
- •Foreword
- •Preface
- •Contents
- •About the Editors
- •Contributors
- •1: Tracheobronchial Anatomy
- •Trachea
- •Introduction
- •External Morphology
- •Internal Morphology
- •Mucous Layer
- •Blood Supply
- •Anatomo-Clinical Relationships
- •Bronchi
- •Main Bronchi
- •Bronchial Division
- •Left Main Bronchus (LMB)
- •Right Main Bronchus (RMB)
- •Blood Supply
- •References
- •2: Flexible Bronchoscopy
- •Introduction
- •History
- •Description
- •Indications and Contraindications
- •Absolute Contraindications
- •Procedure Preparation
- •Technique of FB Procedure
- •Complications of FB Procedure
- •Basic Diagnostic Procedures
- •Bronchoalveolar Lavage (BAL)
- •Transbronchial Lung Biopsy (TBLB)
- •Transbronchial Needle Aspiration (TBNA)
- •Bronchial Brushings
- •Advanced Diagnostic Bronchoscopy
- •EBUS-TBNA
- •Ultrathin Bronchoscopy
- •Transbronchial Lung Cryobiobsy (TBLC)
- •Therapeutic Procedures Via FB
- •LASER Bronchoscopy
- •Electrocautery
- •Argon Plasma Coagulation (APC)
- •Cryotherapy
- •Photodynamic Therapy
- •Airway Stent Placement
- •Endobronchial Valve Placement
- •Conclusion
- •References
- •History and Historical Perspective
- •Indications and Contraindications
- •Procedure Description
- •Procedure Planning
- •Target Approximation
- •Sampling
- •Complications
- •Future Directions
- •Summary and Recommendations
- •References
- •4: Rigid Broncoscopy
- •Innovations
- •Ancillary Equipment
- •Rigid Bronchoscopy Applications
- •Laser Bronchoscopy
- •Tracheobronchial Prosthesis
- •Transbronchial Needle Aspiration (TBNA)
- •Rigid Bronchoscope in Other Treatments for Bronchial Obstruction
- •Mechanical Debridement
- •Pediatric Rigid Bronchoscopy
- •Tracheobronchial Dilatation
- •Foreign Bodies Removal
- •Other Indications
- •Complications
- •The Procedure
- •Some Conclusions
- •References
- •History and Historical Perspective
- •Indications and Contraindications
- •Preprocedural Evaluation and Preparation
- •Physical Examination
- •Procedure-Related Indications
- •Application of the Technique
- •Topical Anesthesia
- •Anesthesia of the Nasal Mucosa and Nasopharynx
- •Anesthesia of the Mouth and Oropharynx
- •Superior Laryngeal Nerve Block
- •Recurrent Laryngeal Nerve Block (RLN)
- •Conscious Sedation
- •Monitored Anesthesia Care (MAC)
- •General Anesthesia
- •Monitoring the Depth of Anesthesia
- •Interventional Bronchoscopy Suites
- •Airway Devices
- •Laryngeal Mask Airway (LMA)
- •Endotracheal Tube (ETT)
- •Rigid Bronchoscope
- •Modes of Ventilation
- •Spontaneous Ventilation
- •Assisted Ventilation
- •Noninvasive Positive Pressure Ventilation (NIV)
- •Positive Pressure Controlled Mechanical Ventilation
- •Jet Ventilation
- •Electronic Mechanical Jet Ventilation
- •Postprocedure Care
- •Special Consideration
- •Anesthesia for Peripheral Diagnostic and Therapeutic Bronchoscopy
- •Anesthesia for Interventional Bronchoscopic Procedures During the COVID-19 Pandemic
- •Summary and Recommendations
- •Conclusion
- •References
- •Background
- •Curricular Structure and Delivery
- •What Is a Bronchoscopy Curriculum?
