
- •Foreword
- •Preface
- •Contents
- •About the Editors
- •Contributors
- •1: Tracheobronchial Anatomy
- •Trachea
- •Introduction
- •External Morphology
- •Internal Morphology
- •Mucous Layer
- •Blood Supply
- •Anatomo-Clinical Relationships
- •Bronchi
- •Main Bronchi
- •Bronchial Division
- •Left Main Bronchus (LMB)
- •Right Main Bronchus (RMB)
- •Blood Supply
- •References
- •2: Flexible Bronchoscopy
- •Introduction
- •History
- •Description
- •Indications and Contraindications
- •Absolute Contraindications
- •Procedure Preparation
- •Technique of FB Procedure
- •Complications of FB Procedure
- •Basic Diagnostic Procedures
- •Bronchoalveolar Lavage (BAL)
- •Transbronchial Lung Biopsy (TBLB)
- •Transbronchial Needle Aspiration (TBNA)
- •Bronchial Brushings
- •Advanced Diagnostic Bronchoscopy
- •EBUS-TBNA
- •Ultrathin Bronchoscopy
- •Transbronchial Lung Cryobiobsy (TBLC)
- •Therapeutic Procedures Via FB
- •LASER Bronchoscopy
- •Electrocautery
- •Argon Plasma Coagulation (APC)
- •Cryotherapy
- •Photodynamic Therapy
- •Airway Stent Placement
- •Endobronchial Valve Placement
- •Conclusion
- •References
- •History and Historical Perspective
- •Indications and Contraindications
- •Procedure Description
- •Procedure Planning
- •Target Approximation
- •Sampling
- •Complications
- •Future Directions
- •Summary and Recommendations
- •References
- •4: Rigid Broncoscopy
- •Innovations
- •Ancillary Equipment
- •Rigid Bronchoscopy Applications
- •Laser Bronchoscopy
- •Tracheobronchial Prosthesis
- •Transbronchial Needle Aspiration (TBNA)
- •Rigid Bronchoscope in Other Treatments for Bronchial Obstruction
- •Mechanical Debridement
- •Pediatric Rigid Bronchoscopy
- •Tracheobronchial Dilatation
- •Foreign Bodies Removal
- •Other Indications
- •Complications
- •The Procedure
- •Some Conclusions
- •References
- •History and Historical Perspective
- •Indications and Contraindications
- •Preprocedural Evaluation and Preparation
- •Physical Examination
- •Procedure-Related Indications
- •Application of the Technique
- •Topical Anesthesia
- •Anesthesia of the Nasal Mucosa and Nasopharynx
- •Anesthesia of the Mouth and Oropharynx
- •Superior Laryngeal Nerve Block
- •Recurrent Laryngeal Nerve Block (RLN)
- •Conscious Sedation
- •Monitored Anesthesia Care (MAC)
- •General Anesthesia
- •Monitoring the Depth of Anesthesia
- •Interventional Bronchoscopy Suites
- •Airway Devices
- •Laryngeal Mask Airway (LMA)
- •Endotracheal Tube (ETT)
- •Rigid Bronchoscope
- •Modes of Ventilation
- •Spontaneous Ventilation
- •Assisted Ventilation
- •Noninvasive Positive Pressure Ventilation (NIV)
- •Positive Pressure Controlled Mechanical Ventilation
- •Jet Ventilation
- •Electronic Mechanical Jet Ventilation
- •Postprocedure Care
- •Special Consideration
- •Anesthesia for Peripheral Diagnostic and Therapeutic Bronchoscopy
- •Anesthesia for Interventional Bronchoscopic Procedures During the COVID-19 Pandemic
- •Summary and Recommendations
- •Conclusion
- •References
- •Background
- •Curricular Structure and Delivery
- •What Is a Bronchoscopy Curriculum?
- •Tradition, Teaching Styles, and Beliefs
- •Using Assessment Tools to Guide the Educational Process
- •The Ethics of Teaching
- •When Learners Teach: The Journey from Novice to Mastery and Back Again
- •The Future Is Now
- •References
- •Interventional Procedure
- •Assessment of Flow–Volume Curve
- •Dyspnea
- •Analysis of Pressure–Pressure Curve
- •Conclusions
- •References
- •Introduction
- •Adaptations of the IP Department
- •Environmental Control
- •Personal Protective Equipment
- •Procedure Performance
- •Bronchoscopy in Intubated Patients
- •Other Procedures in IP Unit
- •References
- •Introduction
- •Safety
- •Patient Safety
- •Provider Safety
- •Patient Selection and Screening
- •Lung Cancer Diagnosis and Staging
- •Inpatients
- •COVID-19 Clearance
- •COVID Clearance: A Role for Bronchoscopy
- •Long COVID: A Role for Bronchoscopy
- •Preparing for the Next Pandemic
- •References
- •Historical Perspective
- •Indications and Contraindications
- •Evidence-Based Review
- •Summary and Recommendations
- •References
- •Introduction
- •Clinical Presentation
- •Diagnosis
- •Treatment
- •History and Historical Perspectives
- •Indications and Contraindications
- •Benign and Malignant Tumors
- •Tumors with Uncertain Prognosis
- •Application of the Technique
- •Evidence Based Review
- •Summary and Recommendations
- •References
- •12: Cryotherapy and Cryospray
- •Introduction
- •Historical Perspective
- •Equipment
- •Cryoadhesion
- •Indications
- •Cryorecanalization
- •Cryoadhesion and Foreign Body Removal
- •Cryoadhesion and Mucus Plugs/Blood Clot Retrieval
- •Endobronchial Cryobiopsy
- •Transbronchial Cryobiopsy for Lung Cancer
- •Safety Concerns and Contraindications
- •Cryoablation
- •Indications
- •Evidence
- •Safety Concerns and Contraindications
