
The Nitro Group in Organic Synthesis
.pdf
7.2 R–H FROM R–NO2 201
Because the α-nitroketones are prepared by the acylation of nitroalkanes (see Section 5.2), by the oxidation of β-nitro alcohols (Section 3.2.3), or by the nitration of enol acetates (Section 2.2.5), denitration of α-nitro ketones provides a useful method for the preparation of ketones (Scheme 7.10). A simple synthesis of cyclopentenone derivatives is shown in Eq. 7.66.76
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
O |
||||||
MeO |
|
|
NO2 |
|
|
O |
|
|
O2N |
|
|
|
|
|
(CH2)7CO2Me |
||||||||
|
|
|
|
|
|
||||||||||||||||||
|
|
|
|
|
|
|
|
t-BuOK |
|
|
|
|
|
|
|
||||||||
|
|
|
+ |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
OMe |
|
|
|
N |
|
(CH2)7CO2Me |
DMSO |
|
|
|
||||||||||||||
|
OMe |
N |
|
|
OMe |
||||||||||||||||||
|
|
||||||||||||||||||||||
|
|
|
|
|
|
|
O |
|
|
|
|
|
|
|
|
|
|||||||
|
|
|
|
|
|
|
80% |
||||||||||||||||
|
|
|
1) Bu3SnH, AIBN |
|
|
|
|
(CH2)7CO2Me |
|
|
|
O |
|||||||||||
|
|
|
|
|
|
|
|
|
|
||||||||||||||
|
|
|
|
|
|
|
MeONa |
|
|
|
|
|
|
|
|
|
(CH2)6CO2Me |
||||||
|
|
|
|
|
|
|
H |
|
|
|
|
|
|
|
|
||||||||
|
|
|
2) H+ |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||
|
|
|
|
|
|
|
O |
(7.66) |
|||||||||||||||
|
|
|
|
|
|
73% |
|
||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||
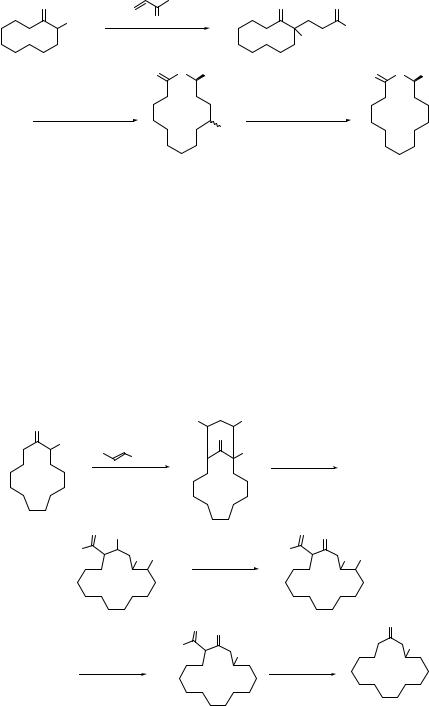
Magnus and coworker have presented a new strategy for the preparation of taxane diterpenes by using nitro-aldol reaction and denitration as key steps (see Scheme 7.11).77
The high acidity of α-nitroketones makes it possible to perform the Henry reactions or Michael additions under extremely mild conditions. The reaction proceeds in the presence of catalytic amounts of Ph3P to give the C–C bond formation products under nearly neutral conditions. Thus, 1,5-dicarbonyl compounds78 and α-methylenecarbonyl compounds79 are prepared by the denitration of α-nitroketones, as shown in Eqs. 7.67 and 7.68, respectively.
|
|
|
O |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
O |
O |
|||||||||||
|
|
|
|
|
H |
Ph3P |
|
|
|
|
|
|
|
Me |
|
|
|
||||||||||||||||
n-C7H15 |
|
|
|
|
Me + |
|
|
|
|
|
|
n-C7H15 |
|
|
|
|
H |
||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||
|
|
|
|
|
|
|
|
O |
|
|
|
|
|
|
|
|
|
|
|
|
|
NO2 |
|
|
|
||||||||
|
|
|
|
|
NO2 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
87% |
|
|
|
||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
O |
|
O |
|
|
|
||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||
|
|
|
|
|
|
|
|
Bu3SnH, AIBN |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||
|
|
|
|
|
|
|
n-C7H15 |
|
|
|
|
|
|
H |
(7.67) |
||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||||||||
|
|
|
|
|
|
|
|
|
|
benzene |
|
|
|
|
|
|
|
|
|
||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Me |
|
|
|
|
|
|
|
|
|
|
|
|
|||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
87% |
|
|
|
|
|
|
|
|
|
|
|
|
|
||||
O |
|
|
|
|
|
O |
1) HCHO, Ph3P |
O |
|
|
O |
|
|
|
|||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
2) Ac2O |
|
|
|
|
Me |
|
|
|
|
|
|
|
C6H13 |
|
|
|
||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||
Me |
|
|
|
|
|
|
|
C6H13 |
3) Bu3SnH, AIBN |
|
|
|
|
|
|
|
|
|
(7.68) |
||||||||||||||
|
|
|
|
|
|
|
NO2 |
4) DBU |
|
|
|
|
|
|
|
|
70% (overall) |
|
|
|
|||||||||||||
Ballini and coworkers have reported a simple synthesis of 1-phenylheptane-1,5-dione based on the strategy of the Michael addition and denitration as shown in Eq. 7.69).80 The product is a natural product that is isolated from fungus.