- •Tradition, Teaching Styles, and Beliefs
- •Using Assessment Tools to Guide the Educational Process
- •The Ethics of Teaching
- •When Learners Teach: The Journey from Novice to Mastery and Back Again
- •The Future Is Now
- •References
- •Interventional Procedure
- •Assessment of Flow–Volume Curve
- •Dyspnea
- •Analysis of Pressure–Pressure Curve
- •Conclusions
- •References
- •Introduction
- •Adaptations of the IP Department
- •Environmental Control
- •Personal Protective Equipment
- •Procedure Performance
- •Bronchoscopy in Intubated Patients
- •Other Procedures in IP Unit
- •References
- •Introduction
- •Safety
- •Patient Safety
- •Provider Safety
- •Patient Selection and Screening
- •Lung Cancer Diagnosis and Staging
- •Inpatients
- •COVID-19 Clearance
- •COVID Clearance: A Role for Bronchoscopy
- •Long COVID: A Role for Bronchoscopy
- •Preparing for the Next Pandemic
- •References
- •Historical Perspective
- •Indications and Contraindications
- •Evidence-Based Review
- •Summary and Recommendations
- •References
- •Introduction
- •Clinical Presentation
- •Diagnosis
- •Treatment
- •History and Historical Perspectives
- •Indications and Contraindications
- •Benign and Malignant Tumors
- •Tumors with Uncertain Prognosis
- •Application of the Technique
- •Evidence Based Review
- •Summary and Recommendations
- •References
- •12: Cryotherapy and Cryospray
- •Introduction
- •Historical Perspective
- •Equipment
- •Cryoadhesion
- •Indications
- •Cryorecanalization
- •Cryoadhesion and Foreign Body Removal
- •Cryoadhesion and Mucus Plugs/Blood Clot Retrieval
- •Endobronchial Cryobiopsy
- •Transbronchial Cryobiopsy for Lung Cancer
- •Safety Concerns and Contraindications
- •Cryoablation
- •Indications
- •Evidence
- •Safety Concerns and Contraindications
- •Cryospray
- •Indications
- •Evidence
- •Safety Concerns and Contraindications
- •Advantages of Cryotherapy
- •Limitations
- •Future Research Directions
- •References
- •13: Brachytherapy
- •History and Historical Perspective
- •Indications and Contraindications
- •Application of the Technique
- •Evidence-Based Review
- •Adjuvant Treatment
- •Palliative Treatment
- •Complications
- •Summary and Recommendations
- •References
- •14: Photodynamic Therapy
- •Introduction
- •Photosensitizers
- •First-Generation Photosensitizers
- •M-Tetrahidroxofenil Cloro (mTHPC) (Foscan®)
- •PDT Reaction
- •Tumor Damage Process
- •Procedure
- •Indications
- •Curative PDT Indications
- •Palliative PDT Indications
- •Contraindications
- •Rationale for Use in Early-Stage Lung Cancer
- •Rationale
- •PDT in Combination with Other Techniques for Advanced-Stage Non-small Cell Lung Cancer
- •Commentary
- •Complementary Endoscopic Methods for PDT Applications
- •New Perspectives
- •Other PDT Applications
- •Conclusions
- •References
- •15: Benign Airways Stenosis
- •Etiology
- •Congenital Tracheal Stenosis
- •Iatrogenic
- •Infectious
- •Idiopathic Tracheal Stenosis
- •Distal Bronchial Stenosis
- •Diagnosis Methods
- •Patient History
- •Imaging Techniques
- •Bronchoscopy
- •Pulmonary Function Test
- •Treatment
- •Endoscopic Treatment
- •Dilatation
- •Laser Therapy
- •Stents
- •How to Proceed
- •Stent Placement
- •Placing a Montgomery T Tube
- •The Rule of Twos for Benign Tracheal Stenosis (Fig. 15.23)
- •Surgery
- •Summary and Recommendations
