
- •Foreword
- •Preface
- •Contents
- •About the Editors
- •Contributors
- •1: Tracheobronchial Anatomy
- •Trachea
- •Introduction
- •External Morphology
- •Internal Morphology
- •Mucous Layer
- •Blood Supply
- •Anatomo-Clinical Relationships
- •Bronchi
- •Main Bronchi
- •Bronchial Division
- •Left Main Bronchus (LMB)
- •Right Main Bronchus (RMB)
- •Blood Supply
- •References
- •2: Flexible Bronchoscopy
- •Introduction
- •History
- •Description
- •Indications and Contraindications
- •Absolute Contraindications
- •Procedure Preparation
- •Technique of FB Procedure
- •Complications of FB Procedure
- •Basic Diagnostic Procedures
- •Bronchoalveolar Lavage (BAL)
- •Transbronchial Lung Biopsy (TBLB)
- •Transbronchial Needle Aspiration (TBNA)
- •Bronchial Brushings
- •Advanced Diagnostic Bronchoscopy
- •EBUS-TBNA
- •Ultrathin Bronchoscopy
- •Transbronchial Lung Cryobiobsy (TBLC)
- •Therapeutic Procedures Via FB
- •LASER Bronchoscopy
- •Electrocautery
- •Argon Plasma Coagulation (APC)
- •Cryotherapy
- •Photodynamic Therapy
- •Airway Stent Placement
- •Endobronchial Valve Placement
- •Conclusion
- •References
- •History and Historical Perspective
- •Indications and Contraindications
- •Procedure Description
- •Procedure Planning
- •Target Approximation
- •Sampling
- •Complications
- •Future Directions
- •Summary and Recommendations
- •References
- •4: Rigid Broncoscopy
- •Innovations
- •Ancillary Equipment
- •Rigid Bronchoscopy Applications
- •Laser Bronchoscopy
- •Tracheobronchial Prosthesis
- •Transbronchial Needle Aspiration (TBNA)
- •Rigid Bronchoscope in Other Treatments for Bronchial Obstruction
- •Mechanical Debridement
- •Pediatric Rigid Bronchoscopy
- •Tracheobronchial Dilatation
- •Foreign Bodies Removal
- •Other Indications
- •Complications
- •The Procedure
- •Some Conclusions
- •References
- •History and Historical Perspective
- •Indications and Contraindications
- •Preprocedural Evaluation and Preparation
- •Physical Examination
- •Procedure-Related Indications
- •Application of the Technique
- •Topical Anesthesia
- •Anesthesia of the Nasal Mucosa and Nasopharynx
- •Anesthesia of the Mouth and Oropharynx
- •Superior Laryngeal Nerve Block
- •Recurrent Laryngeal Nerve Block (RLN)
- •Conscious Sedation
- •Monitored Anesthesia Care (MAC)
- •General Anesthesia
- •Monitoring the Depth of Anesthesia
- •Interventional Bronchoscopy Suites
- •Airway Devices
- •Laryngeal Mask Airway (LMA)
- •Endotracheal Tube (ETT)
- •Rigid Bronchoscope
- •Modes of Ventilation
- •Spontaneous Ventilation
- •Assisted Ventilation
- •Noninvasive Positive Pressure Ventilation (NIV)
- •Positive Pressure Controlled Mechanical Ventilation
- •Jet Ventilation
- •Electronic Mechanical Jet Ventilation
- •Postprocedure Care
- •Special Consideration
- •Anesthesia for Peripheral Diagnostic and Therapeutic Bronchoscopy
- •Anesthesia for Interventional Bronchoscopic Procedures During the COVID-19 Pandemic
- •Summary and Recommendations
- •Conclusion
- •References
- •Background
- •Curricular Structure and Delivery
- •What Is a Bronchoscopy Curriculum?
