total synthesis of kainic acid, as shown in Eq. 7.101. The tandem Michael addition of a nitrogen nucleophile to nitrodiene gives the requisite framework. The radical denitration does not give the desired product, but it gives a mixture of regioand stereoisomer. On the other hand, the Pd(0)-mediated denitration proceeds with high regioand stereoselectivity.141 In a similar way, other proteinogenic amino acids having a pyrrolidine dicarboxylic acid ring are prepared (Eq. 7.102).142
|
|
|
|
CO2Et |
|
|
|
Bn |
|
|
|
|
NO2 |
|
|
|
|
|
N |
|
|
|
|
|
+ |
H |
EtOH |
|
|
OH |
|
|
|
||
|
|
|
|
|
|
|
|||||
|
|
|
|
|
|
O2N |
|
|
|
|
|
|
OBz |
|
HO |
Yt · 15h |
|
|
|
|
|
||
|
|
|
NHBn |
|
|
|
CO2Et |
|
|
|
|
|
|
|
|
Bn |
|
|
|
H |
|
|
|
|
|
|
|
|
|
|
88% |
|
|
||
|
|
|
|
N |
|
|
|
N |
|
|
|
|
Pd(0), HCO2NH4 |
OH |
|
|
|
OH |
|
||||
|
THF |
|
|
H |
|
|
|
|
|
|
(7.101) |
|
|
|
|
CO2Et |
|
|
|
|
CO2H |
|
|
|
|
|
|
|
|
|
|
|
|
||
|
|
|
|
100% |
|
|
|
(–)-α-kainic acid |
|
||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
PMB |
|
|
|
|
CO2Et |
|
|
PMB |
|
|
MeO |
C N |
O |
|
|
|
|
|
|
|
|
|
2 |
|
|
|
|
|
|
MeO2C N O |
EtOH |
|
|
|
NO2 |
CO |
Et |
||
|
+ |
|
|
|
|
|
|
|
2 |
||
|
|
|
|
|
|
|
|
|
|
|
|
HN |
H |
|
|
NO2 |
RT |
|
|
|
|
CH2OH |
|
CH2OH |
|
|
|
|
|
|
|
N |
|||
Bn |
|
|
|
|
|
|
|
||||
|
|
|
|
|
|
|
|
Bn |
|
|
|
|
|
|
|
|
PMB |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
MeO2C |
N O |
|
|
|
80% |
|
|
|
|
|
Pd (0) |
|
|
CO2Et |
|
|
|
||
|
|
|
HCO2NH4 |
|
|
|
|
|
|
(7.102) |
|
|
|
|
THF |
|
N |
CH2OH |
|
|
|
||
|
|
|
|
|
|
|
|
|
|
|
|
Bn 90%
7.3 ALKENES FROM R–NO2
Elimination of the NO2 group via radical process or via ionic process from aliphatic nitro compounds affords a useful method for the preparation of olefins. Nitro compounds, with two radical leaving groups at vicinal positions, are prone to undergo radical elimination to give olefins. The nitro group at a β-position with respect to an electron-withdrawing group is readily eliminated to give α,β-unsaturated compounds on treatment with a base (Scheme 7.21).