- •References
- •16: Endobronchial Prostheses
- •Introduction
- •Indications
- •Extrinsic Compression
- •Intraluminal Obstruction
- •Stump Fistulas
- •Esophago-respiratory Fistulas (ERF)
- •Expiratory Central Airway Collapse
- •Physiologic Rationale for Airway Stent Insertion
- •Stent Selection Criteria
- •Stent-Related Complications
- •Granulation Tissue
- •Stent Fracture
- •Migration
- •Contraindications
- •Follow-Up and Patient Education
- •References
- •Introduction
- •Overdiagnosis
- •False Positives
- •Radiation
- •Risk of Complications
- •Lung Cancer Screening Around the World
- •Incidental Lung Nodules
- •Management of Lung Nodules
- •References
- •Introduction
- •Minimally Invasive Procedures
- •Mediastinoscopy
- •CT-Guided Transthoracic Biopsy
- •Fluoroscopy-Guided Transthoracic Biopsies
- •US-Guided Transthoracic Biopsy
- •Thoracentesis and Pleural Biopsy
- •Thoracentesis
- •Pleural Biopsy
- •Surgical or Medical Thoracoscopy
- •Image-Guided Pleural Biopsy
- •Closed Pleural Biopsy
- •Image-Guided Biopsies for Extrathoracic Metastases
- •Tissue Acquisition, Handling and Processing
- •Implications of Tissue Acquisition
- •Guideline Recommendations for Tissue Acquisition in Mediastinal Staging
- •Methods to Overcome Challenges in Tissue Acquisition and Genotyping
- •Rapid on-Site Evaluation (ROSE)
- •Sensitive Genotyping Assays
- •Liquid Biopsy
- •Summary, Recommendations and Highlights
- •References
- •History
- •Data Source and Methodology
- •Tumor Size
- •Involvement of the Main Bronchus
- •Atelectasis/Pneumonitis
- •Nodal Staging
- •Proposal for the Revision of Stage Groupings
- •Small Cell Lung Cancer (SCLC)
- •Discussion
- •Methodology
- •T Descriptors
- •N Descriptors
- •M Descriptors
- •Summary
- •References
- •Introduction
- •Historical Perspective
- •Fluoroscopy
- •Radial EBUS Mini Probe (rEBUS)
- •Ultrasound Bronchoscope (EBUS)
- •Virtual Bronchoscopy
- •Trans-Parenchymal Access
- •Cone Beam CT (CBCT)
- •Lung Vision
- •Sampling Instruments
- •Conclusions
- •References
- •History and Historical Perspective
- •Narrow Band Imaging (NBI)
- •Dual Red Imaging (DRI)
- •Endobronchial Ultrasound (EBUS)
- •Optical Coherence Tomography (OCT)
- •Indications and Contraindications
- •Confocal Laser Endomicroscopy and Endocytoscopy
- •Raman Spectrophotometry
- •Application of the Technique
- •Supplemental Technology for Diagnostic Bronchoscopy
- •Evidence-Based Review
- •Summary and Recommendations, Highlight of the Developments During the Last Three Years (2013 on)
- •References
- •Introduction
- •History and Historical Perspective
- •Endoscopic AF-OCT System
- •Preclinical Studies
- •Clinical Studies
- •Lung Cancer
- •Asthma
- •Airway and Lumen Calibration
- •Obstructive Sleep Apnea
- •Future Applications
- •Summary
- •References
- •23: Endobronchial Ultrasound
- •History and Historical Perspective
- •Equipment
- •Technique
- •Indication, Application, and Evidence
- •Convex Probe Ultrasound
- •Equipment
- •Technique
- •Indication, Application, and Evidence
- •CP-EBUS for Malignant Mediastinal or Hilar Adenopathy
- •CP-EBUS for the Staging of Non-small Cell Lung Cancer
- •CP-EBUS for Restaging NSCLC After Neoadjuvant Chemotherapy
- •Complications
- •Summary
- •References
- •Introduction
- •What Is Electromagnetic Navigation?
