
- •Preface
- •Acknowledgments
- •Contents
- •1.1 Introduction
- •1.2 Normal Embryology
- •1.3 Abnormalities of the Kidney
- •1.3.1 Renal Agenesis
- •1.3.2 Renal Hypoplasia
- •1.3.3 Supernumerary Kidneys
- •1.3.5 Polycystic Kidney Disease
- •1.3.6 Simple (Solitary) Renal Cyst
- •1.3.7 Renal Fusion and Renal Ectopia
- •1.3.8 Horseshoe Kidney
- •1.3.9 Crossed Fused Renal Ectopia
- •1.4 Abnormalities of the Ureter
- •1.5 Abnormalities of the Bladder
- •1.6 Abnormalities of the Penis and Urethra in Males
- •1.7 Abnormalities of Female External Genitalia
- •Further Reading
- •2.1 Introduction
- •2.2 Pathophysiology
- •2.3 Etiology of Hydronephrosis
- •2.5 Clinical Features
- •2.6 Investigations and Diagnosis
- •2.7 Treatment
- •2.8 Antenatal Hydronephrosis
- •Further Reading
- •3.1 Introduction
- •3.2 Embryology
- •3.3 Pathophysiology
- •3.4 Etiology of PUJ Obstruction
- •3.5 Clinical Features
- •3.6 Diagnosis and Investigations
- •3.7 Management of Newborns with PUJ Obstruction
- •3.8 Treatment
- •3.9 Post-operative Complications and Follow-Up
- •Further Reading
- •4: Renal Tumors in Children
- •4.1 Introduction
- •4.2 Wilms’ Tumor
- •4.2.1 Introduction
- •4.2.2 Etiology
- •4.2.3 Histopathology
- •4.2.4 Nephroblastomatosis
- •4.2.5 Clinical Features
- •4.2.6 Risk Factors for Wilms’ Tumor
- •4.2.7 Staging of Wilms Tumor
- •4.2.8 Investigations
- •4.2.9 Prognosis and Complications of Wilms Tumor
- •4.2.10 Surgical Considerations
- •4.2.11 Surgical Complications
- •4.2.12 Prognosis and Outcome
- •4.2.13 Extrarenal Wilms’ Tumors
- •4.3 Mesoblastic Nephroma
- •4.3.1 Introduction
- •4.3.3 Epidemiology
- •4.3.5 Clinical Features
- •4.3.6 Investigations
- •4.3.7 Treatment and Prognosis
- •4.4 Clear Cell Sarcoma of the Kidney (CCSK)
- •4.4.1 Introduction
- •4.4.2 Pathophysiology
- •4.4.3 Clinical Features
- •4.4.4 Investigations
- •4.4.5 Histopathology
- •4.4.6 Treatment
- •4.4.7 Prognosis
- •4.5 Malignant Rhabdoid Tumor of the Kidney
- •4.5.1 Introduction
- •4.5.2 Etiology and Pathophysiology
- •4.5.3 Histologic Findings
- •4.5.4 Clinical Features
- •4.5.5 Investigations and Diagnosis
- •4.5.6 Treatment and Outcome
- •4.5.7 Mortality/Morbidity
- •4.6 Renal Cell Carcinoma in Children
- •4.6.1 Introduction
- •4.6.2 Histopathology
- •4.6.4 Staging
- •4.6.5 Clinical Features
- •4.6.6 Investigations
- •4.6.7 Management
- •4.6.8 Prognosis
- •4.7 Angiomyolipoma of the Kidney
- •4.7.1 Introduction
- •4.7.2 Histopathology
- •4.7.4 Clinical Features
- •4.7.5 Investigations
- •4.7.6 Treatment and Prognosis
- •4.8 Renal Lymphoma
- •4.8.1 Introduction
- •4.8.2 Etiology and Pathogenesis
- •4.8.3 Diagnosis
- •4.8.4 Clinical Features
- •4.8.5 Treatment and Prognosis
- •4.9 Ossifying Renal Tumor of Infancy
- •4.10 Metanephric Adenoma
- •4.10.1 Introduction
- •4.10.2 Histopathology
- •4.10.3 Diagnosis
- •4.10.4 Clinical Features
- •4.10.5 Treatment
- •4.11 Multilocular Cystic Renal Tumor
- •Further Reading
- •Wilms’ Tumor
- •Mesoblastic Nephroma
- •Renal Cell Carcinoma in Children
- •Angiomyolipoma of the Kidney
- •Renal Lymphoma
- •Ossifying Renal Tumor of Infancy
- •Metanephric Adenoma
- •Multilocular Cystic Renal Tumor
- •5.1 Introduction
- •5.2 Embryology