- •Tradition, Teaching Styles, and Beliefs
- •Using Assessment Tools to Guide the Educational Process
- •The Ethics of Teaching
- •When Learners Teach: The Journey from Novice to Mastery and Back Again
- •The Future Is Now
- •References
- •Interventional Procedure
- •Assessment of Flow–Volume Curve
- •Dyspnea
- •Analysis of Pressure–Pressure Curve
- •Conclusions
- •References
- •Introduction
- •Adaptations of the IP Department
- •Environmental Control
- •Personal Protective Equipment
- •Procedure Performance
- •Bronchoscopy in Intubated Patients
- •Other Procedures in IP Unit
- •References
- •Introduction
- •Safety
- •Patient Safety
- •Provider Safety
- •Patient Selection and Screening
- •Lung Cancer Diagnosis and Staging
- •Inpatients
- •COVID-19 Clearance
- •COVID Clearance: A Role for Bronchoscopy
- •Long COVID: A Role for Bronchoscopy
- •Preparing for the Next Pandemic
- •References
- •Historical Perspective
- •Indications and Contraindications
- •Evidence-Based Review
- •Summary and Recommendations
- •References
- •Introduction
- •Clinical Presentation
- •Diagnosis
- •Treatment
- •History and Historical Perspectives
- •Indications and Contraindications
- •Benign and Malignant Tumors
- •Tumors with Uncertain Prognosis
- •Application of the Technique
- •Evidence Based Review
- •Summary and Recommendations
- •References
- •12: Cryotherapy and Cryospray
- •Introduction
- •Historical Perspective
- •Equipment
- •Cryoadhesion
- •Indications
- •Cryorecanalization
- •Cryoadhesion and Foreign Body Removal
- •Cryoadhesion and Mucus Plugs/Blood Clot Retrieval
- •Endobronchial Cryobiopsy
- •Transbronchial Cryobiopsy for Lung Cancer
- •Safety Concerns and Contraindications
- •Cryoablation
- •Indications
- •Evidence
- •Safety Concerns and Contraindications
- •Cryospray
- •Indications
- •Evidence
- •Safety Concerns and Contraindications
- •Advantages of Cryotherapy
- •Limitations
- •Future Research Directions
- •References
- •13: Brachytherapy
- •History and Historical Perspective
- •Indications and Contraindications
- •Application of the Technique
- •Evidence-Based Review
- •Adjuvant Treatment
- •Palliative Treatment
- •Complications
- •Summary and Recommendations
- •References
- •14: Photodynamic Therapy
- •Introduction
- •Photosensitizers
- •First-Generation Photosensitizers
- •M-Tetrahidroxofenil Cloro (mTHPC) (Foscan®)
- •PDT Reaction
- •Tumor Damage Process
- •Procedure
- •Indications
- •Curative PDT Indications
- •Palliative PDT Indications
- •Contraindications
- •Rationale for Use in Early-Stage Lung Cancer
- •Rationale
- •PDT in Combination with Other Techniques for Advanced-Stage Non-small Cell Lung Cancer
- •Commentary
- •Complementary Endoscopic Methods for PDT Applications
- •New Perspectives
- •Other PDT Applications
- •Conclusions
- •References
- •15: Benign Airways Stenosis
- •Etiology
- •Congenital Tracheal Stenosis
- •Iatrogenic
- •Infectious
- •Idiopathic Tracheal Stenosis
- •Distal Bronchial Stenosis
- •Diagnosis Methods
- •Patient History
- •Imaging Techniques
- •Bronchoscopy
- •Pulmonary Function Test
- •Treatment
- •Endoscopic Treatment
- •Dilatation
- •Laser Therapy
- •Stents
- •How to Proceed
- •Stent Placement
- •Placing a Montgomery T Tube
- •The Rule of Twos for Benign Tracheal Stenosis (Fig. 15.23)
- •Surgery
- •Summary and Recommendations
- •References
- •16: Endobronchial Prostheses
- •Introduction
- •Indications
- •Extrinsic Compression
- •Intraluminal Obstruction
- •Stump Fistulas
- •Esophago-respiratory Fistulas (ERF)
- •Expiratory Central Airway Collapse
- •Physiologic Rationale for Airway Stent Insertion
- •Stent Selection Criteria
- •Stent-Related Complications
- •Granulation Tissue
- •Stent Fracture
- •Migration
- •Contraindications
- •Follow-Up and Patient Education
- •References
- •Introduction
- •Overdiagnosis
- •False Positives
- •Radiation
- •Risk of Complications
- •Lung Cancer Screening Around the World
- •Incidental Lung Nodules
