
Baer M., Billing G.D. (eds.) - The role of degenerate states in chemistry (Adv.Chem.Phys. special issue, Wiley, 2002)
.pdf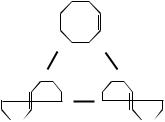
474 |
yehuda haas and shmuel zilberg |
Cyclootene loop
i |
i |
Figure 34. Anchors for the cis–trans isomerization
i
of cyclooctene.
contains a conical intersection. In this case, the ring closure makes the two trans isomers distinguishable—being enantiomers, they belong to two different anchors. The proposed loop is consistent with the fact that the only reaction observed is cis–trans isomerization—the enclosed conical intersection is expected to be low lying, and to lead smoothly only to the trans isomers and back to the cis reactant.
Butadiene
UNSUBSTITUTED BUTADIENE. Butadiene anchors were presented in Figures 1(3) and 13. The basic tetrahedral character of the conical intersection (as for H4) is expected to be maintained, when considering the re-pairing of four electrons. However, the situation is more complicated (and the photochemistry much richer), since here p electrons are involved rather than s electrons as in H4. It is therefore necessary to consider the consequences of the p-orbital rotation, en route to a new sigma bond.
As shown in Figure 13, which is completely analogous to Figure 2, three independent spin pairing schemes exist: in the planar butadiene, the pairing is {12,34}; in cyclobutene, {14,23}. The third possibility {13,24} corresponds to bicyclobutane. Since the loop between these three structures is an i3 one (for Hu¨ckel-type reactions), a conical intersection must be present inside the it. Irradiation of butadiene is thus expected to lead to both cyclobutene and bicyclobutane. The latter is not observed at room temperature, but is found when the reaction is carried out in cryogenic matrices, where highly strained structures may be observed due to rapid cooling [83,85]. A room temperature experiment that may reveal the bicyclobutane anchor, is the scrambling of carbon atoms expected to be found in recovered butadiene, using labeled 13C isotopes. A given spin-pairing scheme (anchor) accommodates different conformers (Fig. 1), which may also be revealed in low-temperature

conical intersections in molecular photochemistry |
475 |
||||||
|
|
|
TABLE I |
|
|
|
|
|
The Phase Change Upon Cyclization of Different s-cis Cyclobutadiene |
|
|||||
|
|
|
Isomersa |
|
|
|
|
|
Product |
XII |
XIII |
XIV |
XV |
|
|
|
Reactant |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
VIII |
i |
i |
p |
p |
|
|
|
IX |
i |
i |
p |
p |
|
|
|
X |
p |
p |
i |
i |
|
|
|
XI |
p |
p |
i |
i |
|
|
a The p stands for phase-preserving reaction, the i for phase inverting.
experiments [83,85]. For example, photochemical s-cis/s-trans isomerization of butadiene is observed.
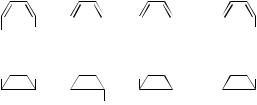
SUBSTITUTED BUTADIENES. The consequences of p-type orbitals rotations, become apparent when substituents are added. Many structural isomers of butadiene can be formed (Structures VIII–XI), and the electrocylic ring-closure reaction to form cyclobutene can be phase inverting or preserving if the motion is conrotatory or disrotatory, respectively. The four cyclobutene structures XII– XV of cyclobutene may be formed by cyclization. Table I shows the different possibilities for the cyclization of the four isomers VIII–XI. These structures are shown in Figure 35.
In a similar way Table II summarizes how the phase changes upon interconversion among the isomers. Inspection of the two tables shows that for any loop containing three of the possible isomers (open chain and cyclobutene ones), the phase either does not change, or changes twice. Thus, there cannot be a conical intersection inside any of these loops; in other words, photochemical transformations between these species only cannot occur via a conical intersection, regardless of the nature of the excited state.
TABLE II
The Phase Change Upon Interconversion Reactions Between Different
s-cis Cyclobutadiene Isomersa
Product |
VIII |
IX |
X |
XI |
Reactant |
|
|
|
|
|
|
|
|
|
VIII |
|
p |
i |
i |
IX |
p |
|
i |
i |
X |
i |
i |
|
p |
XI |
i |
i |
p |
|
a The p stands for the phase-preserving reaction, the i for the phase inverting.