- •SuperDimension Navigation System (EMN-SD)
- •Computerized Tomography
- •Computer Interphase
- •The Edge Catheter: Extended Working Channel (EWC)
- •Procedural Steps
- •Planning
- •Detecting Anatomical Landmarks
- •Pathway Planning
- •Saving the Plan and Exiting
- •Registration
- •Real-Time Navigation
- •SPiN System Veran Medical Technologies (EMN-VM)
- •Procedure
- •Planning
- •Navigation
- •Biopsy
- •Complications
- •Limitations
- •Summary
- •References
- •Introduction
- •Image Acquisition
- •Hardware
- •Practical Considerations
- •Radiation Dose
- •Mobile CT Studies
- •Future Directions
- •Conclusion
- •References
- •26: Robotic Assisted Bronchoscopy
- •Historical Perspective
- •Evidence-Based Review
- •Diagnostic Yield
- •Monarch RAB
- •Ion Endoluminal Robotic System
- •Summary
- •References
- •History and Historical Perspective
- •Indications and Contraindications
- •General
- •Application of the Technique
- •Preoperative Care
- •Patient’s Position and Operative Field
- •Incision and Initial Dissection
- •Palpation
- •Biopsy
- •Control of Haemostasis and Closure
- •Postoperative Care
- •Complications
- •Technical Variants
- •Extended Cervical Mediastinoscopy
- •Mediastinoscopic Biopsy of Scalene Lymph Nodes
- •Inferior Mediastinoscopy
- •Mediastino-Thoracoscopy
- •Video-Assisted Mediastinoscopic Lymphadenectomy
- •Transcervical Extended Mediastinal Lymphadenectomy
- •Evidence-Based Review
- •Summary and Recommendations
- •References
- •Introduction
- •Case 1
- •Adrenal and Hepatic Metastases
- •Brain
- •Bone
- •Case 1 Continued
- •Biomarkers
- •Case 1 Concluded
- •Case 2
- •Chest X-Ray
- •Computerized Tomography
- •Positive Emission Tomography
- •Magnetic Resonance Imaging
- •Endobronchial Ultrasound with Transbronchial Needle Aspiration
- •Transthoracic Needle Aspiration
- •Transbronchial Needle Aspiration
- •Endoscopic Ultrasound with Needle Aspiration
- •Combined EUS-FNA and EBUS-TBNA
- •Case 2 Concluded
- •Case 3
- •Standard Cervical Mediastinoscopy
- •Extended Cervical Mediastinoscopy
- •Anterior Mediastinoscopy
- •Video-Assisted Thoracic Surgery
- •Case 3 Concluded
- •Case 4
- •Summary
- •References
- •29: Pleural Anatomy
- •Pleural Embryonic Development
- •Pleural Histology
- •Cytological Characteristics
- •Mesothelial Cells Functions
- •Pleural Space Defense Mechanism
- •Pleura Macroscopic Anatomy
- •Visceral Pleura (Pleura Visceralis or Pulmonalis)
- •Parietal Pleura (Pleura Parietalis)
- •Costal Parietal Pleura (Costalis)
- •Pleural Cavity (Cavitas Thoracis)
- •Pleural Apex or Superior Pleural Sinus [12–15]
- •Anterior Costal-Phrenic Sinus or Cardio-Phrenic Sinus
- •Posterior Costal-Phrenic Sinus
- •Cost-Diaphragmatic Sinus or Lateral Cost-Phrenic Sinus
- •Fissures18
- •Pleural Vascularization
- •Parietal Pleura Lymphatic Drainage
- •Visceral Pleura Lymphatic Drainage
- •Pleural Innervation
- •References
- •30: Chest Ultrasound
- •Introduction
- •The Technique
- •The Normal Thorax
- •Chest Wall Pathology
- •Pleural Pathology
- •Pleural Thickening
- •Pneumothorax
- •Pulmonary Pathology
- •Extrathoracic Lymph Nodes
- •COVID and Chest Ultrasound
- •Conclusions
- •References
- •Introduction
- •History of Chest Tubes
- •Overview of Chest Tubes
- •Contraindications for Chest Tube Placement
- •Chest Tube Procedural Technique
- •Special Considerations
- •Pneumothorax
- •Empyema
- •Hemothorax
- •Chest Tube Size Considerations
- •Pleural Drainage Systems
- •History of and Introduction to Indwelling Pleural Catheters
- •Indications and Contraindications for IPC Placement
- •Special Considerations
- •Non-expandable Lung
- •Chylothorax
- •Pleurodesis
- •Follow-Up and IPC Removal
- •IPC-Related Complications and Management
- •Competency and Training
- •Summary
- •References
- •32: Empyema Thoracis
- •Historical Perspectives
- •Incidence
- •Epidemiology
- •Pathogenesis
- •Clinical Presentation
- •Radiologic Evaluation
- •Biochemical Analysis
- •Microbiology
- •Non-operative Management
- •Prognostication
- •Surgical Management
- •Survivorship
- •Summary and Recommendations
- •References
- •Evaluation
- •Initial Intervention