7.3.1 Radical Elimination
In 1971, Kornblum and coworkers reported a new synthesis of tetra-substituted alkenes from vicinal dinitro compounds.143 The requisite vicinal dinitro compounds are prepared by the
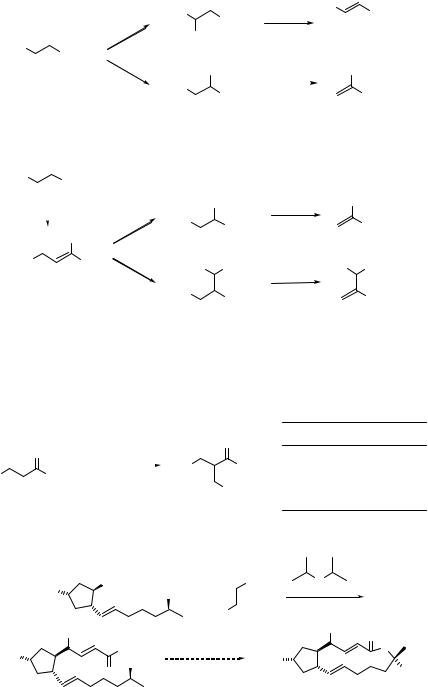
|
|
|
|
7.3 |
ALKENES FROM R–NO2 |
219 |
||||||
|
|
|
|
O2N |
|
|
|
E |
Et |
|||
|
|
|
|
|
|
|
CO |
|||||
|
|
|
|
CO2Et |
|
|
|
|
2 |
|
||
|
|
|
base |
|
|
|
|
|
|
α,β-unsaturated |
||
|
|
|
E |
|
|
|
||||||
O2N |
|
|
|
|
|
|
compounds |
|
||||
|
|
|
|
|
|
|
|
|
|
|
||
|
CO2Et |
|
|
|
|
|
|
|
|
|
|
|
β |
|
2LDA |
|
|
|
|
|
|
|
|
|
|
α |
E |
|
|
|
E |
|
||||||
|
|
|
|
|
|
|
|
|||||
|
|
|
|
O2N |
|
|
|
|
|
CO2Et |
||
|
|
|
|
|
|
|
|
|
||||
|
|
|
|
CO2Et |
|
|
|
|||||
|
|
|
|
Scheme 7.22. |
|
|
|
|
|
|||
O2N |
|
CO2Me |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
LDA |
|
THF-HMPA |
|
R |
DBU |
|
|
R |
|
|||
|
|
|
|
|||||||||
|
RX |
O2N |
|
|
|
|
|
|||||
|
|
|
|
|
|
|
CO2Me |
|||||
|
|
|
|
|
|
|||||||
|
|
OLi |
|
CO2Me |
|
|
|
|||||
|
|
|
66–80% |
|
|
|
85–90% |
|
||||
|
|
|
|
|
|
|
|
|||||
O2N |
|
OMe |
RCHO |
HO R |
|
|
|
HO R |
(7.118) |
|||
|
|
DBU |
|
|
|
|||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
O2N |
|
|
|
CO2Me |
||||
|
|
|
|
CO2Me |
|
|
|
|||||
|
|
|
|
48–84% |
|
|
|
|
|
|||
The alkylation of dianion of methyl 3-nitropropionate requires 5 equiv of HMPA. HMPA is a listed mutagen and should not be used in industry or in academia. 1,3-Dimethyl-3,4,5,6-tetra- hydropyrimidine (DMPU)164 and quinuclidine N-oxide (QNO)165 are recommended as replacements of HMPA (Eq. 7.119).
|
|
|
|
O |
Additive (equiv) |
Yield (%) |
|
O |
|
|
None |
8 |
|
|
LDA, THF, –78 ºC |
|
||||
|
|
|
|
|
||
O N |
OMe |
|
O2N |
OMe |
HMPA (5) |
85 |
|
||||||
PhCH2Br |
|
|
|
|||
2 |
|
|
|
Ph |
DMPU (10) |
85 |
|
|
|
|
|||
|
|
|
|
QNO (5) |
78 |
|
|
|
|
|
|
||
|
|
|
|
|
|
(7.119) |
The reaction shown in Eq. 7.120 has been applied to a total synthesis of (+)-brefeldin-A.166
R1O |
CHO |
CO2Me |
N |
OR2 |
+ |
H |
|
|
|
DMSO |
|
|
|
O2N |
|
|
|
|
|
|
OH |
|
OH |
|
|
O |
|
R1O |
OMe |
HO |
O |
2 |
|
||
|
OR |
|
H |
|
O |
|
|
|
|
|
(+)-Brefeldin-A |
|
54% |
|
(7.120) |
Esters and ketones bearing β-nitro groups can be prepared in many ways. For example, the Diels-Alder reaction of methyl β-nitroacrylate is one typical case. Various cyclic dienes are prepared by this route, and the reactions of Eq. 7.121167 and Eq. 7.122168 are exemplified.