|
|
|
|
|
|
|
|
O |
|
||||
|
O |
|
|
|
1) Ph3P |
|
|
|
|
|
|
||
|
+ |
|
|
Ph |
|
|
|
||||||
|
|
|
NO2 |
|
|
|
|
|
|||||
Ph |
|
|
|
|
2) Bu3SnH, AIBN |
|
|
|
|||||
|
|
O |
|
|
O |
(7.69) |
|||||||
|
|
|
|
|
|||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
50% (overall)




206 SUBSTITUTION AND ELIMINATION OF NO2 IN R–NO2 |
|
|
|
|
|
|
|
|||||||||||
|
|
|
|
|
|
O2N |
OH |
|
|
|
|
|
|
OH |
||||
HO |
|
|
|
|
|
|
|
|
|
|
OH |
|||||||
1) |
NO2 |
Bu3SnH, AIBN |
||||||||||||||||
|
|
|
|
|
|
OH |
||||||||||||
|
|
|
|
|
|
|||||||||||||
|
|
|
|
|
|
|
|
|
|
|
N |
|
||||||
HN |
|
|
|
2) Swern oxidation |
|
|
|
|
|
|
|
|
||||||
|
|
|
|
N |
|
toluene |
|
|
(7.72) |
|||||||||
|
|
|||||||||||||||||
|
|
|
|
|
|
|||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
Bn |
|||||
Bn |
OTHP |
|
|
Bn |
|
70% |
||||||||||||
|
|
|
|
|
|
|
|
|||||||||||
|
|
|
|
|
|
|
|
|
|
|
||||||||
Dauzonne has reported a simple synthesis of flavanones by radical denitration and dehalogenation of 3-chloro-2,3-dihydro-3-nitro-2-aryl-4H-1-benzopyran-4-ones,92 which are readily prepared by the reaction of salicylaldehydes with 1-chloro-1-nitro-2-arylethenes (Eq. 7.73).93
O |
O |
|
|
|
|
||
NO2 |
Bu3SnH, AIBN |
|
|
Cl |
(7.73) |
||
benzene |
|||
|
Ph |
||
|
O |
O Ph
97%
Sequential Michael additions are versatile methods for the construction of cyclic compounds. Although a variety of these reactions have been developed, the use of alcohols as nucleophiles for the Michael addition to nitroalkenes has been little studied. Recently, Ikeda and coworkers have reported an elegant synthesis of octahydrobenzo[b]furans via the sequential Michael addition of 1-nitro-cyclohexene with methyl 4-hydroxy-2-butynoate in the presence of t-BuOK followed by radical denitration (Eq. 7.74).94
CO2Me |
|
|
|
CO2Me |
|
|
|
CO |
Me |
|||
|
|
O N |
|
|
2 |
|
||||||
NO2 |
|
|
t-BuOK |
|
2 |
|
|
1) Bu3SnH, |
|
|
|
|
+ |
|
|
|
|
|
AIBN, toluene |
|
|
(7.74) |
|||
|
|
THF, 0 ºC |
|
O |
2) H+ |
|
||||||
|
|
|
|
|
||||||||
|
|
|
|
|
|
|
|
|
O |
|
||
HO |
|
|
100% |
|
|
|
86% |
|
||||
|
|
|
|
|
|
|
|
|
||||
The Diels-Alder reaction followed by radical denitration provides a useful strategy for construction of six-membered compounds, in which the nitro group accelerates the reaction and also controls the regio-chemistry of the addition (Eq. 7.75).95
O |
O |
O |
|
|
|
+ |
|
Bu3SnH |
|
AIBN |
|
benzene |
|
|
reflux |
O2N |
Me |
NO2 |
Me |
|
|
83% |
86% (7.75) |
The intramolecular Diels-Alder reaction of nitrotrienes proceeds stereoselectively in the presence of LiClO4 in diethyl ether to give one stereoisomer from endo selectivity. The nitro group is removed from the adduct with Bu3SnH (Eq. 7.76).96