- •5.4 Histologic Findings
- •5.7 Associated Anomalies
- •5.8 Clinical Features
- •5.9 Investigations
- •5.10 Treatment
- •Further Reading
- •6: Congenital Ureteral Anomalies
- •6.1 Etiology
- •6.2 Clinical Features
- •6.3 Investigations and Diagnosis
- •6.4 Duplex (Duplicated) System
- •6.4.1 Introduction
- •6.4.3 Clinical Features
- •6.4.4 Investigations
- •6.4.5 Treatment and Prognosis
- •6.5 Ectopic Ureter
- •6.5.1 Introduction
- •6.5.3 Clinical Features
- •6.5.4 Diagnosis
- •6.5.5 Surgical Treatment
- •6.6 Ureterocele
- •6.6.1 Introduction
- •6.6.3 Clinical Features
- •6.6.4 Investigations and Diagnosis
- •6.6.5 Treatment
- •6.6.5.1 Surgical Interventions
- •6.8 Mega Ureter
- •Further Reading
- •7: Congenital Megaureter
- •7.1 Introduction
- •7.3 Etiology and Pathophysiology
- •7.4 Clinical Presentation
- •7.5 Investigations and Diagnosis
- •7.6 Treatment and Prognosis
- •7.7 Complications
- •Further Reading
- •8.1 Introduction
- •8.2 Pathophysiology
- •8.4 Etiology of VUR
- •8.5 Clinical Features
- •8.6 Investigations
- •8.7 Management
- •8.7.1 Medical Treatment of VUR
- •8.7.2 Antibiotics Used for Prophylaxis
- •8.7.3 Anticholinergics
- •8.7.4 Surveillance
- •8.8 Surgical Therapy of VUR
- •8.8.1 Indications for Surgical Interventions
- •8.8.2 Indications for Surgical Interventions Based on Age at Diagnosis and the Presence or Absence of Renal Lesions
- •8.8.3 Endoscopic Injection
- •8.8.4 Surgical Management
- •8.9 Mortality/Morbidity
- •Further Reading
- •9: Pediatric Urolithiasis
- •9.1 Introduction
- •9.2 Etiology
- •9.4 Clinical Features
- •9.5 Investigations
- •9.6 Complications of Urolithiasis
- •9.7 Management
- •Further Reading
- •10.1 Introduction
- •10.2 Embryology of Persistent Müllerian Duct Syndrome
- •10.3 Etiology and Inheritance of PMDS
- •10.5 Clinical Features
- •10.6 Treatment
- •10.7 Prognosis
- •Further Reading
- •11.1 Introduction
- •11.2 Physiology and Bladder Function
- •11.2.1 Micturition
- •11.3 Pathophysiological Changes of NBSD
- •11.4 Etiology and Clinical Features
- •11.5 Investigations and Diagnosis
- •11.7 Management
- •11.8 Clean Intermittent Catheterization
- •11.9 Anticholinergics
- •11.10 Botulinum Toxin Type A
- •11.11 Tricyclic Antidepressant Drugs
- •11.12 Surgical Management
- •Further Reading
- •12.1 Introduction
- •12.2 Etiology
- •12.3 Pathophysiology
- •12.4 Clinical Features
- •12.5 Investigations and Diagnosis
- •12.6 Management
- •Further Reading
- •13.1 Introduction
- •13.2 Embryology
- •13.3 Epispadias
- •13.3.1 Introduction
- •13.3.2 Etiology
- •13.3.4 Treatment
- •13.3.6 Female Epispadias
- •13.3.7 Surgical Repair of Female Epispadias
- •13.3.8 Prognosis
- •13.4 Bladder Exstrophy
- •13.4.1 Introduction
- •13.4.2 Associated Anomalies
- •13.4.3 Principles of Surgical Management of Bladder Exstrophy
- •13.4.4 Evaluation and Management
- •13.5 Cloacal Exstrophy
- •13.5.1 Introduction
- •13.5.2 Skeletal Changes in Cloacal Exstrophy
- •13.5.3 Etiology and Pathogenesis
- •13.5.4 Prenatal Diagnosis
- •13.5.5 Associated Anomalies
- •13.5.8 Surgical Reconstruction
- •13.5.9 Management of Urinary Incontinence
- •13.5.10 Prognosis
- •13.5.11 Complications
- •Further Reading
- •14.1 Introduction
- •14.2 Etiology