- •Management of Lung Nodules
- •References
- •Introduction
- •Minimally Invasive Procedures
- •Mediastinoscopy
- •CT-Guided Transthoracic Biopsy
- •Fluoroscopy-Guided Transthoracic Biopsies
- •US-Guided Transthoracic Biopsy
- •Thoracentesis and Pleural Biopsy
- •Thoracentesis
- •Pleural Biopsy
- •Surgical or Medical Thoracoscopy
- •Image-Guided Pleural Biopsy
- •Closed Pleural Biopsy
- •Image-Guided Biopsies for Extrathoracic Metastases
- •Tissue Acquisition, Handling and Processing
- •Implications of Tissue Acquisition
- •Guideline Recommendations for Tissue Acquisition in Mediastinal Staging
- •Methods to Overcome Challenges in Tissue Acquisition and Genotyping
- •Rapid on-Site Evaluation (ROSE)
- •Sensitive Genotyping Assays
- •Liquid Biopsy
- •Summary, Recommendations and Highlights
- •References
- •History
- •Data Source and Methodology
- •Tumor Size
- •Involvement of the Main Bronchus
- •Atelectasis/Pneumonitis
- •Nodal Staging
- •Proposal for the Revision of Stage Groupings
- •Small Cell Lung Cancer (SCLC)
- •Discussion
- •Methodology
- •T Descriptors
- •N Descriptors
- •M Descriptors
- •Summary
- •References
- •Introduction
- •Historical Perspective
- •Fluoroscopy
- •Radial EBUS Mini Probe (rEBUS)
- •Ultrasound Bronchoscope (EBUS)
- •Virtual Bronchoscopy
- •Trans-Parenchymal Access
- •Cone Beam CT (CBCT)
- •Lung Vision
- •Sampling Instruments
- •Conclusions
- •References
- •History and Historical Perspective
- •Narrow Band Imaging (NBI)
- •Dual Red Imaging (DRI)
- •Endobronchial Ultrasound (EBUS)
- •Optical Coherence Tomography (OCT)
- •Indications and Contraindications
- •Confocal Laser Endomicroscopy and Endocytoscopy
- •Raman Spectrophotometry
- •Application of the Technique
- •Supplemental Technology for Diagnostic Bronchoscopy
- •Evidence-Based Review
- •Summary and Recommendations, Highlight of the Developments During the Last Three Years (2013 on)
- •References
- •Introduction
- •History and Historical Perspective
- •Endoscopic AF-OCT System
- •Preclinical Studies
- •Clinical Studies
- •Lung Cancer
- •Asthma
- •Airway and Lumen Calibration
- •Obstructive Sleep Apnea
- •Future Applications
- •Summary
- •References
- •23: Endobronchial Ultrasound
- •History and Historical Perspective
- •Equipment
- •Technique
- •Indication, Application, and Evidence
- •Convex Probe Ultrasound
- •Equipment
- •Technique
- •Indication, Application, and Evidence
- •CP-EBUS for Malignant Mediastinal or Hilar Adenopathy
- •CP-EBUS for the Staging of Non-small Cell Lung Cancer
- •CP-EBUS for Restaging NSCLC After Neoadjuvant Chemotherapy
- •Complications
- •Summary
- •References
- •Introduction
- •What Is Electromagnetic Navigation?
- •SuperDimension Navigation System (EMN-SD)
- •Computerized Tomography
- •Computer Interphase
- •The Edge Catheter: Extended Working Channel (EWC)
- •Procedural Steps
- •Planning
- •Detecting Anatomical Landmarks
- •Pathway Planning
- •Saving the Plan and Exiting
- •Registration
- •Real-Time Navigation
- •SPiN System Veran Medical Technologies (EMN-VM)
- •Procedure
- •Planning
- •Navigation
- •Biopsy
- •Complications
- •Limitations
- •Summary
- •References
- •Introduction
- •Image Acquisition
- •Hardware
- •Practical Considerations
- •Radiation Dose
- •Mobile CT Studies
- •Future Directions
- •Conclusion
- •References
- •26: Robotic Assisted Bronchoscopy
- •Historical Perspective
- •Evidence-Based Review
- •Diagnostic Yield
- •Monarch RAB
- •Ion Endoluminal Robotic System
- •Summary
- •References
- •History and Historical Perspective
- •Indications and Contraindications
- •General
- •Application of the Technique
- •Preoperative Care
- •Patient’s Position and Operative Field
- •Incision and Initial Dissection
- •Palpation
- •Biopsy
- •Control of Haemostasis and Closure
- •Postoperative Care
- •Complications
- •Technical Variants
- •Extended Cervical Mediastinoscopy
- •Mediastinoscopic Biopsy of Scalene Lymph Nodes
- •Inferior Mediastinoscopy