476 |
|
|
|
yehuda haas and shmuel zilberg |
|
|
||||||||||
2 |
3 |
2 |
3 |
2 |
3 |
|
2 |
3 |
|
|||||||
1 |
|
|
|
4 a 1 |
|
|
4 b 1 |
|
|
4 |
b a 1 |
|
4 |
|||
a |
|
|
b |
|
|
a |
|
|
|
|
|
|
b |
|||
VIII |
|
|
IX |
|
|
X |
|
|
XI |
|
|
|||||
a |
|
|
b |
|
|
a 2 |
3 |
|
2 |
3 |
b |
|||||
|
|
|
|
|
|
|
|
1 |
|
|
|
4 |
1 |
|
|
4 |
|
|
|
|
a |
|
|
b |
|
|
b |
a |
|
|
|||
XII |
XIII |
XIV |
|
|
XV |
|
||||||||||
Figure 35. Structures VIII–XV.
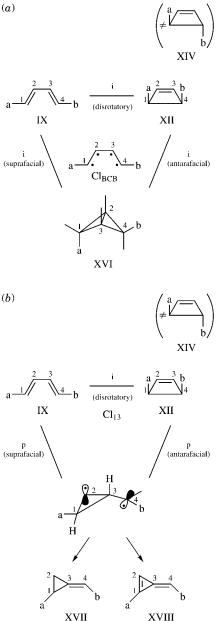
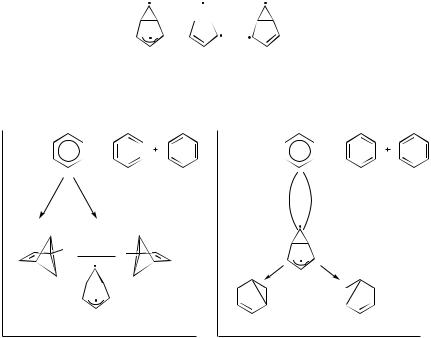
With bicyclobutane as the third anchor, several different conical intersections may be found for certain reactant–product pairs, which in turn can be identified using Tables I and II. For example, structure IX may cyclize to XII or XIII, but not to XIV or XV, as shown in Figure 36. In a similar way, isomer VIII may convert to either X or XI but not to IV. In each of these cases, a second product that can be traced to either the cyclopropyl biradical or bicyclobutane (BCB) form of the third anchor (Fig. 1) is also formed. Although the relative yield of the products cannot be estimated from this analysis, these selection rules are strict for reactions in which conical intersections are involved, and they can predict which pairs of products are possible. In Figure 36a and 36b, the two possible modes of ring closure are shown: suprafacial and antarafacial—the BCBs (or cyclopropyl biradicals) formed are different isomers. In Figure 36a, we show the conical intersection as a tetra radicaloid, allowing re-pairing of all four electrons. The biradical form was chosen in order to explain more clearly the thermal formation of the two final products—cyclopropyl derivatives XVII and XVIII. This result may generalized as follows: Reactions in which all four p electrons participate, cannot result in cis–trans isomerization or cyclobutane formation only. These photochemical transformations involving a four-electron system must be accompanied by an additional, fairly strained one in which there is a cyclopropyl ring, or by a bicyclobutane product.
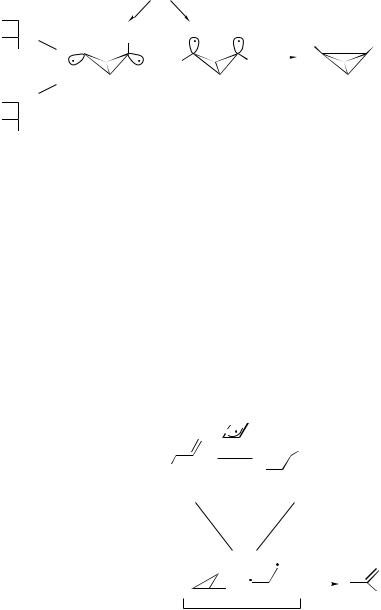
THE CYCLOBUTADIENE–TETRAHEDRANE SYSTEM. A related reaction is the photo-
isomerization of cyclobutadiene (CBD). It was found that unsubstituted CBD does not react in an argon matrix upon irradiation, while the tri-butyl substituted derivative forms the corresponding tetrahedrane [86,87]. These results may be understood on the basis of a conical intersection enclosed by the loop shown in Figure 37. The analogy with the butadiene loop (Fig. 13) is obvious. The two CBDs and the biradical shown in the figure are the three anchors in this system. With small substituents, the two lobes containing the lone electrons can be far
conical intersections in molecular photochemistry |
477 |
Figure 36. Possible butadiene photochemical reactions.