- •Cryospray
- •Indications
- •Evidence
- •Safety Concerns and Contraindications
- •Advantages of Cryotherapy
- •Limitations
- •Future Research Directions
- •References
- •13: Brachytherapy
- •History and Historical Perspective
- •Indications and Contraindications
- •Application of the Technique
- •Evidence-Based Review
- •Adjuvant Treatment
- •Palliative Treatment
- •Complications
- •Summary and Recommendations
- •References
- •14: Photodynamic Therapy
- •Introduction
- •Photosensitizers
- •First-Generation Photosensitizers
- •M-Tetrahidroxofenil Cloro (mTHPC) (Foscan®)
- •PDT Reaction
- •Tumor Damage Process
- •Procedure
- •Indications
- •Curative PDT Indications
- •Palliative PDT Indications
- •Contraindications
- •Rationale for Use in Early-Stage Lung Cancer
- •Rationale
- •PDT in Combination with Other Techniques for Advanced-Stage Non-small Cell Lung Cancer
- •Commentary
- •Complementary Endoscopic Methods for PDT Applications
- •New Perspectives
- •Other PDT Applications
- •Conclusions
- •References
- •15: Benign Airways Stenosis
- •Etiology
- •Congenital Tracheal Stenosis
- •Iatrogenic
- •Infectious
- •Idiopathic Tracheal Stenosis
- •Distal Bronchial Stenosis
- •Diagnosis Methods
- •Patient History
- •Imaging Techniques
- •Bronchoscopy
- •Pulmonary Function Test
- •Treatment
- •Endoscopic Treatment
- •Dilatation
- •Laser Therapy
- •Stents
- •How to Proceed
- •Stent Placement
- •Placing a Montgomery T Tube
- •The Rule of Twos for Benign Tracheal Stenosis (Fig. 15.23)
- •Surgery
- •Summary and Recommendations
- •References
- •16: Endobronchial Prostheses
- •Introduction
- •Indications
- •Extrinsic Compression
- •Intraluminal Obstruction
- •Stump Fistulas
- •Esophago-respiratory Fistulas (ERF)
- •Expiratory Central Airway Collapse
- •Physiologic Rationale for Airway Stent Insertion
- •Stent Selection Criteria
- •Stent-Related Complications
- •Granulation Tissue
- •Stent Fracture
- •Migration
- •Contraindications
- •Follow-Up and Patient Education
- •References
- •Introduction
- •Overdiagnosis
- •False Positives
- •Radiation
- •Risk of Complications
- •Lung Cancer Screening Around the World
- •Incidental Lung Nodules
- •Management of Lung Nodules
- •References
- •Introduction
- •Minimally Invasive Procedures
- •Mediastinoscopy
- •CT-Guided Transthoracic Biopsy
- •Fluoroscopy-Guided Transthoracic Biopsies
- •US-Guided Transthoracic Biopsy
- •Thoracentesis and Pleural Biopsy
- •Thoracentesis
- •Pleural Biopsy
- •Surgical or Medical Thoracoscopy
- •Image-Guided Pleural Biopsy
- •Closed Pleural Biopsy
- •Image-Guided Biopsies for Extrathoracic Metastases
- •Tissue Acquisition, Handling and Processing
- •Implications of Tissue Acquisition
- •Guideline Recommendations for Tissue Acquisition in Mediastinal Staging
- •Methods to Overcome Challenges in Tissue Acquisition and Genotyping
- •Rapid on-Site Evaluation (ROSE)
- •Sensitive Genotyping Assays
- •Liquid Biopsy
- •Summary, Recommendations and Highlights
- •References
- •History
- •Data Source and Methodology
- •Tumor Size
- •Involvement of the Main Bronchus
- •Atelectasis/Pneumonitis
- •Nodal Staging
- •Proposal for the Revision of Stage Groupings
- •Small Cell Lung Cancer (SCLC)
- •Discussion
- •Methodology
- •T Descriptors
- •N Descriptors
- •M Descriptors
- •Summary
- •References
- •Introduction
- •Historical Perspective
- •Fluoroscopy
- •Radial EBUS Mini Probe (rEBUS)
- •Ultrasound Bronchoscope (EBUS)
- •Virtual Bronchoscopy
- •Trans-Parenchymal Access
- •Cone Beam CT (CBCT)
- •Lung Vision
- •Sampling Instruments
- •Conclusions
- •References
- •History and Historical Perspective
- •Narrow Band Imaging (NBI)
- •Dual Red Imaging (DRI)
- •Endobronchial Ultrasound (EBUS)
- •Optical Coherence Tomography (OCT)
- •Indications and Contraindications
- •Confocal Laser Endomicroscopy and Endocytoscopy
- •Raman Spectrophotometry
- •Application of the Technique
- •Supplemental Technology for Diagnostic Bronchoscopy
- •Evidence-Based Review
- •Summary and Recommendations, Highlight of the Developments During the Last Three Years (2013 on)
- •References
- •Introduction
- •History and Historical Perspective
- •Endoscopic AF-OCT System
- •Preclinical Studies
- •Clinical Studies
- •Lung Cancer
- •Asthma
- •Airway and Lumen Calibration
- •Obstructive Sleep Apnea
- •Future Applications
- •Summary
- •References
- •23: Endobronchial Ultrasound
- •History and Historical Perspective
- •Equipment
- •Technique
- •Indication, Application, and Evidence
- •Convex Probe Ultrasound
- •Equipment
- •Technique
- •Indication, Application, and Evidence
- •CP-EBUS for Malignant Mediastinal or Hilar Adenopathy
- •CP-EBUS for the Staging of Non-small Cell Lung Cancer
- •CP-EBUS for Restaging NSCLC After Neoadjuvant Chemotherapy
- •Complications
- •Summary
- •References
- •Introduction
- •What Is Electromagnetic Navigation?