- •Mediastino-Thoracoscopy
- •Video-Assisted Mediastinoscopic Lymphadenectomy
- •Transcervical Extended Mediastinal Lymphadenectomy
- •Evidence-Based Review
- •Summary and Recommendations
- •References
- •Introduction
- •Case 1
- •Adrenal and Hepatic Metastases
- •Brain
- •Bone
- •Case 1 Continued
- •Biomarkers
- •Case 1 Concluded
- •Case 2
- •Chest X-Ray
- •Computerized Tomography
- •Positive Emission Tomography
- •Magnetic Resonance Imaging
- •Endobronchial Ultrasound with Transbronchial Needle Aspiration
- •Transthoracic Needle Aspiration
- •Transbronchial Needle Aspiration
- •Endoscopic Ultrasound with Needle Aspiration
- •Combined EUS-FNA and EBUS-TBNA
- •Case 2 Concluded
- •Case 3
- •Standard Cervical Mediastinoscopy
- •Extended Cervical Mediastinoscopy
- •Anterior Mediastinoscopy
- •Video-Assisted Thoracic Surgery
- •Case 3 Concluded
- •Case 4
- •Summary
- •References
- •29: Pleural Anatomy
- •Pleural Embryonic Development
- •Pleural Histology
- •Cytological Characteristics
- •Mesothelial Cells Functions
- •Pleural Space Defense Mechanism
- •Pleura Macroscopic Anatomy
- •Visceral Pleura (Pleura Visceralis or Pulmonalis)
- •Parietal Pleura (Pleura Parietalis)
- •Costal Parietal Pleura (Costalis)
- •Pleural Cavity (Cavitas Thoracis)
- •Pleural Apex or Superior Pleural Sinus [12–15]
- •Anterior Costal-Phrenic Sinus or Cardio-Phrenic Sinus
- •Posterior Costal-Phrenic Sinus
- •Cost-Diaphragmatic Sinus or Lateral Cost-Phrenic Sinus
- •Fissures18
- •Pleural Vascularization
- •Parietal Pleura Lymphatic Drainage
- •Visceral Pleura Lymphatic Drainage
- •Pleural Innervation
- •References
- •30: Chest Ultrasound
- •Introduction
- •The Technique
- •The Normal Thorax
- •Chest Wall Pathology
- •Pleural Pathology
- •Pleural Thickening
- •Pneumothorax
- •Pulmonary Pathology
- •Extrathoracic Lymph Nodes
- •COVID and Chest Ultrasound
- •Conclusions
- •References
- •Introduction
- •History of Chest Tubes
- •Overview of Chest Tubes
- •Contraindications for Chest Tube Placement
- •Chest Tube Procedural Technique
- •Special Considerations
- •Pneumothorax
- •Empyema
- •Hemothorax
- •Chest Tube Size Considerations
- •Pleural Drainage Systems
- •History of and Introduction to Indwelling Pleural Catheters
- •Indications and Contraindications for IPC Placement
- •Special Considerations
- •Non-expandable Lung
- •Chylothorax
- •Pleurodesis
- •Follow-Up and IPC Removal
- •IPC-Related Complications and Management
- •Competency and Training
- •Summary
- •References
- •32: Empyema Thoracis
- •Historical Perspectives
- •Incidence
- •Epidemiology
- •Pathogenesis
- •Clinical Presentation
- •Radiologic Evaluation
- •Biochemical Analysis
- •Microbiology
- •Non-operative Management
- •Prognostication
- •Surgical Management
- •Survivorship
- •Summary and Recommendations
- •References
- •Evaluation
- •Initial Intervention
- •Pleural Interventions for Recurrent Symptomatic MPE
- •Especial Circumstances
- •References
- •34: Medical Thoracoscopy
- •Introduction
- •Diagnostic Indications for Medical Thoracoscopy
- •Lung Cancer
- •Mesothelioma
- •Other Tumors
- •Tuberculosis
- •Therapeutic Indications
- •Pleurodesis of Pneumothorax
- •Thoracoscopic Drainage
- •Drug Delivery
- •Procedural Safety and Contraindications
- •Equipment
- •Procedure
- •Pre-procedural Preparations and Considerations
- •Procedural Technique [32]
- •Medical Thoracoscopy Versus VATS
- •Conclusion
- •References
- •Historical Perspective
- •Indications and Contraindications
- •Evidence-Based Review
- •Endobronchial Valves
- •Airway Bypass Tracts
- •Coils
- •Other Methods of ELVR
- •Summary and Recommendations
- •References
- •36: Bronchial Thermoplasty
- •Introduction
- •Mechanism of Action
- •Trials
- •Long Term: Ten-Year Study
- •Patient Selection
- •Bronchial Thermoplasty Procedure
- •Equipment
- •Pre-procedure
- •Bronchoscopy
- •Post-procedure
- •Conclusion
- •References
- •Introduction
- •Bronchoalveolar Lavage (BAL)