- •14.3 Clinical Features
- •14.4 Associated Anomalies
- •14.5 Diagnosis
- •14.6 Treatment and Prognosis
- •Further Reading
- •15: Cloacal Anomalies
- •15.1 Introduction
- •15.2 Associated Anomalies
- •15.4 Clinical Features
- •15.5 Investigations
- •Further Reading
- •16: Urachal Remnants
- •16.1 Introduction
- •16.2 Embryology
- •16.4 Clinical Features
- •16.5 Tumors and Urachal Remnants
- •16.6 Management
- •Further Reading
- •17: Inguinal Hernias and Hydroceles
- •17.1 Introduction
- •17.2 Inguinal Hernia
- •17.2.1 Incidence
- •17.2.2 Etiology
- •17.2.3 Clinical Features
- •17.2.4 Variants of Hernia
- •17.2.6 Treatment
- •17.2.7 Complications of Inguinal Herniotomy
- •17.3 Hydrocele
- •17.3.1 Embryology
- •17.3.3 Treatment
- •Further Reading
- •18: Cloacal Exstrophy
- •18.1 Introduction
- •18.2 Etiology and Pathogenesis
- •18.3 Associated Anomalies
- •18.4 Clinical Features and Management
- •Further Reading
- •19: Posterior Urethral Valve
- •19.1 Introduction
- •19.2 Embryology
- •19.3 Pathophysiology
- •19.5 Clinical Features
- •19.6 Investigations and Diagnosis
- •19.7 Management
- •19.8 Medications Used in Patients with PUV
- •19.10 Long-Term Outcomes
- •19.10.3 Bladder Dysfunction
- •19.10.4 Renal Transplantation
- •19.10.5 Fertility
- •Further Reading
- •20.1 Introduction
- •20.2 Embryology
- •20.4 Clinical Features
- •20.5 Investigations
- •20.6 Treatment
- •20.7 The Müllerian Duct Cyst
- •Further Reading
- •21: Hypospadias
- •21.1 Introduction
- •21.2 Effects of Hypospadias
- •21.3 Embryology
- •21.4 Etiology of Hypospadias
- •21.5 Associated Anomalies
- •21.7 Clinical Features of Hypospadias
- •21.8 Treatment
- •21.9 Urinary Diversion
- •21.10 Postoperative Complications
- •Further Reading
- •22: Male Circumcision
- •22.1 Introduction
- •22.2 Anatomy and Pathophysiology
- •22.3 History of Circumcision
- •22.4 Pain Management
- •22.5 Indications for Circumcision
- •22.6 Contraindications to Circumcision
- •22.7 Surgical Procedure
- •22.8 Complications of Circumcision
- •Further Reading
- •23: Priapism in Children
- •23.1 Introduction
- •23.2 Pathophysiology
- •23.3 Etiology
- •23.5 Clinical Features
- •23.6 Investigations
- •23.7 Management
- •23.8 Prognosis
- •23.9 Priapism and Sickle Cell Disease
- •23.9.1 Introduction
- •23.9.2 Epidemiology
- •23.9.4 Pathophysiology
- •23.9.5 Clinical Features
- •23.9.6 Treatment
- •23.9.7 Prevention of Stuttering Priapism
- •23.9.8 Complications of Priapism and Prognosis
- •Further Reading
- •24.1 Introduction
- •24.2 Embryology and Normal Testicular Development and Descent
- •24.4 Causes of Undescended Testes and Risk Factors
- •24.5 Histopathology
- •24.7 Clinical Features and Diagnosis
- •24.8 Treatment
- •24.8.1 Success of Surgical Treatment
- •24.9 Complications of Orchidopexy
- •24.10 Infertility and Undescended Testes
- •24.11 Undescended Testes and the Risk of Cancer
- •Further Reading
- •25: Varicocele
- •25.1 Introduction
- •25.2 Etiology
- •25.3 Pathophysiology
- •25.4 Grading of Varicoceles
- •25.5 Clinical Features
- •25.6 Diagnosis
- •25.7 Treatment
- •25.8 Postoperative Complications
- •25.9 Prognosis
- •Further Reading
- •26.1 Introduction
- •26.2 Etiology and Risk Factors