- •Pleural Interventions for Recurrent Symptomatic MPE
- •Especial Circumstances
- •References
- •34: Medical Thoracoscopy
- •Introduction
- •Diagnostic Indications for Medical Thoracoscopy
- •Lung Cancer
- •Mesothelioma
- •Other Tumors
- •Tuberculosis
- •Therapeutic Indications
- •Pleurodesis of Pneumothorax
- •Thoracoscopic Drainage
- •Drug Delivery
- •Procedural Safety and Contraindications
- •Equipment
- •Procedure
- •Pre-procedural Preparations and Considerations
- •Procedural Technique [32]
- •Medical Thoracoscopy Versus VATS
- •Conclusion
- •References
- •Historical Perspective
- •Indications and Contraindications
- •Evidence-Based Review
- •Endobronchial Valves
- •Airway Bypass Tracts
- •Coils
- •Other Methods of ELVR
- •Summary and Recommendations
- •References
- •36: Bronchial Thermoplasty
- •Introduction
- •Mechanism of Action
- •Trials
- •Long Term: Ten-Year Study
- •Patient Selection
- •Bronchial Thermoplasty Procedure
- •Equipment
- •Pre-procedure
- •Bronchoscopy
- •Post-procedure
- •Conclusion
- •References
- •Introduction
- •Bronchoalveolar Lavage (BAL)
- •Technical Aspects of BAL Procedure
- •ILD Cell Patterns and Diagnosis from BAL
- •Technical Advises for Conventional TLB and TLB-C in ILD
- •Future Directions
- •References
- •Introduction
- •The Pediatric Airway
- •Advanced Diagnostic Procedures
- •Endobronchial Ultrasound
- •Virtual Navigational Bronchoscopy
- •Cryobiopsy
- •Therapeutic Procedures
- •Dilation Procedures
- •Thermal Techniques
- •Mechanical Debridement
- •Endobronchial Airway Stents
- •Metallic Stents
- •Silastic Stents
- •Novel Stents
- •Endobronchial Valves
- •Bronchial Thermoplasty
- •Discussion
- •References
- •Introduction
- •Etiology
- •Congenital ADF
- •Malignant ADF
- •Cancer Treatment-Related ADF
- •Benign ADF
- •Iatrogenic ADF
- •Diagnosis
- •Treatment Options
- •Endoscopic Techniques
- •Stents
- •Clinical Results
- •Stent Complications
- •Other Available Stents
- •Other Endoscopic Methods
- •References
- •Introduction
- •Anatomy and Physiology of Swallowing
- •Functional Physiology of Swallowing
- •Epidemiology and Risk Factors
- •Types of Foreign Bodies
- •Organic
- •Inorganic
- •Mineral
- •Miscellaneous
- •Clinical Presentation
- •Acute FB
- •Retained FB
- •Radiologic Findings
- •Bronchoscopy
- •Airway Management
- •Rigid Vs. Flexible Bronchoscopy
- •Retrieval Procedure
- •Instruments
- •Grasping Forceps
- •Baskets
- •Balloons
- •Suction Instruments
- •Ablative Therapies
- •Cryotherapy
- •Laser Therapy
- •Electrocautery and APC
- •Surgical Management
- •Complications
- •Bleeding and Hemoptysis
- •Distal Airway Impaction
- •Iron Pill Aspiration
- •Follow-Up and Sequelae
- •Conclusion
- •References
- •Vascular Origin of Hemoptysis
- •History and Historical Perspective
- •Diagnostic Bronchoscopy
- •Therapeutic Bronchoscopy
- •General Measures
- •Therapeutic Bronchoscopy
- •Evidence-Based Review
- •Summary
- •Recommendations
- •References
- •History
- •“The Glottiscope” (1807)
- •“The Esophagoscope” (1895)
- •The Rigid Bronchoscope (1897–)
- •The Flexible Bronchoscope (1968–)
- •Transbronchial Lung Biopsy (1972) (Fig. 42.7)
- •Laser Therapy (1981–)
- •Endobronchial Stents (1990–)
- •Electromagnetic Navigation (2003–)
- •Bronchial Thermoplasty (2006–)
- •Endobronchial Microwave Therapy (2004–)
- •American Association for Bronchology and Interventional Pulmonology (AABIP) and Journal of Bronchology and Interventional Pulmonology (JOBIP) (1992–)
- •References
- •Index

Aero-Digestive Fistulas: |
39 |
Endoscopic Approach |
Alicia N. Rodríguez and José Pablo Díaz-Jiménez
Introduction
Aero-digestive stulas (ADF) are pathological communications between any part of the digestive system (more commonly the esophagus) and the respiratory tract, congenital or acquired, resulting from various causes but mainly from tumor invasion through the esophagus and the tracheal or bronchial walls. They are often called tracheoesophageal, bronchoesophageal, or esophagealpulmonary stulas, and they can be benign or malignant in origin. In the last case, they represent serious complications of neoplasms that arise in the esophagus, lung, or mediastinum.