- •Technical Aspects of BAL Procedure
- •ILD Cell Patterns and Diagnosis from BAL
- •Technical Advises for Conventional TLB and TLB-C in ILD
- •Future Directions
- •References
- •Introduction
- •The Pediatric Airway
- •Advanced Diagnostic Procedures
- •Endobronchial Ultrasound
- •Virtual Navigational Bronchoscopy
- •Cryobiopsy
- •Therapeutic Procedures
- •Dilation Procedures
- •Thermal Techniques
- •Mechanical Debridement
- •Endobronchial Airway Stents
- •Metallic Stents
- •Silastic Stents
- •Novel Stents
- •Endobronchial Valves
- •Bronchial Thermoplasty
- •Discussion
- •References
- •Introduction
- •Etiology
- •Congenital ADF
- •Malignant ADF
- •Cancer Treatment-Related ADF
- •Benign ADF
- •Iatrogenic ADF
- •Diagnosis
- •Treatment Options
- •Endoscopic Techniques
- •Stents
- •Clinical Results
- •Stent Complications
- •Other Available Stents
- •Other Endoscopic Methods
- •References
- •Introduction
- •Anatomy and Physiology of Swallowing
- •Functional Physiology of Swallowing
- •Epidemiology and Risk Factors
- •Types of Foreign Bodies
- •Organic
- •Inorganic
- •Mineral
- •Miscellaneous
- •Clinical Presentation
- •Acute FB
- •Retained FB
- •Radiologic Findings
- •Bronchoscopy
- •Airway Management
- •Rigid Vs. Flexible Bronchoscopy
- •Retrieval Procedure
- •Instruments
- •Grasping Forceps
- •Baskets
- •Balloons
- •Suction Instruments
- •Ablative Therapies
- •Cryotherapy
- •Laser Therapy
- •Electrocautery and APC
- •Surgical Management
- •Complications
- •Bleeding and Hemoptysis
- •Distal Airway Impaction
- •Iron Pill Aspiration
- •Follow-Up and Sequelae
- •Conclusion
- •References
- •Vascular Origin of Hemoptysis
- •History and Historical Perspective
- •Diagnostic Bronchoscopy
- •Therapeutic Bronchoscopy
- •General Measures
- •Therapeutic Bronchoscopy
- •Evidence-Based Review
- •Summary
- •Recommendations
- •References
- •History
- •“The Glottiscope” (1807)
- •“The Esophagoscope” (1895)
- •The Rigid Bronchoscope (1897–)
- •The Flexible Bronchoscope (1968–)
- •Transbronchial Lung Biopsy (1972) (Fig. 42.7)
- •Laser Therapy (1981–)
- •Endobronchial Stents (1990–)
- •Electromagnetic Navigation (2003–)
- •Bronchial Thermoplasty (2006–)
- •Endobronchial Microwave Therapy (2004–)
- •American Association for Bronchology and Interventional Pulmonology (AABIP) and Journal of Bronchology and Interventional Pulmonology (JOBIP) (1992–)
- •References
- •Index

342 |
S. Gasparini and L. Zuccatosta |
|
|
Torrington et al. [2] did not nd by preoperative bronchoscopy any relevant information that changed the stage of the diseases and that obviate the need for surgery. On the contrary, in a study on 64 patients with peripheral bronchogenic carcinoma, Arsitizabal et al. [3] detected by bronchoscopy unsuspected endobronchial lesions on CT in 17% of patients and three of these patients had a nodule less than 3 cm in size. Chhajed et al., retrospectively, assessed the role of bronchoscopy in nodules less than 3 cm and they observed additional endobronchial tumors manifestations in 8% of cases [4]. In another study, Schwarz et al. [5] detected unsuspected endobronchial involvement in 5.5% of 181 patients with peripheral lung cancer, and fexible bronchoscopy changed the planned surgical approach in ve cases. Furthermore, the authors found during bronchoscopy anatomic variant of the bronchial tree in 15 cases, and this might be of interest for the surgical strategy.
These studies provide some evidence that bronchoscopy still plays an important role in the preoperative workup of PPLs and in the clinical practice the endoscopic evaluation is generally routinely performed before surgery for peripheral pulmonary lesions. Considering also the lower incidence of complications of the transbronchial approach in comparison to the percutaneous biopsy, any effort must be made to use bronchoscopy to obtain a cytohistological diagnosis of a PPL, avoiding further and more risky procedures.