- •26.3 Diagnosis
- •26.4 Intermittent Testicular Torsion
- •26.6 Effects of Testicular Torsion
- •26.7 Clinical Features
- •26.8 Treatment
- •26.9.1 Introduction
- •26.9.2 Etiology of Extravaginal Torsion
- •26.9.3 Clinical Features
- •26.9.4 Treatment
- •26.10 Torsion of the Testicular or Epididymal Appendage
- •26.10.1 Introduction
- •26.10.2 Embryology
- •26.10.3 Clinical Features
- •26.10.4 Investigations and Treatment
- •Further Reading
- •27: Testicular Tumors in Children
- •27.1 Introduction
- •27.4 Etiology of Testicular Tumors
- •27.5 Clinical Features
- •27.6 Staging
- •27.6.1 Regional Lymph Node Staging
- •27.7 Investigations
- •27.8 Treatment
- •27.9 Yolk Sac Tumor
- •27.10 Teratoma
- •27.11 Mixed Germ Cell Tumor
- •27.12 Stromal Tumors
- •27.13 Simple Testicular Cyst
- •27.14 Epidermoid Cysts
- •27.15 Testicular Microlithiasis (TM)
- •27.16 Gonadoblastoma
- •27.17 Cystic Dysplasia of the Testes
- •27.18 Leukemia and Lymphoma
- •27.19 Paratesticular Rhabdomyosarcoma
- •27.20 Prognosis and Outcome
- •Further Reading
- •28: Splenogonadal Fusion
- •28.1 Introduction
- •28.2 Etiology
- •28.4 Associated Anomalies
- •28.5 Clinical Features
- •28.6 Investigations
- •28.7 Treatment
- •Further Reading
- •29: Acute Scrotum
- •29.1 Introduction
- •29.2 Torsion of Testes
- •29.2.1 Introduction
- •29.2.3 Etiology
- •29.2.4 Clinical Features
- •29.2.5 Effects of Torsion of Testes
- •29.2.6 Investigations
- •29.2.7 Treatment
- •29.3 Torsion of the Testicular or Epididymal Appendage
- •29.3.1 Introduction
- •29.3.2 Embryology
- •29.3.3 Clinical Features
- •29.3.4 Investigations and Treatment
- •29.4.1 Introduction
- •29.4.2 Etiology
- •29.4.3 Clinical Features
- •29.4.4 Investigations and Treatment
- •29.5 Idiopathic Scrotal Edema
- •29.6 Testicular Trauma
- •29.7 Other Causes of Acute Scrotum
- •29.8 Splenogonadal Fusion
- •Further Reading
- •30.1 Introduction
- •30.2 Imperforate Hymen
- •30.3 Vaginal Atresia
- •30.5 Associated Anomalies
- •30.6 Embryology
- •30.7 Clinical Features
- •30.8 Investigations
- •30.9 Management
- •Further Reading
- •31: Disorders of Sexual Development
- •31.1 Introduction
- •31.2 Embryology
- •31.3 Sexual and Gonadal Differentiation
- •31.5 Evaluation of a Newborn with DSD
- •31.6 Diagnosis and Investigations
- •31.7 Management of Patients with DSD
- •31.8 Surgical Corrections of DSD
- •31.9 Congenital Adrenal Hyperplasia (CAH)
- •31.10 Androgen Insensitivity Syndrome (Testicular Feminization Syndrome)
- •31.13 Gonadal Dysgenesis
- •31.15 Ovotestis Disorders of Sexual Development
- •31.16 Other Rare Disorders of Sexual Development
- •Further Reading
- •Index

310 |
11 Neurogenic Bladder Sphincter Dysfunction |
|
|
•Urodynamics studies should be performed no earlier than 6 weeks after the injury, to allow stabilization of the extent of the neurologic injury
•Renal ultrasonography should be part of the assessment
•Voiding cystography is done in those with signs of potential risk (i.e. sphincter dyssynergy or poor detrusor compliance).
•The aim is to achieve low detrusor filling and voiding pressures with complete emptying and in the absence of this, these patients should continue on CIC in addition to anticholinergics.