- •SuperDimension Navigation System (EMN-SD)
- •Computerized Tomography
- •Computer Interphase
- •The Edge Catheter: Extended Working Channel (EWC)
- •Procedural Steps
- •Planning
- •Detecting Anatomical Landmarks
- •Pathway Planning
- •Saving the Plan and Exiting
- •Registration
- •Real-Time Navigation
- •SPiN System Veran Medical Technologies (EMN-VM)
- •Procedure
- •Planning
- •Navigation
- •Biopsy
- •Complications
- •Limitations
- •Summary
- •References
- •Introduction
- •Image Acquisition
- •Hardware
- •Practical Considerations
- •Radiation Dose
- •Mobile CT Studies
- •Future Directions
- •Conclusion
- •References
- •26: Robotic Assisted Bronchoscopy
- •Historical Perspective
- •Evidence-Based Review
- •Diagnostic Yield
- •Monarch RAB
- •Ion Endoluminal Robotic System
- •Summary
- •References
- •History and Historical Perspective
- •Indications and Contraindications
- •General
- •Application of the Technique
- •Preoperative Care
- •Patient’s Position and Operative Field
- •Incision and Initial Dissection
- •Palpation
- •Biopsy
- •Control of Haemostasis and Closure
- •Postoperative Care
- •Complications
- •Technical Variants
- •Extended Cervical Mediastinoscopy
- •Mediastinoscopic Biopsy of Scalene Lymph Nodes
- •Inferior Mediastinoscopy
- •Mediastino-Thoracoscopy
- •Video-Assisted Mediastinoscopic Lymphadenectomy
- •Transcervical Extended Mediastinal Lymphadenectomy
- •Evidence-Based Review
- •Summary and Recommendations
- •References
- •Introduction
- •Case 1
- •Adrenal and Hepatic Metastases
- •Brain
- •Bone
- •Case 1 Continued
- •Biomarkers
- •Case 1 Concluded
- •Case 2
- •Chest X-Ray
- •Computerized Tomography
- •Positive Emission Tomography
- •Magnetic Resonance Imaging
- •Endobronchial Ultrasound with Transbronchial Needle Aspiration
- •Transthoracic Needle Aspiration
- •Transbronchial Needle Aspiration
- •Endoscopic Ultrasound with Needle Aspiration
- •Combined EUS-FNA and EBUS-TBNA
- •Case 2 Concluded
- •Case 3
- •Standard Cervical Mediastinoscopy
- •Extended Cervical Mediastinoscopy
- •Anterior Mediastinoscopy
- •Video-Assisted Thoracic Surgery
- •Case 3 Concluded
- •Case 4
- •Summary
- •References
- •29: Pleural Anatomy
- •Pleural Embryonic Development
- •Pleural Histology
- •Cytological Characteristics
- •Mesothelial Cells Functions
- •Pleural Space Defense Mechanism
- •Pleura Macroscopic Anatomy
- •Visceral Pleura (Pleura Visceralis or Pulmonalis)
- •Parietal Pleura (Pleura Parietalis)
- •Costal Parietal Pleura (Costalis)
- •Pleural Cavity (Cavitas Thoracis)
- •Pleural Apex or Superior Pleural Sinus [12–15]
- •Anterior Costal-Phrenic Sinus or Cardio-Phrenic Sinus
- •Posterior Costal-Phrenic Sinus
- •Cost-Diaphragmatic Sinus or Lateral Cost-Phrenic Sinus
- •Fissures18
- •Pleural Vascularization
- •Parietal Pleura Lymphatic Drainage
- •Visceral Pleura Lymphatic Drainage
- •Pleural Innervation
- •References
- •30: Chest Ultrasound
- •Introduction
- •The Technique
- •The Normal Thorax
- •Chest Wall Pathology
- •Pleural Pathology
- •Pleural Thickening
- •Pneumothorax
- •Pulmonary Pathology
- •Extrathoracic Lymph Nodes
- •COVID and Chest Ultrasound
- •Conclusions
- •References
- •Introduction
- •History of Chest Tubes
- •Overview of Chest Tubes
- •Contraindications for Chest Tube Placement
- •Chest Tube Procedural Technique
- •Special Considerations
- •Pneumothorax
- •Empyema
- •Hemothorax
- •Chest Tube Size Considerations
- •Pleural Drainage Systems
- •History of and Introduction to Indwelling Pleural Catheters
- •Indications and Contraindications for IPC Placement
- •Special Considerations
- •Non-expandable Lung
- •Chylothorax
- •Pleurodesis
- •Follow-Up and IPC Removal
- •IPC-Related Complications and Management
- •Competency and Training
- •Summary
- •References
- •32: Empyema Thoracis
- •Historical Perspectives
- •Incidence
- •Epidemiology
- •Pathogenesis
- •Clinical Presentation
- •Radiologic Evaluation
- •Biochemical Analysis
- •Microbiology
- •Non-operative Management
- •Prognostication
- •Surgical Management
- •Survivorship
- •Summary and Recommendations
- •References
- •Evaluation
- •Initial Intervention
- •Pleural Interventions for Recurrent Symptomatic MPE
- •Especial Circumstances
- •References
- •34: Medical Thoracoscopy
- •Introduction
- •Diagnostic Indications for Medical Thoracoscopy
- •Lung Cancer
- •Mesothelioma
- •Other Tumors
- •Tuberculosis
- •Therapeutic Indications
- •Pleurodesis of Pneumothorax
- •Thoracoscopic Drainage
- •Drug Delivery
- •Procedural Safety and Contraindications
- •Equipment
- •Procedure
- •Pre-procedural Preparations and Considerations
- •Procedural Technique [32]
- •Medical Thoracoscopy Versus VATS
- •Conclusion
- •References
- •Historical Perspective
- •Indications and Contraindications
- •Evidence-Based Review
- •Endobronchial Valves
- •Airway Bypass Tracts
- •Coils
- •Other Methods of ELVR
- •Summary and Recommendations
- •References
- •36: Bronchial Thermoplasty
- •Introduction
- •Mechanism of Action
- •Trials
- •Long Term: Ten-Year Study
- •Patient Selection
- •Bronchial Thermoplasty Procedure
- •Equipment
- •Pre-procedure
- •Bronchoscopy
- •Post-procedure
- •Conclusion
- •References
- •Introduction
- •Bronchoalveolar Lavage (BAL)
- •Technical Aspects of BAL Procedure
- •ILD Cell Patterns and Diagnosis from BAL
- •Technical Advises for Conventional TLB and TLB-C in ILD
- •Future Directions
- •References
- •Introduction
- •The Pediatric Airway
- •Advanced Diagnostic Procedures
- •Endobronchial Ultrasound
- •Virtual Navigational Bronchoscopy
- •Cryobiopsy
- •Therapeutic Procedures
- •Dilation Procedures
- •Thermal Techniques
- •Mechanical Debridement
- •Endobronchial Airway Stents
- •Metallic Stents
- •Silastic Stents
- •Novel Stents
- •Endobronchial Valves
- •Bronchial Thermoplasty
- •Discussion
- •References
- •Introduction
- •Etiology
- •Congenital ADF
- •Malignant ADF
- •Cancer Treatment-Related ADF
- •Benign ADF
- •Iatrogenic ADF
- •Diagnosis
- •Treatment Options
- •Endoscopic Techniques
- •Stents
- •Clinical Results
- •Stent Complications
- •Other Available Stents
- •Other Endoscopic Methods
- •References
- •Introduction
- •Anatomy and Physiology of Swallowing
- •Functional Physiology of Swallowing
- •Epidemiology and Risk Factors
- •Types of Foreign Bodies
- •Organic
- •Inorganic
- •Mineral
- •Miscellaneous
- •Clinical Presentation
- •Acute FB
- •Retained FB
- •Radiologic Findings
- •Bronchoscopy
- •Airway Management
- •Rigid Vs. Flexible Bronchoscopy
- •Retrieval Procedure
- •Instruments
- •Grasping Forceps
- •Baskets
- •Balloons
- •Suction Instruments
- •Ablative Therapies
- •Cryotherapy
- •Laser Therapy
- •Electrocautery and APC
- •Surgical Management
- •Complications
- •Bleeding and Hemoptysis
- •Distal Airway Impaction
- •Iron Pill Aspiration
- •Follow-Up and Sequelae
- •Conclusion
- •References
- •Vascular Origin of Hemoptysis
- •History and Historical Perspective
- •Diagnostic Bronchoscopy
- •Therapeutic Bronchoscopy
- •General Measures
- •Therapeutic Bronchoscopy
- •Evidence-Based Review
- •Summary
- •Recommendations
- •References
- •History
- •“The Glottiscope” (1807)
- •“The Esophagoscope” (1895)
- •The Rigid Bronchoscope (1897–)
- •The Flexible Bronchoscope (1968–)
- •Transbronchial Lung Biopsy (1972) (Fig. 42.7)
- •Laser Therapy (1981–)
- •Endobronchial Stents (1990–)
- •Electromagnetic Navigation (2003–)
- •Bronchial Thermoplasty (2006–)
- •Endobronchial Microwave Therapy (2004–)
- •American Association for Bronchology and Interventional Pulmonology (AABIP) and Journal of Bronchology and Interventional Pulmonology (JOBIP) (1992–)
- •References
- •Index

498 |
T. L. Ferguson et al. |
|
|
nodal involvement [71]. In a systematic review from 2013 of 238 patients, the reported median sensitivity of the Chamberlain procedure was 71% and the NPV was 91% [12]. With the invention of video-assisted thoracic surgery (VATS), this has largely replaced the Chamberlain procedure for accessing the aortopulmonary window (station 5) and para-aortic (station 6) lymph nodes. A retrospective cohort study was done in 2007 which included 112 patients with clinically suspected N2 disease in lymph node stations 5 and 6. Thirty-nine patients underwent VATS which concluded in the correct diagnosis in 100% [74].