Historical Perspective
It is well known that, although a PPL is not visible by bronchoscopy, sampling instruments can be inserted through the working channel of the scope and pushed in the peripheral airways, making possible biopsy of lesions localized even at subpleural level. In patients with localized PPL the use of a guidance technique is mandatory, since blind samples or bronchoalveolar lavage have a very low diagnostic yield [1].
The transbronchial approach to PPLs was rst described in 1959, before the advent of the fexi-
ble bronchoscope. Tsuboi et al. [6], using “Metras” catheters with various bended shape to be inserted in all the lobar bronchi, were able to introduce a curette in the lung periphery under fuoroscopic guidance, providing diagnosis of lung cancer in 81.6% of 158 cases of peripheral lung carcinoma. In the 1970s, after the advent of the fexible bronchoscope, the technique became easier, it spread widely around the world, and a large number of studies were published, always using fuoroscopy as a guidance system.
In the rst studies the sampling instruments used were mainly brushing and forceps biopsy, while in the 1980s and 1990s also the use of fexible transbronchial needles (TBNA) was introduced [7].
Based on a large number of studies carried out with fuoroscopic guidance, it became evident that the diagnostic sensitivity with this technique was not optimal, due to the dif culty or the inability of fuoroscopy to visualize small lesions or soft attenuations like ground glass opacity or PPLs located in areas fuoroscopically hidden by mediastinal structures.
To overcome such limits, to improve the diagnostic yield of the transbronchial approach to PPLs, and to reduce radiation exposure to patients and staff, in the last decades technological developments led to the introduction of new and innovative guidance systems.
In 2002 the use of virtual bronchoscopy navigation to identify the route to the lesion was described [8].
In the same year, Herth et al. [9] introduced the use of ultrasound fexible mini probes adequate to be inserted in the working channel of the scope and to be pushed in the periphery of the bronchial tree. The radial echo mini probes (rEBUS) are able to recognize the hypoechogenic signal of a solid lesion. To facilitate the bioptic approach with the use of ultrasound mini probe, in 2004 Kurimoto et al. [10] introduced the use of a guide sheath inserted together with the probe in the working channel of the bronchoscope. Once the location of the lesion is identi ed, the probe is withdrawn leaving the sheath in site, in this way providing guidance for the introduction of biopsy forceps.

20 Bronchoscopy Role in the Evaluation of Peripheral Pulmonary Lesions: An Overview |
343 |
|
|
In 2005 the use of the electromagnetic navigation system (EMN) was introduced [11]. This technology uses low frequency electromagnetic waves that are able to localize a sensor probe introduced through the bronchoscope in the airways; the dynamic position of the probe is then superimposed, using a dedicated software, on previously acquired CT images.
In recent years, further and new modalities of guidance have been proposed.
Cone-beam computed tomography (CBCT) is a radiographic imaging method that allows three- dimensional images, with the use of a cone- shaped X-ray beam produced by a C-arm rotating around the patient and able to acquire a complete data volume set in a single rotation. This technology, already used in other elds of medicine, has been introduced in interventional pulmonology as a guidance system for the bronchoscopic approach to PPLs [12, 13].
More recently, a novel navigation and guidance system that utilizes multimodal image fusion of preoperative CT and intraoperative fuoroscopy to enable real-time augmented fuoroscopic images has been proposed (LungVision system, Body Vision Medical Ltd) [14, 15]. This system may be used with a standard C-arm fuoroscopy, without the need of an expensive cone beam CT.
Some authors utilized together two or more of the above-mentioned guidance systems, in this way integrating the advantages of each technique (rEBUS plus virtual navigation bronchoscopy, rEBUS plus EMN, rEBUS plus fuoroscopy, EMN plus CBCT, EMN plus, and rEBUS plus CBCT), trying to demonstrate that the combination of multiple guidance modalities can increase the diagnostic yield [16].
One of the last techniques proposed in the history of the transbronchial approach to PPLs is the so-called “trans-parenchymal nodule access,” that was developed with the aim of allowing lesion centering, independently from its relationship with bronchial tree. This method is based on a hole done in the bronchial wall with a coring needle, allowing to create a tunnel into the parenchyma, from the bronchus to the lesion. A dedicated virtual navigation system is required, to
create a 3D model of airways and to calculate the best vessel free point where the hole may safely be performed [17].
Regardless of introduction of different guidance systems, progress in the transbronchial approach to PPLs is related also to development of new bronchoscopes, such as ultrathin instruments that can be pushed more peripherally in the airways. The rst publication regarding the usefulness of a thin bronchoscope for PPLs in adult patients was reported by Prakash in 1985 [18].