11.5Investigations and Diagnosis
•Urinalysis, and urine culture
•Serum electrolytes, BUN and creatinine levels
•Abdominal ultrasonography
•Lateral spine radiography is important to look for vertebral anomalies especially sacral agenesis.
•Magnetic resonance imaging
•Voiding cystourethrography (Figs. 11.4, 11.5, 11.6, and 11.7):
–A voiding cystourethrogram can assess bladder neck and urethral function (internal and external sphincter) during filling and voiding phases.
–A voiding cystourethrogram can identify a urethral diverticulum, urethral obstruction, and vesicoureteral reflux.
•Ultrasonography of the spinal canal can be useful in infants younger than 5 months; however, once the vertebrae begin to ossify, ultrasonography becomes much less sensitive.
•Measure of residual urine:
–The postvoid residual urine (PVR) measurement is important.
–If the PVR is high, the bladder may be acontractile or the bladder outlet may be obstructed.
–Both of these conditions will cause urinary retention with overflow incontinence.
•Uroflow rate:
–Uroflow rate is volume of urine voided per unit of time.
–It is used to evaluate bladder outlet obstruction.
–Low uroflow rate may reflect urethral obstruction, a weak detrusor, or a combination of both.
–This test alone cannot distinguish an obstruction from a contractile detrusor.
•Urodynamic studies are essential for the diagnosis and management of children with neurogenic bladder.
•It is important to determine several urodynamic parameters, including:
–Bladder capacity
–Bladder compliance
–Intravesical-filling pressure
–Intravesical pressure at the moment of urethral leakage
–Presence or absence of reflex
–Detrusor activity
–Competence of the internal and external sphincteric mechanisms
–Degree of coordination of the detrusor and sphincteric mechanisms
–Voiding pattern
–Postvoiding residual urine volume
–Detrusor and abdominal storage
–Voiding pressures
–Urine flow rate
–Postvoiding residual volume
–The relationship between detrusor contraction and the urinary sphincter
–If contrast is instilled in the bladder, the anatomy can be imaged during voiding
•A urodynamic study consists of the followings:
–The child is catheterized with a triplelumen urodynamic catheter after lubricating the urethra with 1 % liquid lidocaine.
–The intravesical pressure is recorded first.
–The bladder is drained and the residual urine carefully measured and the residual volume pressure is determined.
–This helps determine detrusor compliance at natural filling and is more accurate than cystometric compliance measured during even slow filling of the bladder.
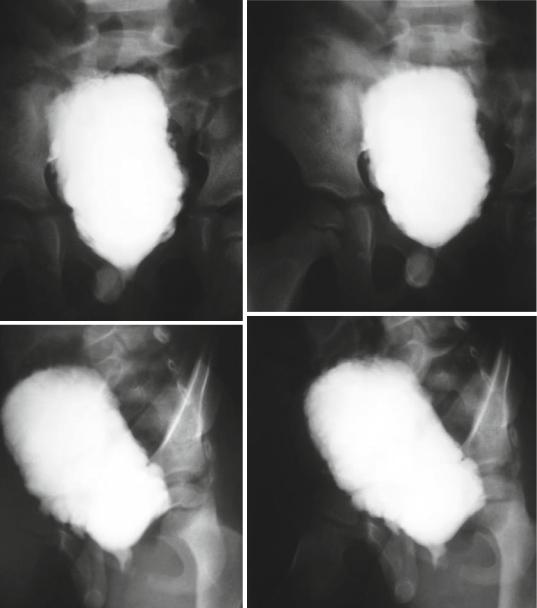
11.5 Investigations and Diagnosis |
311 |
|
|
Figs. 11.4, 11.5, 11.6, and 11.7 Micturating cystourethrograms showing neurogenic bladder
–A small balloon catheter is passed into the rectum to measure intra-abdominal pressure during the filling and emptying phases of the study.
–The side-hole port of the urethral pressure channel is positioned at the highest point of resistance in the urethra and kept in place.
–This measures resistance throughout bladder filling and emptying to determine the leak point pressure.
–External urethral sphincter electromyography (EMG) is performed using a 24-gauge concentric needle electrode inserted perineally in boys or para-urethrally in girls and advanced into the skeletal muscle component of the sphincter until individual motor unit action potentials are seen or heard on a standard EMG recorder.