Video-Assisted Thoracic Surgery
The rst practical use of thoracoscopy was reported in 1910 by Dr. Jacobaeus when he used a thoracoscope to investigate and treat pleural effusions. The rst thoracoscopes were similar to the rst endoscopes. There were several limitations which included a lack of magni cation, only the operator can see the structures clearly, and the function of the assisted instruments were lacking. With the invention of the video-assisted imaging system, surgeons were able to magnify the image but also share the image with other surgeons simultaneously. The minimal requirements to perform a VATS include a rigid telescope, a light source with cable, a camera, and an imaging processor [75].
VATS for mediastinal staging is usually performed when needle techniques fail or are unable to access the lymph nodes in question. One major advantage of VATS over needle techniques is the direct visualization of the lung and mediastinal structures, including lymph node stations. It can provide a complete staging including TNM (Fig. 28.1). The major disadvantage of VATS is increased morbidity and mortality associated with surgery. In a 2013 meta-analysis of 4 studies, the reported median sensitivity was 99% (range 58–100%) and the negative predictive value was 96% (range 88–100%) for staging the mediastinum [12].
Surgical vs Minimally Invasive
Techniques
When evaluating a patient with lung cancer, accurate staging is what dictates treatment and management. Invasive biopsy is still the main modality for diagnosis and staging, which can be done surgically or with minimally invasive techniques. The guidelines recommend that minimally invasive needle techniques are the rst modality of choice to con rm mediastinal involvement in accessible lymph nodes stations. This recommendation is based on the availability of these technologies (EBUS-TBNA, EUS-FNA, etc.) and the appropriate skill of the operator. If minimally invasive techniques are negative and there is still a high clinical suspicion of disease, surgical biopsy is then recommended ( [12], Table 28.2).
Case 3 Concluded
Patient was evaluated by Cardiothoracic Surgery and underwent PFTs which were favorable for surgery. She underwent wedge resection that showed squamous cell carcinoma and subsequently underwent lobectomy with mediastinal lymph node dissection with curative intent.
Case 4
A 64-year-old man, former smoker with a 45-pack year smoking history, presented with a productive cough, dyspnea, and tachycardia. A chest CT showed a 1.4 cm right upper lobe nodule without lymphadenopathy along with centrilobular emphysema. A subsequent PET-CT revealed increased uptake in with an SUV of 3.3 from the right upper lobe nodule with no mediastinal or hilar uptake (Figs. 28.9 and 28.10). Patient had a clinical stage IA tumor. Pulmonary function tests showed moderate obstruction with FEV1 of 60%. Given his high-risk nature; A referral to cardiothoracic surgery was placed. After evaluation, patient underwent a right upper lobectomy in the
Данная книга находится в списке для перевода на русский язык сайта https://meduniver.com/
28 Lung Cancer Staging Methods: A Practical Approach |
499 |
|
|
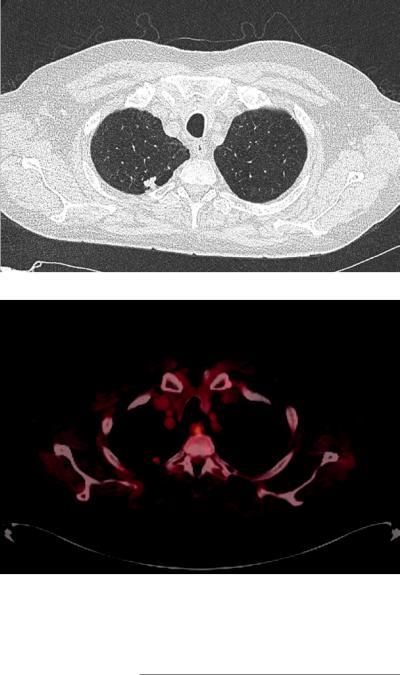
Fig. 28.9 CT chest showing 1.4 cm right upper lobe nodule with surrounding emphysema
Fig. 28.10 PET CT showing slightly increased uptake in right upper lobe nodule with SUV 3.3
operating room for curative intent. Pathology showed aT1bN0M0 squamous cell carcinoma.
When there is a high probability that the lesion in question is lung cancer with a normal clinical examination and no suspicious extrathoracic abnormalities on chest CT, the patient should be considered for curative intent treatment. The next step is to obtain a PET-CT to evaluate for mediastinal uptake and distant metastasis. If the PET shows negative nodal involvement, invasive perioperative evaluation of the mediastinal nodes is not required [12]. Ideally, the patient should be
evaluated by a thoracic surgeon or a multidisciplinary team for consideration of surgical resection.