conical intersections in molecular photochemistry |
479 |
and theoretically [89]. Orbital symmetry rules [52] predict that the major photochemical pathway be a [1,3] suprafacial sigmatropic shift that preserves the molecular stereochemistry. It is found experimentally that a [1,3] shift does indeed take place, but in addition, a cyclopropane derivative is obtained, and that the [1,3] shift reaction is indeed stereospecific—the methyl group migrates via a supra path, with retention of the configuration. No evidence for an antara path products was reported. This system provides an example of the H-allyl conical intersection: The transition between A and B is via an allyl type transition state. This is a four electron antiaromatic transition state, which is an out-of-phase combination of the two bond alternating structures of the reactant and product. Anchor C may include both the biradical and the cyclopropane derivative—they have the same spin-pairing scheme. All three reactions are phase inverting, the loop is an i3 one and contains a conical intersection.
If A transforms to B by an antara-type process (a Mo¨bius four electron reaction), the phase would be preserved in the reaction and in the complete loop (An i2p loop), and no conical intersection is possible for this case. In that case, the only way to equalize the energies of the ground and excited states, is along a trajectory that increases the separation between atoms in the molecule. Indeed, the two are computed to meet only at infinite interatomic distances, that is, upon dissociation [89].
BENZENE–BENZVALENE ISOMERIZATION. The photochemical valence isomerization of benzene to form benzvalene [90, p. 357] is another example in which allyl radical structures (Fig. 12) play a central role. The system is analyzed in Figure 39. In order to follow the pattern of the previous examples, we show the in Figure 39a two benzvalene isomers as two anchors, and benzene as the third. This is an example of type C loop shown in Figure 11. The two benzvalene isomers, are connected via the shown allylic prebenzvalene TS structure, which is the out-of-phase combination of the two allyl structures shown at the top of the figure. The benzvalene ! benzene transformation is thermally allowed (phase preserving) [91]. The coordinate connecting benzene with the allyl-type transition state is phase preserving. Thus, the phase-change rule predicts the existence of a conical intersection in the region enclosed by these anchors. High-level computations indicate that the prebenzvalene moiety is in fact an intermediate [92]. In that case, the two-anchor loop shown in Figure 39b applies, with the same results.
In a photochemical experiment, irradiation of benzene leads to S1, which connects to the ground-state surface via the conical intersection shown. Benzene, the much more stable species, is expected to be recovered preferentially, but the prebenzvalene structure which transforms to benzvalene is also formed. Another possible route from the prebenzvalene, along a different coordinate, will lead to fulvene [90, p.357] after a hydrogen-atom transfer from
480 |
yehuda haas and shmuel zilberg |
Allyl-type TS or intermediate
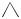 −
−
|
{13,26,45} |
{15,26,34} |
|
(a) |
|
(b) |
|
Qp |
Qp |
|
|
|
|
|
|
{12,34,56} |
{16,23,45} |
{12,34,56} |
{16,23,45} |
pp
11
|
5 |
6 |
i |
3 |
|
|
|
|
|
|
|
|
2 |
|
|
|
|
4 |
3 |
|
|
5 |
4 |
|
|
|
|
|
|
1 |
|
1 |
|||
|
2 |
|
|
6 |
|
|
||
|
|
|
|
|
|
|
||
|
|
|
|
6 |
2 |
6 |
2 |
|
{13,26,45} |
|
|
|
|||||
|
|
{15,26,34} |
3 |
|
3 |
|||
|
|
|
|
|
5 |
5 |
||
|
|
|
TS |
|
|
4 |
|
4 |
|
|
|
|
|
Qi |
|
|
Qi |
Figure 39. Benzene to benzvalene reaction. (a) Assuming that the prebenzvalene structure is a transition state. The two benzvalene isomers are anchors. (b) Assuming that prebenzvalene is an intermediate. A two-anchor loop results, compare Figure 12.
one of the carbon atoms to another. This scenario, which is observed experimentally [90], was corroborated computationally [92].