Most of the malignant ADF arise from digestive tumors and only a small proportion is due to lung tumors. Other tumors such as thyroid, larynx, or mediastinal metastatic nodes represent only a very low percentage of causes of ADF. Ninety percent of patients with malignant ADF have advanced or metastatic disease with high Eastern Cooperative Oncology Group (ECOG) scores.
Authors have no confict of interest to declare.
A. N. Rodríguez
School of Medicine, National University of Mar del Plata, Buenos Aires, Argentina
J. P. Díaz-Jiménez (*)
Interventional Pulmonary Department, Hospital Universitari de Bellvitge, Hospitalet de Llobregat, Barcelona, Spain
e-mail: pablodiaz@pablodiaz.org
Spontaneous closure of the stula is rare. The evolution is usually morbid due to the dramatic clinical implications and subsequent infections. Once symptoms of respiratory infection begin, in most cases, these complications cause aspiration pneumonia leading to sepsis, mediastinitis, acute respiratory distress syndrome (ARDS), and a fatal outcome. The prognosis is serious and the patient usually dies within a few weeks.
The main goals of management of a patient with ADF should be to treat recurrent infections with antibiotics and seal the communication between the esophagus and the airway, and ensure proper enteral feeding.
Non-malignant causes can occur as a consequence of treatments such as surgery, radiotherapy, chemotherapy, or radiotherapy associated with some angiogenic drugs, resections with laser, manipulations with esophageal or tracheobronchial prostheses, infectious diseases, granulomatous processes, chest trauma, necrosis caused by pressure from the cuff of the endotracheal intubation or tracheostomy tube, foreign bodies, and/or ingestion of caustics. Surgical repair, when possible, is the best option for non-malignant ADF.
Etiology
Congenital ADF
They are due to failure of the embryonic organs to fully form and are usually associated with other
© The Author(s), under exclusive license to Springer Nature Switzerland AG 2023 |
669 |
J. P. Díaz-Jiménez, A. N. Rodríguez (eds.), Interventions in Pulmonary Medicine, https://doi.org/10.1007/978-3-031-22610-6_39
Данная книга находится в списке для перевода на русский язык сайта https://meduniver.com/
670 |
A. N. Rodríguez and J. P. Díaz-Jiménez |
|
|
congenital abnormalities, vertebral, esophageal,
or anal atresias, and especially cardiac abnormalities as part of the vertebral defects, anal atresia, cardiac defects, tracheo-esophageal stula, renal anomalies, and limb abnormalities, vertebral defects, anal atresia, tracheo-esophageal stula with esophageal atresia, and radial and renal dysplasia (VACTERL/VATER) Association [1] and are usually diagnosed on all in early childhood. They arise when there are abnormalities in the formation of the laryngotracheal tubes (which divide into trachea and esophagus) in the fourth week of embryonic development [2].
Congenital stulas are rare and usually occur in 0.04% of all births. Their discussion is out of the scope of the present chapter.
Malignant ADF
Most acquired aero-digestive stulas result from cancer, and emerge due to tumor invasion or from a complication of oncological treatments such as surgery, radiotherapy/chemotherapy, laser resections, necrosis caused by the pressures produced by intubation, or direct pressure coming from esophageal or tracheobronchial prostheses.
The pathogenesis of the stulas is favored by the proximity of both neighboring organs. On the one hand, the trachea and the left main bronchus are closely linked to the esophagus, so tumors originating in any of these adjoining parts can invade the thin layer that separates them and end up communicating both organs.
On the other hand, the mediastinal nodes, especially in the subcarinal area, can also invade and communicate with the airway and the digestive tract, since they are part of the lymphatic drainage on both systems.
From the anatomical point of view, both benign and malignant ADF are located in 57% of cases between the esophagus and the trachea and 37% between the esophagus and one or both of the main bronchi. Fistulas between the esophagus and the lung parenchyma are usually the least frequent, close to 10% [3]. Quite less frequent are bronchopleural, bronchovascular, and bronchoperitoneal stulas.
Martini et al. [4] reported the largest study on ADF and showed that it occurred in 5% of 1943
esophageal cancer patients, 0.16% of 5714 lung cancer patients, and 15% of 41 tracheal cancer patients.