Summary
When evaluating patients suspected of having lung cancer, the rst step is to acquire a thorough history, complete exam, and radiologic data to guide further treatment. Once imaging is obtained, further diagnostic workup is usually

500 |
T. L. Ferguson et al. |
|
|
warranted with tissue sampling to acquire a histological diagnosis and accurate stage. CT scan of the chest is usually the rst imaging test of choice, followed usually by PET-CT if further information is required. PET-CT has proven to be invaluable in aiding physicians by providing information about the tumor, mediastinum, and metastases, both within the chest and beyond. Brain MRI has proven to be superior to CT in identifying metastases to the brain and can be utilized to search for metastatic disease. Once imaging is completed, the next step in diagnosis is usually tissue acquisition by needle techniques and these include EBUS-TBNA, EUS-FNA, TTNA, TBNA, and navigational bronchoscopy with use of RP-EBUS. When patients have stage I or II disease, surgery is usually the best option and patients may undergo surgical staging of the mediastinum. Surgical staging includes standard cervical mediastinoscopy, extended cervical mediastinoscopy, anterior mediastinoscopy (Chamberlain Procedure), and VATS. Regardless of technique, molecular analysis and immunohistochemical staining have become crucial in the treatment for NSCLC and should be evaluated.
References
1.\Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–49. https:// onlinelibrary.wiley.com/doi/abs/10.3322/caac.21660. https://doi.org/10.3322/caac.21660.
2.\Dyba T, Randi G, Bray F, et al. The European cancer burden in 2020: incidence and mortality estimates for 40 countries and 25 major cancers. Eur J Cancer. 1990;2021(157):308–47. https://doi.org/10.1016/j. ejca.2021.07.039.
3.\Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018;68:7–30. https://doi. org/10.3322/caac.21442.
4.\de Koning HJ, van der Aalst, Carlijn M, de Jong PA, et al. Reduced lung-cancer mortality with volume CT screening in a randomized trial. N Engl J Med. 2020;382(6):503–13. https://doi.org/10.1056/ NEJMoa1911793.
5.\US Preventive Services Task Force, Krist AH, Davidson KW, et al. Screening for lung cancer: US preventive services task force recommendation state-
ment. JAMA. 2021;325(10):962–70. https://escholarship.org/uc/item/4d08218t
6.\Goldstraw P, Chansky K, Crowley J, et al. The IASLC lung cancer staging project: proposals for revision of the TNM stage groupings in the forthcoming (eighth) edition of the TNM classi cation for lung cancer. J Thorac Oncol. 2016;11(1):39–51. https://doi. org/10.1016/j.jtho.2015.09.009.
7.\Ettinger DS, Wood DE, Aisner DL, et al. NCCN guidelines insights: Non–Small cell lung cancer, version 2.2021. J Natl Compr Cancer Netw. 2021;19(3):254– 66. https://doi.org/10.6004/jnccn.2021.0013.
8.\Kocher F, Hilbe W, Seeber A, et al. Longitudinal analysis of 2293 NSCLC patients: A comprehensive study from the TYROL registry. Lung Cancer. 2015;87(2):193–200. https://www.sciencedirect.com/ science/article/pii/S0169500214005169. https://doi. org/10.1016/j.lungcan.2014.12.006.
9.\Riihimäki M, Hemminki A, Fallah M, et al. Metastatic sites and survival in lung cancer. Lung Cancer. 2014;86(1):78–84. https://www.sciencedirect.com/ science/article/pii/S0169500214003201. https://doi. org/10.1016/j.lungcan.2014.07.020.
10.\Kanaji N, Watanabe N, Kita N, et al. Paraneoplastic syndromes associated with lung cancer. World J Clin Oncol. 2014;5(3):197–223. https://doi.org/10.5306/ wjco.v5.i3.197.
11.\Spiro SG, Gould MK, Colice GL. Initial evaluation of the patient with lung cancer: symptoms, signs, laboratory tests, and paraneoplastic syndromes: ACCP evidenced-based clinical practice guidelines (2nd edition). Chest. 2007;132:149S–60S.
12.\Silvestri GA, Gonzalez AV, Jantz MA, et al. Methods for staging non-small cell lung cancer: diagnosis and management of lung cancer, 3rd ed: American college of chest physicians evidence-based clinical practice guidelines. Chest. 2013;143(5 Suppl):e211S–50S.
. https://www.ncbi.nlm.nih.gov/pubmed/23649440. https://doi.org/10.1378/chest.12-2355.
13.\Varela G, Thomas PA. Surgical management of advanced non-small cell lung cancer. J Thorac Dis. 2014;6(Suppl 2):S217–23. https://www.ncbi.nlm. nih.gov/pubmed/24868439. https://doi.org/10.3978/j. issn.2072-1439.2014.04.34.
14.\Surveillance Research Program, National Cancer Institute SEER*Stat software (seer.cancer.gov/seerstat) SEER 18 2011–2017.
15.\Kowalski DM, Cho BC, Lubiniecki GM, et al. Pembrolizumab versus chemotherapy for previously untreated, PD-L1-expressing, locally advanced or metastatic non-small-cell lung cancer (KEYNOTE-042): A randomised, open-label, controlled, phase 3 trial. Lancet. 2019;393(10183):1819–30. https://doi. org/10.1016/S0140-6736(18)32409-7.
16.\Li J, Xu W, Kong F, Sun X, Zuo X. Meta-analysis: accuracy of 18FDG PET-CT for distant metastasis staging in lung cancer patients. Surg Oncol. 2013;22(3):151–5. https://doi.org/10.1016/j. suronc.2013.04.001.
Данная книга находится в списке для перевода на русский язык сайта https://meduniver.com/
28 Lung Cancer Staging Methods: A Practical Approach |
501 |
|
|
17.\Søgaard R, Fischer BMB, Mortensen J, Højgaard L, Lassen U. Preoperative staging of lung cancer with
PET/CT: |
cost-effectiveness |
evaluation |
alongside |
||
a randomized controlled trial. Eur J Nucl Med Mol |
|||||
Imaging. 2011;38(5):802–9. https://doi.org/10.1007/ |
|||||
s00259-010-1703-y. |
|
|
|
||
18.\Silvestri |
GA, |
Littenberg B, |
Colice GL. The |
clini- |
|
cal evaluation |
for detecting |
metastatic |
lung |
can- |
|
cer : A meta-analysis. Am J Respir Crit Care Med. 1995;152(1):225–30.
19.\Sun Y, Yu H, Ma J, Lu P. The role of 18F-FDG PET/ CT integrated imaging in distinguishing malignant from benign pleural effusion. PLoS One. 2016;11(8):e0161764. https://www.ncbi.nlm.nih.gov/ pubmed/27560933. https://doi.org/10.1371/journal. pone.0161764.