(7.76)
Thus, radical denitration has developed as a reliable tool in organic synthesis and has been mainly carried out using tin hydride in total syntheses of natural products. There is one report in which NaTeH was used for removing the nitro group. Norslanadione, a biologically active


208 SUBSTITUTION AND ELIMINATION OF NO2 IN R–NO2
Table 7.3. Radical denitration with Bu3SnH
|
|
|
Yield (%) |
|
|
|
|
|
|
|
|
|
Yield (%) |
|
||
RNO2 |
|
|
RH |
Ref. |
|
RNO2 |
|
|
|
|
|
RH |
Ref. |
|||
Cl |
|
|
|
S |
S |
|
|
|
|
Me |
|
|
|
|||
|
|
NO2 |
87 |
100 |
|
|
|
|
|
|
|
95 |
112 |
|||
Cl |
|
|
|
|
NO2 |
Me |
|
|||||||||
|
Cl |
|
|
|
|
|
|
|
|
|
||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||
O |
|
|
|
O |
O |
|
O |
|
|
|
|
|
|
|||
|
|
|
|
|
|
|
|
|
|
|
|
|||||
|
|
OMe |
|
|
|
|
|
|
|
|
|
|
||||
|
|
78 |
104 |
O |
|
O |
|
|
NO2 |
|
78 |
107 |
||||
|
|
Cl |
|
|
|
|
|
|
|
|
||||||
|
|
ClNO2 |
|
|
|
|
|
|
|
P(OEt)2 |
|
|
|
|||
|
|
|
|
|
|
|
|
|
|
O |
|
|
|
|
||
|
NO2 |
|
|
|
|
OAc |
|
|
|
|
|
|
|
|||
O O |
|
|
|
|
|
O |
|
|
|
|
|
|
||||
|
CO2Me |
78 |
103 |
AcO |
|
|
|
OMe |
|
83 |
106 |
|||||
H |
|
|
|
|
|
|
|
|||||||||
|
6 |
O2N |
AcO |
H |
|
|
||||||||||
|
|
|
|
|
|
Me |
|
|
|
|
|
|
||||
|
NO2 |
|
|
|
|
O2N O |
|
|
|
|||||||
|
|
|
O |
O |
|
|
|
|
|
|
|
|||||
Me |
|
CO2Me |
75 |
103 |
|
|
|
|
|
|
|
63 |
108 |
|||
O |
|
6 |
|
|
Ph |
O |
|
|
|
O |
|
|
|
|||
|
|
|
|
|
|
AcO H |
|
|
|
|
|
|
||||
|
|
CH2Ph |
|
|
O |
O |
|
O |
|
|
|
|
|
|
||
|
|
|
|
|
|
|
|
|
|
|
|
|||||
|
|
|
|
|
|
|
|
|
NO2 |
|
|
|
||||
O2N |
CO2Me |
80 |
102 |
O |
|
O |
|
|
|
58 |
72 |
|||||
|
|
F |
|
|
|
|
|
|
|
|
|
CN |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||
Me |
|
NO2 |
31 |
101 |
O2N |
CO2Et |
|
O Si |
|
87 |
116 |
|||||
|
|
O |
|
|
|
|
|
|
|
|
||||||
F3C |
O |
|
|
|
O |
|
|
|
|
|
|
|
|
|||
|
|
|
|
O |
|
|
|
|
|
|
|
|
|
|
||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||
|
|
|
80 |
105 |
|
Et |
|
|
|
|
|
|
85 |
115 |
||
N |
NO2 |
C4H9 |
C C CH2 CH CHPh |
|||||||||||||
|
|
|
|
|||||||||||||
|
|
O |
|
|
|
O NO2 |
|
|
|
|
|
|
|
|
||
OH NO2 |
64 |
109 |
|
NO2 |
NHCbz |
|
65 |
117 |
||||||||
(S) |
|
|
|
CO2Et |
|
|||||||||||
|
|
|
|
|
|
|
|
|||||||||
|
|
|
|
|
|
|
|
|
|
O |
|
|
|
|
||
|
|
O |
|
|
|
|
HN |
|
|
Me |
|
|
|
|||
|
|
|
|
|
|
|
|
|
|
|
|
|||||
|
|
NO2 |
|
|
HOCH2 |
|
|
|
|
|
|
|
|
|||
|
|
59 |
30 |
RO |
|
O |
|
|
N |
|
|
82 |
118 |
|||
|
|
OH |
|
|
O |
|
|
|
|
|
|
|||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||
MeO |
|
|
|
|
|
NO2 |
|
|
|
|
|
|
|
|
||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||
|
|
Me |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Me |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