Burt et al. [5], in an excellent retrospective study including 207 patients with ADF, showed that for most of the aero-digestive stulas, primary tumor site was esophagus in 161 (77%), lung in 33 (16%), trachea in 5 (2%), metastatic nodes in 4 (2%), larynx in 3 (1%), and thyroid in 1. The risk of developing malignant ADF was also higher in patients with esophageal carcinoma. The incidence of ADF in patients with esophageal cancer was 4.5%, and 0.3% in patients with primary lung cancer.
Balazs et al. [6], in a series of 264 patients with malignant stulas, found that 243 had esophageal cancer, 19 had lung tumors, and 2 had mediastinal tumors.
Of all malignant esophageal-pulmonary stulas, 92% have esophageal cancer and 7% have lung cancer. Other neoplasms of different origin, such as thyroid carcinoma, larynx, lymphomas, or malignant mediastinal nodules, correspond to only a small percentage of these stulas [7].
Cancer Treatment-Related ADF
Some therapies used for cancer can result in ADF. Bevacizumab is a monoclonal antibody directed at vascular endothelial growth factor (VEGF). In combination with paclitaxel and carboplatin, it is indicated for the treatment of advanced non-small cell lung carcinoma (NSCLC). This angiogenic agent has been shown to cause the formation of tracheal or bronchoesophageal stulas when administered in combination with radiochemotherapy [8].
Some publications warn of the danger of causing esophageal respiratory stulas due to the combination of bevacizumab and radiochemotherapy for treating both non-small and small cell lung cancer. In a report on small cell carcinoma treated patients, 2/29 developed tracheoesophageal stula and another patient died of aero-digestive hemorrhage. Of the group of ve patients with advanced non-small cell carcinoma, treated with a combination of bevacizumab and radiochemotherapy, two developed tracheoesophageal stula. With these ndings, both trials were closed to additional enrollment.
39 Aero-Digestive Fistulas: Endoscopic Approach |
671 |
|
|
All patients and treating physicians were noti ed of these potential safety issues and protocolbased treatment was stopped. Genentech and appropriate regulatory authorities including the U.S. Food and Drug Administration and National Cancer Institute were noti ed. Consequently, a black box warning was issued in the bevacizumab label [9].
As suggested by this and many other reports [9–12], the use of antiangiogenic therapies such as bevacizumab, and especially when associated with concomitant radiotherapy in patients with thoracic malignant neoplasms, can lead to the formation of stulas (which in most cases tend to be fatal), even months after completion of radiotherapy treatment. This complication may be common to all VEGF pathway inhibitors, advising that the safety of bevacizumab treatments in patients previously treated with radiotherapy should be studied with extreme caution, suggesting that these cases should only be included in carefully designed clinical trials [13].
Regarding safety of endoscopic procedures in patients who have been treated with antiangiogenic agents (AAs), Kachaami et al. [14] reported a retrospective multicenter study of a consecutive case series of 445 cancer patients, from 5 oncology hospitals, who underwent endoscopy within 31 days of antiangiogenic agents’ administration.
The most common types of cancer were colorectal, lung, and breast, and most patients had stage III or IV disease at the time of diagnosis.
Among the 445 patients who received a total of 545 endoscopies, 3 procedure-related adverse events (0.7%) occurred within 30 days of the procedure. Two of them were minor, and a third patient developed pancreatitis. The authors concluded that, in this study, the rate of adverse events related to endoscopy procedures in patients with antiangiogenic agents (AAs) seemed to be low, only 0.7% when performed in a specialized cancer center.
The use of immunotherapy agents for advanced lung adenocarcinoma has been associated with rapid development of ADF in a report. In this particular case, after mechanical debulking and stent placement, the patient received nivolumab and 12 days after that, a tracheoesophageal stula was diagnosed related to a metastatic
lymph node [14]. As lung cancer treatments become more complex, special attention should be paid on possible unusual complications.