20.\Brady MJ, Thomas J, Wong TZ, Franklin KM, Ho LM, Paulson EK. Adrenal nodules at FDG PET/CT in patients known to have or suspected of having lung cancer: A proposal for an ef cient diagnostic algorithm. Radiology. 2009;250(2):523–30. https://www. ncbi.nlm.nih.gov/pubmed/19188319. https://doi. org/10.1148/radiol.2502080219.
21.\Wu Q, |
Luo W, Zhao Y, Xu F, Zhou Q. The util- |
ity of |
18F-FDG PET/CT for the diagnosis of |
adrenal metastasis in lung cancer: A PRISMAcompliant meta-analysis. Nucl Med Commun. 2017;38(12):1117–24. https://www.ncbi.nlm.nih. gov/pubmed/28953208. https://doi.org/10.1097/ MNM.0000000000000757.
22.\Choi SH, Kim SY, Park SH, Kim KW, Lee JY, Lee SS, Lee MG. Diagnostic performance of CT, gadoxetate disodium-enhanced MRI, and PET/CT for the diagnosis of colorectal liver metastasis: systematic review and meta-analysis. J Magn Reson Imaging. 2018;47(5):1237–50. https://doi.org/10.1002/jmri. 25852. Epub 2017 Sep 13
23.\Detterbeck FC, Mazzone PJ, Naidich DP, Bach PB. Screening for lung cancer: diagnosis and management of lung cancer, 3rd ed: American college of chest physicians evidence-based clinical practice guidelines. Chest. 2013;143(5 Suppl):e78S–92S. https://www.ncbi.nlm.nih.gov/pubmed/23649455. https://doi.org/10.1378/chest.12-2350.
24.\Lee HY, Lee KS, Kim B, et al. Diagnostic ef - cacy of PET/CT plus brain MR imaging for detection of extrathoracic metastases in patients with lung adenocarcinoma. J Korean Med Sci. 2009;24(6):1132–8. https://www.ncbi.nlm.nih. gov/pubmed/19949671. https://doi.org/10.3346/ jkms.2009.24.6.1132.
25.\Li Y, Jin G, Su D. Comparison of gadolinium- enhanced MRI and 18FDG PET/PET-CT for the diagnosis of brain metastases in lung cancer patients: A meta-analysis of 5 prospective studies. Oncotarget. 2017;8(22):35743–9. https://www. ncbi.nlm.nih.gov/pubmed/28415747. https://doi. org/10.18632/oncotarget.16182.
26.\Qu X, Huang X,Yan W, Wu L, Dai K. A meta-analysis of 18FDG-PET–CT, 18FDG-PET, MRI and bone scintigraphy for diagnosis of bone metastases in patients
with lung cancer. Eur J Radiol. 2012;81(5):1007–15. 10.1016/j.ejrad.2011.01.126
27.\VanderLaan PA, Yamaguchi N, Folch E, et al.
Success and |
failure rates |
of tumor |
genotyp- |
ing techniques |
in routine |
pathological |
samples |
with non-small-cell lung cancer. Lung Cancer. 2014;84(1):39–44. https://www.clinicalkey.es/ playcontent/1-s2.0-S016950021400049X. https://doi. org/10.1016/j.lungcan.2014.01.013.
28.\Rivera MP, Mehta AC, Wahidi MM. Establishing the diagnosis of lung cancer: diagnosis and management of lung cancer, 3rd ed: American college of chest physicians evidence-based clinical practice guidelines. Chest. 2013;143(5 Suppl):e142S–65S. . https://www. ncbi.nlm.nih.gov/pubmed/23649436. https://doi. org/10.1378/chest.12-2353.
29.\Lynch TJ, Bell DW, Sordella R, et al. Activating mutations in the epidermal growth factor receptor underlying responsiveness of non-small-cell lung cancer to ge tinib. N Engl J Med. 2004;350(21):2129–39. http:// content.nejm.org/cgi/content/abstract/350/21/2129. https://doi.org/10.1056/NEJMoa040938.
30.\Gutierrez ME, Choi K, Lanman RB, et al. Genomic pro ling of advanced non-small cell lung cancer in community settings: gaps and opportunities. Clin Lung Cancer. 2017;18(6):651–9. https://www.clini- calkey.es/playcontent/1-s2.0-S1525730417301092. https://doi.org/10.1016/j.cllc.2017.04.004.
31.\Silvestri GA, Gould MK, Margolis ML, et al. Noninvasive staging of non-small cell lung cancer: ACCP evidenced-based clinical practice guidelines (2nd edition). Chest. 2007;132(3 Suppl):178S–201S. https://www.ncbi.nlm.nih.gov/pubmed/17873168
32.\Pretreatment evaluation of non-small-cell lung cancer. The American Thoracic Society and The European Respiratory Society. Am J Respir Crit Care Med. 1997;156(1):320–32. https://doi.org/10.1164/ ajrccm.156.1.ats156.1.
33.\Wahl RL, Hutchins GD, Buchsbaum DJ, Liebert M, Grossman HB, Fisher S. 18F-2-deoxy-2- fuoro-D-glucose uptake into human tumor xenografts. feasibility studies for cancer imaging with positron-emission tomography. Cancer. 1991;67(6):1544–50. https://doi.org/10.1002/1097- 0142(19910315)67:6<1544::AID-CNCR2820670614 >3.0.CO;2-0.
34.\Paesmans M, Garcia C, Wong CO, et al. Primary tumour standardised uptake value is prognostic in nonsmall cell lung cancer: A multivariate pooled analysis of individual data. Eur Respir J. 2015;46(6):1751–61. https://www. narcis.nl/publication/RecordID/oai:cris.maas- trichtuniversity.nl:publications%2F2c3174a7- 5568-45e4-b08f-d2aa911cc6b0. https://doi. org/10.1183/13993003.00099-2015.
35.\Maziak DE, Darling GE, Levine MN, et al. Positron emission tomography in staging early lung cancer: A randomized trial. Ann Intern Med. 2009;151(4):221–8. https://www.ncbi. nlm.nih.gov/pubmed/19581636. https://doi.org/ 10.7326/0003-4819-151-4-200908180-00132.
502 |
T. L. Ferguson et al. |
|
|
36.\van Tinteren H, Hoekstra OS, Smit EF, et al. Effectiveness of positron emission tomography in the preoperative assessment of patients with suspected non-small-cell lung cancer: the PLUS multicentre randomised trial. Lancet. 2002;359(9315):1388–92. https://doi.org/10.1016/S0140-6736(02)08352-6.
37.\Viney RC, Boyer MJ, King MT, et al. Randomized controlled trial of the role of positron emission tomography in the management of stage I and II non-small- cell lung cancer. J Clin Oncol. 2004;22(12):2357–62. http://jco.ascopubs.org/content/22/12/2357.abstract. https://doi.org/10.1200/JCO.2004.04.126.