MeO |
|
NO2 |
|
|
|
|
|
|
|
N |
|
|
|
|||
|
O |
85 |
114 |
|
|
NO2 |
O |
|
91 |
120 |
||||||
|
|
|
|
|
||||||||||||
O |
|
|
|
|
|
CO2Et |
|
|
|
|
||||||
|
|
|
|
|
|
|
|
|
|
|
||||||
O |
|
|
|
|
|
Ph |
NO |
|
|
|
|
|
|
|
||
|
|
|
|
|
|
|
2 |
|
|
|
|
|
|
|||
|
|
|
|
|
|
N |
|
|
|
|
|
|
|
|
|
|
|
|
NO2 |
26 |
111 |
|
O |
|
|
|
|
|
|
90 |
121 |
||
|
|
|
|
CPh |
|
|
|
|
||||||||
|
|
|
|
|
|
|
O |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
N |
|
|
|
|
|
|
|
||
|
|
Ph |
|
|
|
|
N Me |
|
|
|
|
|
||||
|
|
N |
91 |
113 |
|
Me |
|
|
|
85 |
122 |
|||||
|
|
|
|
|
|
|
C Me |
|
|
|
||||||
EtO2C ONO2 |
|
|
|
Me |
NO2 |
|
|
|
(continues) |
|||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||

|
|
|
|
|
7.2 |
R–H FROM R–NO2 209 |
|
Table 7.3. Continued |
|
|
|
|
|
|
|
RNO2 |
Yield (%) |
|
|
|
Yield (%) |
|
|
|
RH |
Ref. |
|
RNO2 |
RH |
Ref. |
|
MeO |
|
|
Me |
|
|
|
|
|
|
|
|
|
|
|
|
|
O |
|
O |
|
Me |
|
|
|
58 |
123 |
N |
NO2 |
83 |
124 |
|
|
|
|
|
|
|
||
|
|
|
|
|
|
|
|
|
|
|
|
|
O |
|
|
O2N |
Ph |
|
Because reductive cleavage of aliphatic nitro compounds with Bu3SnH proceeds via alkyl radicals, nitro compounds are also used as precursors to alkyl radicals. Reactions using nitro compounds may have some advantages over other ones, since aliphatic nitro compounds are available from various sources. For example, the sequence of the Michael additions of nitro compounds provides an excellent method for the construction of quaternary carbon compounds (Eq. 7.79).126 Newkome has used this strategy for the construction of dendritic polymers (Eq. 7.80).127
Me |
|
|
|
CO2Et |
|
CO2Et |
Bu |
SnH, AIBN |
Me |
|
|
|
|
|
|||
|
+ |
3 |
|
|
(7.79) |
Me |
CO2Me |
|
Me |
||
NO2 |
|
|
|
CO2Me |
|
|
|
|
|
60% |
|
|
OCH2Ph |
|
HO |
OH |
|
O N |
OCH2Ph + |
CN |
HO |
OH |
|
2 |
|
HO |
OH |
|
|
|
|
|
|
||
|
OCH2Ph |
|
|
|
|
|
Bu3SnH, AIBN |
|
|
|
|
|
benzene, 80 ºC |
|
|
|
|
|
OCH2Ph |
|
|
|
|
NC |
OCH2Ph |
|
HO |
OH |
(7.80) |
|
|
||||
|
OCH2Ph |
|
HO |
OH |
|
|
61% |
|
HO |
OH |
|
Trans-fused bicyclic compounds are prepared by the double Michael reactions of nitro compounds; the nitro group is further alkylated by the radical reaction (Eq. 7.81).128
|
|
|
CN |
|
|
CN |
|
Me |
O |
Me |
O |
|
Bu3SnH, AIBN |
(7.81) |
|
+ |
CO2Et |
|
|
O2N |
|
benzene, 80 ºC |
OEt |
|
OEt |
|
CO |
Et |
|
|
|
2 |
|
|
|
|
|
41% |
|
Giese has used this strategy for the synthesis of sugar derivatives, as shown in Eq. 7.82.129
|
O O |
|
|
|
Bu3SnH |
|
O |
O |
CN |
|
NO2 |
+ |
|
|
O |
|
|||
R |
O |
CN |
AIBN |
R |
|
(7.82) |
|||
|
CN |
|
|
|
CN |
||||
|
|
|
|
|
|
|
|||
|
|
|
|
|
|
|
55% |
|
|
|
|
|
|
|
|
|
|
|