Benign ADF
Benign non-tumoral acquired ADF can result from different conditions:
•\ Traumatic: blunt or penetrating chest trauma [15]
•\ Infammatory diseases/Infectious diseases (tuberculosis, histoplasmosis)
•\ Iatrogenic: postoperative, post radiotherapy, post intubation, post tracheostomy (particularly percutaneous), post stent placement (esophageal or airways)
•\ Esophageal diverticula •\ Caustic ingestion
•\ Foreign body
•\ Other conditions
Infectious conditions such as granulomatosis processes (i.e., tuberculosis [16], actinomycosis, Wegener’s) can result in ADF as well. Other infections can also be responsible: herpetic esophagitis, acquired immunode ciency syndrome (AIDS)-related esophageal candidiasis, cytomegalovirus, or Staphylococcus aureus
[17].
In ammatory processes resulting from ingestion of foreign bodies or corrosive agents [18] or patients with Zenkel’s diverticulum can also be the cause of aero-digestive stulas [19, 20].
Lenz et al. [21] published a retrospective review between 2001 and 2012, of 123 tracheobronchial esophageal stulas: 53% were malignant and 47% were of benign etiology, 60% of which were postoperative. The rest of the benign etiologies were: mediastinal infammation, radiotherapy, esophageal diverticula, caustic ingestion, broncholithiasis, tracheal stenosis, and actinomycosis. However, Shen et al. [22] found in a retrospective study from 1978 to 2007 that 14% of the 35 benign stulas found were due to granulomatous infections. According to Bixby et al. [23], this discrepancy in ADF etiologies between the two studies can be explained by the decline in granulomatous diseases and better
Данная книга находится в списке для перевода на русский язык сайта https://meduniver.com/
672 |
|
A. N. Rodríguez and J. P. Díaz-Jiménez |
|
|
|
treatments for those conditions in the United |
tract for the treatment of tracheal stenosis can |
|
States in recent years compared to the 1970s. |
be the cause of tracheoesophageal stulas |
|
|
|
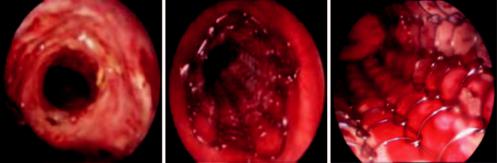
(Fig. 39.1a, b), due to posterior tracheal wall |
Iatrogenic ADF |
|
perforation secondary to stent wire fractures |
|
during extraction maneuvers [30]. |
|
|
|
•\ Tracheostomies or percutaneous tracheosto- |
•\ Injuries resulting from manipulations (espe- |
mies could also be a cause of ADF. Louis et al. |
|
cially during thoracic surgical |
procedures) |
[31] published a case of ADF diagnosed after |
[24, 25] or traumatic tracheal intubations [26] |
removal of the endotracheal tube. Three weeks |
|
that can injure the posterior wall of the trachea |
before, a percutaneous tracheostomy had been |
|
can result in iatrogenic ADF. |
|
performed. The authors enforced the periop- |
•\ Prolonged mechanical ventilation after tra- |
erative use of bronchoscopy as a guide for this |
|
cheal surgical reconstruction is also a reported |
procedure and the need for rigorous learning, |
|
iatrogenic cause for ADF [27]. |
|
since insertion of different-sized cannulas can |
•\ Mediastinoscopy and lymph node biopsies: |
be dif cult. |
|
After these procedures, ADF can arise follow- |
•\ Endotracheal tube balloon is the most frequent |
|
ing the formation of granulomas as a conse- |
cause of tracheoesophageal stula, noted |
|
quence of mediastinitis [28]. Special care |
already by Flege in 1967 [32]. In 1976, Grillo |
|
must be taken in the lower left paratracheal |
et al. described the involvement of both the |
|
station 4 L and in station 7. |
|
trachea and the esophagus in the formation of |
•\ Treatments of esophageal strictures: Placement |
tracheoesophageal stulas, suggesting that the |
|
of prostheses after dilation or resection is |
main component would be the compression |
|
sometimes the cause of ADF, especially at the |
due to the high-pressure cuff on one side and |
|
level of the proximal edge or at the distal edge |
the nasogastric tube on the other [33]. After |
|
of the prosthesis or also at the level of the pre- |
the high-volume, low-pressure cuffs became |
|
viously treated stenosis [29]. |
|
standard, its incidence has decreased but still |
•\ Treatment of tracheal stenosis: The use of |
remains as one of the most important causes, |
|
uncovered metal prostheses in the respiratory |
particularly after prolonged intubation. |
|
a |
b |
c |
Fig. 39.1 (a, b, and c) Posterior tracheal wall perforation secondary to stent wire fractures during extraction maneuvers