38.\Fischer B, Lassen U, Mortensen J, et al. Preoperative staging of lung cancer with combined PET-CT. N Engl J Med. 2009;361(1):32–9. http://content. nejm.org/cgi/content/abstract/361/1/32. https://doi. org/10.1056/NEJMoa0900043.
39.\Fischer BM, Mortensen J, Hansen H, et al. Multimodality approach to mediastinal staging in nonsmall cell lung cancer. Faults and bene ts of PET-CT: A randomised trial. Thorax. 2011;66(4):294–300. https://doi.org/10.1136/thx.2010.154476.
40.\De Wever W, Vankan Y, Stroobants S, Verschakelen J. Detection of extrapulmonary lesions with integrated PET/CT in the staging of lung cancer. Eur Respir J. 2007;29(5):995–1002. http://erj.ersjournals.com/cgi/content/abstract/29/5/995. https://doi. org/10.1183/09031936.00119106.
41.\De Wever W, Ceyssens S, Mortelmans L, et al. Additional value of PET-CT in the staging of lung cancer: comparison with CT alone, PET alone and visual correlation of PET and CT. Eur Radiol. 2007;17(1):23–32. https://www.ncbi.nlm.nih. gov/pubmed/16683115. https://doi.org/10.1007/ s00330-006-0284-4.
42.\Herder G, Kramer H, Hoekstra OS, et al. Traditional versus up-front [18F] Fluorodeoxyglucose–Positron emission tomography staging of Non–Small-cell lung cancer: A dutch cooperative randomized study. J Clin Oncol. 2006;24(12):1800–6. http://jco.ascopubs.org/ content/24/12/1800.abstract. https://doi.org/10.1200/ JCO.2005.02.4695.
43.\Morgensztern D, Goodgame B, Baggstrom MQ, Gao F, Govindan R. The effect of FDG-PET on the stage distribution of non-small cell lung cancer. J Thorac Oncol. 2008;3(2):135–9. https://doi.org/10.1097/ JTO.0b013e3181622c2c.
44.\Gupta NC, Graeber GM, Bishop HA. Comparative ef cacy of positron emission tomography with fuorodeoxyglucose in evaluation of small (<1 cm), intermediate (1 to 3 cm), and large (>3 cm) lymph node lesions. Chest. 2000;117(3):773–8. https://doi. org/10.1378/chest.117.3.773.
45.\Kauczor H, Kreitner K. MRI of the pulmonary parenchyma. Eur Radiol. 1999;9(9):1755–64. https://www. ncbi.nlm.nih.gov/pubmed/10602947. https://doi. org/10.1007/s003300050919.
46.\Webb WR, Gatsonis C, Zerhouni EA, et al. CT and MR imaging in staging non-small cell bronchogenic carcinoma: report of the radiologic diagnostic oncol-
ogy group. Radiology. 1991;178(3):705–13. http:// radiology.rsna.org/content/178/3/705.abstract. https:// doi.org/10.1148/radiology.178.3.1847239.
47.\Wahidi MM, Herth F, Yasufuku K, et al. Technical aspects of endobronchial ultrasound-guided transbronchial needle aspiration: CHEST guideline and expert panel report. Chest. 2016;149(3):816–35. https://www.ncbi.nlm.nih.gov/pubmed/26402427. https://doi.org/10.1378/chest.15-1216.
48.\Grosu HB. EBUS-TBNA for the diagnosis of lymphoma: time to give in? J Bronchology Interv Pulmonol. 2018;25(3):165–6. https://www.ncbi.nlm. nih.gov/pubmed/29944587. https://doi.org/10.1097/ LBR.0000000000000524.
49.\Avasarala SK, Aravena C, Almeida FA. Convex probe endobronchial ultrasound: historical, contemporary, and cutting-edge applications. J Thorac Dis. 2020;12(3):1085–99. https://www.ncbi.nlm.nih. gov/pubmed/32274177. https://doi.org/10.21037/ jtd.2019.10.76.
50.\Herth FJF, Ernst A, Eberhardt R, Vilmann P, Dienemann H, Krasnik M. Endobronchial ultrasound- guided transbronchial needle aspiration of lymph nodes in the radiologically normal mediastinum. Eur Respir J. 2006;28(5):910–4. http://erj.ersjournals. com/cgi/content/abstract/28/5/910. https://doi.org/10. 1183/09031936.06.00124905.
51.\Herth FJF, Eberhardt R, Krasnik M, Ernst A. Endobronchial ultrasound-guided transbronchial needle aspiration of lymph nodes in the radiologically and positron emission tomography-normal mediastinum in patients with lung cancer. Chest. 2008;133(4):887–91. https://doi.org/10.1378/ chest.07-2535.
52.\Tanner NT, Yarmus L, Chen A, et al. Standard bronchoscopy with fuoroscopy vs thin bronchoscopy and radial endobronchial ultrasound for biopsy of pulmonary lesions: A multicenter, prospective, randomized trial. Chest. 2018;154(5):1035–43. https://www. ncbi.nlm.nih.gov/pubmed/30144421. https://doi. org/10.1016/j.chest.2018.08.1026.
53.\Silvestri GA, Feller-Kopman D, Chen A, Wahidi M, Yasufuku K, Ernst A. Latest advances in advanced diagnostic and therapeutic pulmonary procedures. Chest. 2012;142(6):1636–44. https://www.clini- calkey.es/playcontent/1-s2.0-S0012369212607004. https://doi.org/10.1378/chest.12-2326.
54.\Ishida T, Asano F, Yamazaki K, et al. Virtual bronchoscopic navigation combined with endobronchial ultrasound to diagnose small peripheral pulmonary lesions: A randomised trial. Thorax. 2011;66(12):1072–7. https://doi.org/10.1136/thx.2010.145490.
55.\Bo L, Li C, Pan L, et al. Diagnosing a solitary pulmonary nodule using multiple bronchoscopic guided technologies: A prospective randomized study. Lung Cancer. 2019;129:48–54. https://doi.org/10.1016/j. lungcan.2019.01.006.
56.\Berhardt R, Anantham D, Ernst A, Feller-Kopman D, Herth F. Multimodality bronchoscopic diagnosis of peripheral lung lesions: A randomized controlled trial.
Данная книга находится в списке для перевода на русский язык сайта https://meduniver.com/
28 Lung Cancer Staging Methods: A Practical Approach |
503 |
|
|
Am J Respir Crit Care Med. 2007;176(1):36–41. http:// ajrccm.atsjournals.org/cgi/content/abstract/176/1/36. https://doi.org/10.1164/rccm.200612-1866OC.