The rst generation of ultrathin bronchoscopes with a 2.7 mm outer diameter had the limit of a small working channel (0.8 mm), which was not wide enough for allowing the introduction of standard sampling instruments, and were proposed mainly for pediatric use. In the following years, ultrathin bronchoscopes with an outer diameter of 2.8 mm and a working channel of 1.2 mm and, more recently, a 3 mm bronchoscope with a 1.7 working channel were realized [19]. A 1.7 mm working channel allows inserting dedicated fexible transbronchial needles and these instruments have been more extensively used with many of the guidance systems described above, to improve the outcome of the transbronchial approach to PPL.
At the end of this historical review, it must be mentioned the last innovation for the transbronchial diagnosis of PPLs: robotic bronchoscopy (RB). RB may be used with different guidance systems (fuoroscopy, rEBUS, EMN). The advantages are the possibility to more precisely maneuver the scope and instruments into the periphery of the lungs under direct visualization while also ensuring stability during sampling of the target lesions. Speci c chapters of this book are dedicated to ultrathin bronchoscopes and to robotic bronchoscopy and they will provide more detailed data about these fascinating techniques.
Systems of Guidance: Methods
and Results
In this paragraph the technique of fuoroscopic- guided approach to PPLs will be described in detail.
Данная книга находится в списке для перевода на русский язык сайта https://meduniver.com/
344 |
S. Gasparini and L. Zuccatosta |
|
|
Since in the last years a huge number of studies have been published with the new guidance systems (rEBUS, EBUS-TBNA, EMN, CBCT, LungVision) and dedicated chapters of this book describe most of these methods, we will provide for each of these techniques a short overview on some technical aspects and on the diagnostic yields, as obtained by meta-analyses if available.
Fluoroscopy
Despite the advent of more advanced methods, fuoroscopy, due to low cost and to the availability in the majority of hospitals, still remains worldwide the most widely used guidance system for the transbronchial approach to PPLs.
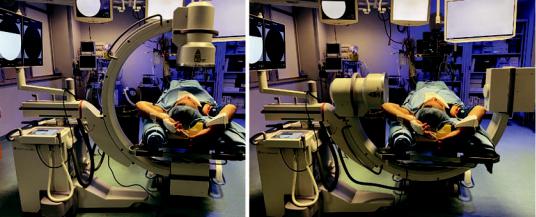
A rotating C-arm or a biplane fuoroscope must be available (Fig. 20.1) to visualize the position of the sampling instrument both, in the posterior–anterior and lateral view (Fig. 20.2). Bi-plan control is mandatory, otherwise the instrument can be misplaced in front or behind the lesion.
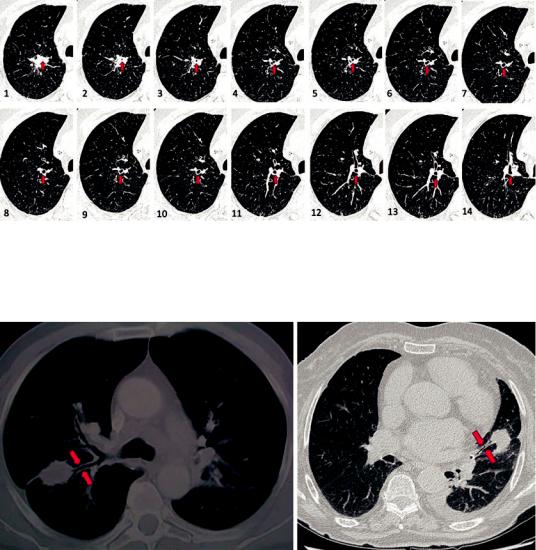
A careful evaluation of CT scan is necessary to precisely identify the bronchus that is leading or that comes closest to the target, before performing bronchoscopy. It is possible to visualize on different adjacent CT slides this bronchus during its pathway. We suggest, starting from the lesion and progressing toward the central air-
ways, to create a “mental navigation” that allows us to gure out the bronchial route to follow (Fig. 20.3).
At this point, bronchoscopic procedures can start. After exploration of the tracheobronchial tree, the tip of the bronchoscope must be wedged into the segmental or subsegmental bronchus that is leading into the lesion. Then, the sampling instrument must be inserted, following its progression at the fuoroscopic screen and trying to direct it toward the lesion. The tip of the bronchoscope should be bended or rotated to nd the most appropriate way to reach the target [20]. After obtaining the centering of the lesion in pos- terior–anterior view, the C-arm must be rotated 90° to verify the correct position in lateral view. For this purpose, it is useful to place the patient’s arms high, above the head, to prevent the humeri from reducing the clarity of fuoroscopic vision (Fig. 20.1).