PHOTOLYSIS OF AMMONIA. Restricting the discussion to neutral species only (ionic ones require high energy, and are not important in the 170–220-nm UV range, where ammonia absorbs strongly), the two low-energy reaction channels to ground state products are
NH3 ! NH2ðX2B1Þ þ Hð2SÞ Dissociation to ground-state atomic hydrogen NH3 ! NHða1 Þ þ H2 Dissociation to molecular hydrogen
The nonbonding electrons of the nitrogen atom are important in determining spin re-pairing, and thus the conical intersections. This is the physical origin of the topicity concept developed by Salem and co-workers [2,30]. Two different spin
conical intersections in molecular photochemistry |
481 |
Figure 40. Ammonia photochemistry. (a) A loop for the NH3 ! NH2ðX2B1Þ þ Hð2SÞ reaction. (b) A loop for the NH3 ! NHða1 Þ þ H2 reaction.
pairing schemes for ammonia were shown in Figure 15. They were based on the observation that the umbrella inversion is a reaction in which electrons are re-paired (a phase inverting reaction). In Figure 40 this reaction forms one leg of two possible Longuet-Higgins loops. In the left-hand one the third anchor is the NH2ð2B1Þ þ Hð2SÞ, as shown in Figure 15 this is an i3 loop. The right-hand loop has the pair H2ð1 þg Þ þ NHða1 Þ as the third anchor: the reactions connecting the two ammonia anchors with it re-pair six electrons—this is an ip2 loop. Both loops therefore encircle conical intersection, and both channels are expected to be photochemically active on the basis of the phase-change rule. Note that the hydrogen-atom dissociation channel requires planarization en route to the conical intersection. This expectation was verified computationaly [93].
INORGANIC COMPLEXES. The cis–trans isomerization of a planar square form of a d8 transition metal complex (e.g., of Pt2þ) is known to be photochemically allowed and thermally forbidden [94]. It was found experimentally [95] to be an intramolecular process, namely, to proceed without any bond-breaking step. Calculations show that the ground and the excited state touch along the reaction coordinate (see Fig. 12 in [96]). Although conical intersections were not mentioned in these papers, the present model appears to apply to these systems.
Consider a metal M bound to four ligands, L1–L4, lying at the corners of a square around the metal. Three anchors can be written for this system, as shown in Figure 41. They consist of all three possible geometrical permutations of pairs of ligands lying across the metal ion. The conical intersection in this case is a tetrahedron (cf. Fig. 2). There are two different (though energetically
|
|
conical intersections in molecular photochemistry |
483 |
||||
Qp |
|
|
|
||||
{12,34,56,78} |
{18,23,45,67} |
|
|||||
|
|
|
|
|
i |
|
|
|
|
|
|
|
|
||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
p |
p |
{18,26,34,57} |
Figure 42. |
The cyclooctateteraene |
|
 Qi to semibullvalene reaction.
Qi to semibullvalene reaction.
loop method is described in some detail [98]. The phase-change rule requires that in order for a conical intersection to be enclosed in a loop, at least one of the reaction coordinates forming it must be phase inverting. Motion along this coordinate leads to an antiaromatic transition state (AATS), discussed in Section III. The coordinate connecting this transition state and the third anchor is phase preserving, (cf. Fig. 5). Thus, the conical intersection lying within the region encircled by the three anchors, may be found by moving first along the phase-inverting reaction coordinate from CHDN to the AATS and then along the phase-preserving coordinate to the third anchor. In practice, the geometry of the AATS is calculated and the system is transported vertically to the first electronically excited-state surface. This state is the twin state of the AATS, and is low lying [28,49]. From this point, relaxation along the steepest gradient downward on the electronically excited state is performed, under two constraints: The system is kept at the same symmetry it had at the AATS geometry (viz., the migrating H atom(s) midway between the original and destination carbon atoms], and the molecule is kept along the phase-preserving reaction coordinate in the direction of the third anchor. Recall that the twin excited state and the third anchor have the same spin-pairing scheme (Section I.C). All other coordinates are optimized for minimum energy—this is a constrained minimum energy path leading to the product. The point at which the system reaches the ground-state potential lies on the CI. It is not necessarily the minimum energy point on the CI; rather, the locus reached by this process is obtained upon moving along the shortest path to the product from the excitedstate surface (at the AATS geometry). The numerical data cited below are for these points on the CI.