57.\Ost DE, Ernst A, Lei X, et al. Diagnostic yield and complications of bronchoscopy for peripheral lung lesions. Results of the AQuIRE registry. Am J Respir Crit Care Med. 2016;193(1):68–77. https://www. ncbi.nlm.nih.gov/pubmed/26367186. https://doi. org/10.1164/rccm.201507-1332OC.
58.\Murgu SD. Robotic assisted-bronchoscopy: technical tips and lessons learned from the initial experience with sampling peripheral lung lesions. BMC Pulm Med. 2019;19(1):89. https://www.ncbi.nlm. nih.gov/pubmed/31072355. https://doi.org/10.1186/ s12890-019-0857-z.
59.\Chen AC, Pastis J, Nicholas J, Mahajan AK, et al. Robotic bronchoscopy for peripheral pulmonary lesions: A multicenter pilot and feasibility study (BENEFIT). Chest. 2021;159(2):845–52. https:// www.ncbi.nlm.nih.gov/pubmed/32822675. https:// doi.org/10.1016/j.chest.2020.08.2047.
60.\Chockalingam A, Hong K. Transthoracic needle aspiration: the past, present and future. J Thorac Dis. 2015;7(Suppl 4):S292–9. https://www.ncbi.nlm.nih. gov/pubmed/26807277. https://doi.org/10.3978/j. issn.2072-1439.2015.12.01.
61.\Birchard KR. Transthoracic needle biopsy semin intervent radiol. 2011;28(1):87–97. https://doi. org/10.1055/s-0031-1273943.
62.\Schreiber G, Mccrory DC. Performance characteristics of different modalities for diagnosis of suspected lung cancer: summary of published evidence. Chest. 2003;123(1):115S–28S. https://www.ncbi.nlm.nih. gov/pubmed/12527571
63.\Wiener RS, Schwartz LM, Woloshin S, Welch HG. Population-based risk for complications after transthoracic needle lung biopsy of a pulmonary nodule: an analysis of discharge records. Ann Intern Med. 2011;155(3):137–44. https://www. ncbi.nlm.nih.gov/pubmed/21810706. https://doi. org/10.7326/0003-4819-155-3-201108020-00003.
64.\Liu Q, Ben S, Xia Y, Wang K, Huang H. Evolution of transbronchial needle aspiration technique. J Thorac Dis. 2015;7(Suppl 4):S224–30. https://www. ncbi.nlm.nih.gov/pubmed/26807269. https://doi. org/10.3978/j.issn.2072-1439.2015.11.31.
65.\Wallace MB, Pascual JMS, Raimondo M, et al. Minimally invasive endoscopic staging of suspected lung cancer. JAMA. 2008;299(5):540–6. https://doi. org/10.1001/jama.299.5.540.
66.\Yamao K, Sawaki A, Mizuno N, Shimizu Y, Yatabe Y, Koshikawa T. Endoscopic ultrasound-guided neneedle aspiration biopsy (EUS-FNAB): past, present, and future. J Gastroenterol. 2005;40(11):1013–23. https://www.ncbi.nlm.nih.gov/pubmed/16322944. https://doi.org/10.1007/s00535-005-1717-6.
67.\Wang Z, Jiang C. Endoscopic ultrasound in the diagnosis of mediastinal diseases. Open Med. 2015;10(1):560–5. http://www.degruyter.com/ doi/10.1515/med-2015-0095. https://doi.org/10.1515/ med-2015-0095.
68.\Colella S, Vilmann P, Konge L, Clementsen PF. Endoscopic ultrasound in the diagnosis and staging of lung cancer. Endosc Ultrasound. 2014;3(4):205– 12. https://www.ncbi.nlm.nih.gov/pubmed/25485267. https://doi.org/10.4103/2303-9027.144510.
69.\Vazquez-Sequeiros E, Levy MJ, Van Domselaar M, et al. Diagnostic yield and safety of endo-
scopic ultrasound guided ne needle |
aspiration |
||
of |
central mediastinal lung |
masses. |
Diagnostic |
and |
therapeutic endoscopy. |
2013;2013:150492–6. |
|
https://www.airitilibrary.com/Publication/alD |
|||
etailedMesh?DocID=P20151216003-201312- |
|||
201703090034-201703090034-8-13. |
https://doi. |
||
org/10.1155/2013/150492. |
|
|
|
70.\Chen L, Li Y, Gao X, et al. High diagnostic accuracy and safety of endoscopic ultrasound-guidedne-needle aspiration in malignant lymph nodes: A systematic review and meta-analysis. Dig Dis Sci. 2021;66(8):2763–75. https://search.proquest. com/docview/2446671513. https://doi.org/10.1007/ s10620-020-06554-2.
71.\Call S, Obiols C, Rami-Porta R. Present indications of surgical exploration of the mediastinum. J Thorac Dis. 2018;10(Suppl 22):S2601–10. https://www.ncbi.nlm. nih.gov/pubmed/30345097. https://doi.org/10.21037/ jtd.2018.03.183.
72.\D'Andrilli A, Maurizi G, Venuta F, Rendina EA. Mediastinal staging: When and how? Gen Thorac Cardiovasc Surg. 2020;68(7):725–32. https://www. ncbi.nlm.nih.gov/pubmed/31797211. https://doi. org/10.1007/s11748-019-01263-8.
73.\Witte B, Wolf M, Hillebrand H, Kriegel E, Huertgen M. Extended cervical mediastinoscopy revisited. Eur J Cardiothorac Surg. 2014;45(1):114–9. https://www. ncbi.nlm.nih.gov/pubmed/23803515. https://doi. org/10.1093/ejcts/ezt313.
74.\Cerfolio RJ, Bryant AS, Eloubeidi MA. Accessing the aortopulmonary window (#5) and the paraaortic (#6) lymph nodes in patients with non-small cell lung cancer. Ann Thorac Surg. 2007;84(3):940– 5. https://www.clinicalkey.es/playcontent/1- s2.0-S0003497507009289. https://doi.org/10.1016/j. athoracsur.2007.04.078.
75.\Luh S, Liu H. Video-assisted thoracic surgery-the past, present status and the future. J Zhejiang Univ Sci B. 2006;7(2):118–28. https://www.airitilibrary. com/Publication/alDetailedMesh?DocID=16731581- 200602-7B-2-118-128-a. https://doi.org/10.1631/ jzus.2006.B0118.

Part VI
Pleural Conditions
Данная книга находится в списке для перевода на русский язык сайта https://meduniver.com/