The results of the published studies using fuoroscopy as a guidance system led to some considerations. Diagnostic yield evaluated by meta-analysis showed an average sensitivity of 78%, but a great heterogeneity of results is reported, ranging from 33% to 88% [21]. The reasons for these different results are linked to several factors.
All the studies demonstrated that the lesion size plays a major role in affecting the outcome. For PPLs less than 2 cm the diagnostic yield can
a |
b |
Fig. 20.1 Rotating C-arm fuoroscope. Postero-anterior (a) and latero-lateral (b) position. The patient’s arms are high, above the head, to prevent the humeri from reducing the clarity of fuoroscopic vision
20 |
Bronchoscopy Role in the Evaluation of Peripheral Pulmonary Lesions: An Overview |
345 |
|
|
|
a |
b |
|
c |
d |
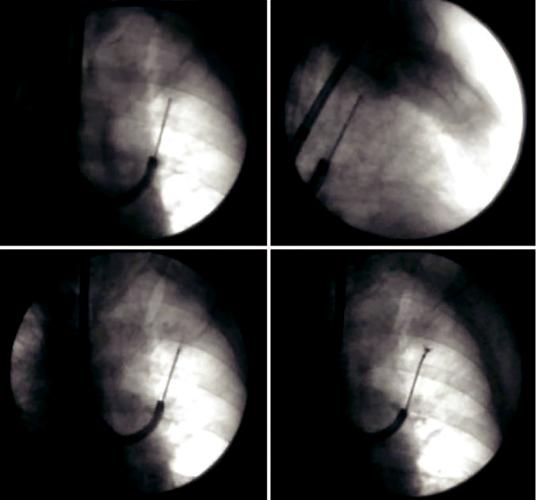
Fig. 20.2 Sampling under fuoroscopic guidance of a peripheral pulmonary nodule located in the left upper lobe. The correct position of the sampling instrument is
veri ed in postero-anterior (a) and in lateral view (b). TBNA (c) and biopsy (d) of the nodule
be estimated between 5% and 64%, while for lesions greater than 2 cm values ranging from 30% to 75% are reported [1, 22]. The diagnostic sensitivity may increase to over 80% for masses with a diameter greater than 4 cm [1].
Other factors that may infuence the results are the sampling instruments used, as discussed later in this chapter, and the operator experience. The location of the lesion may also have a role in affecting results. In a study on 177 patients, Baaklini et al. [23] grouped lesions according to distance from the hilum and yields of bronchos-
copy in central, intermediate, and peripherally located PPLs were 82%, 61%, and 53%, respectively. In the same study the authors found a trend toward higher diagnostic yield when the lesion was located in the right middle lobe and lingula, when compared to all other segments. On the contrary, Radke et al. [24] were unable to nd any correlation between diagnostic yield and the distance between the nearest edge of the lesion and the carina on either the postero-anterior or lateral roentgenogram, nor statistical differences according to the segmental location. In the same
Данная книга находится в списке для перевода на русский язык сайта https://meduniver.com/
346 |
S. Gasparini and L. Zuccatosta |
|
|
Fig. 20.3 Mental navigation. It is possible to identify the bronchus leading into the lesion and follow its pathway (red arrows), to create a “mental navigation” that allows togure out the bronchial route: starting from the lesion
(1–3), following the bronchus in the adjacent CT slides (4–11) up to arriving in a recognizable segmental bronchus (in this case left n.1) (12–14)
Fig. 20.4 Two peripheral lesions with a positive bronchus sign. A bronchus leading into the lesion is well recognizable (red arrows)
study, a precise diagnosis was more likely for malignant than for benign lesions, but in this paper benign PPLs were signi cantly smaller and size may have affected results.
A further element which strongly affects results of the transbronchial approach to PPL is the relationship of the lesion with the bronchial tree. If there is not a bronchus leading into the lesion, it will be dif cult or impossible to insert the sampling instrument into the target. This concept was demonstrated by Naidich et al. [25] that
introduced the de nition of “bronchus sign,” i.e., a bronchus leading to or contained into the lesion visualized by CT scan (Fig. 20.4). In a study on 65 patients, Naidich et al. showed a sensitivity of transbronchial biopsy of 55% if the bronchus sign was present and of only 32% in absence of bronchus sign [25]. The relevance of the bronchus sign, evaluated in several studies also using others and more advanced guidance systems, was con rmed in a meta-analysis by Ali et al. [26], aimed to determining the association between