–The characteristics of the individual motor unit potentials at rest, in response to various
312 |
11 Neurogenic Bladder Sphincter Dysfunction |
|
|
sacral reflexes (i.e. bulbocavernosus, anocutaneous, Valsalva and Credé maneuvers) and bladder filling and emptying are recorded to detect degrees of denervation.
–Next, the bladder is filled through the second port while intravesical pressure is monitored via the third port of the tri-lumen urodynamic catheter.
–The rate of bladder filling is set at 10 % of expected capacity for age.
–The expected bladder capacity in milliliters = age (in years) +30 × 30.
–Detrusor pressure measurements are continuously recorded throughout filling to calculate compliance, and during voiding or leaking to denote emptying pressure.
–Detrusor overactivity:
• This is defined as any short-lived pressure rise of >15 cm H2O from baseline before capacity is reached.
–Sometimes, the urodynamics study is combined with fluoroscopic video-imaging using a dilute radio-opaque contrast agent to visualize the appearance of the bladder wall and bladder neck or to detect the presence of vesicoureteral reflux during the test.
–Alternatively, a radionuclide agent is instilled, with the patient lying above a nuclear camera, to determine at what pressure reflux occurs, when it is known to be present beforehand.
–The study is not considered complete until the child actually urinates or leaks and the ‘voiding’ pressure is measured.
–The small size of the urodynamic catheter does not seem to affect the voiding pressure adversely, even in very young children.
–The normal end filling pressure should be <10 cm H2O, while the normal voiding pressure varies from 55 to 80 cm H2O in boys and from 30 to 65 cm H2O in girls.
–Urodynamic assessment can provide reproducible results in newborns and infants, but it requires attention to mechanical factors and filling rates.
•The main urodynamic study is cystometrography (CMG).
–A small catheter is placed in the bladder, and the bladder is slowly filled with liquid.
–Pressures within the bladder (intravesical) and the abdominal compartment are measured, and by subtracting the abdominal pressure from the intravesical pressure, the pressure generated by the detrusor muscle can be calculated.
–Because the child is monitored through a filling and voiding phase, bladder capacity can be quantified, and the urine flow rate, postvoiding residual volume, and the force generated by a bladder contraction can be measured.
–A filling cystometrogram assesses the bladder capacity, compliance, and the presence of phasic contractions (detrusor instability).
–A voiding cystometrogram (pressure-flow study) simultaneously records the voiding detrusor pressure and the rate of urinary flow. This is the only test able to assess bladder contractility and the extent of a bladder outlet obstruction.
•Electromyography
–If more information is desired, electromyography (EMG) can be used to demonstrate the relationship between the detrusor muscle and the external urinary sphincter.
–Electromyography (EMG) helps to ascertain the presence of coordinated or uncoordinated voiding. Failure of urethral relaxation during bladder contraction results in uncoordinated voiding (detrusor sphincter dyssynergia). During normal voiding, the sphincter relaxes as the detrusor muscle contracts to allow unobstructed urinary flow.
–Spinal cord injury can lead to discoordination so that the sphincter is closed when the detrusor contracts, creating high pressures within the bladder but low flow rates.
–This is known as detrusor-sphincter dyssynergy (DSD).
–EMG allows accurate diagnosis of detrusor sphincter dyssynergia common in spinal cord injuries
–In infants with DSD, increased EMG activity occurs during voiding.
–The presence of DSD places infants at a much greater risk of upper urinary tract deterioration.
11.6 Classification of Neurogenic Bladder |
313 |
|
|
•Fluoroscopy
–Fluoroscopy can be used to perform videourodynamic imaging with contrast enhancement of the bladder, which allows the bladder to be depicted during voiding.
–In addition, reflux may be identified.
–If a closed sphincter is revealed during voiding, this finding strongly suggest the presence of DSD, often obviating the need for EMG studies.
•Videourodynamics
–Videourodynamics is important for evaluation of a patient with incontinence.
–Videourodynamics combines the radiographic findings of voiding cystourethrogram (VCUG) and multichannel urodynamics.
–Videourodynamics enables documentation of lower urinary tract anatomy, such as vesicoureteral reflux and bladder diverticulum, as well as the functional pressure-flow relationship between the bladder and the urethra.
•The urodynamics examination findings are considered normal when there is:
–An appropriate capacity
–A good compliant bladder
–No overactivity
–Normal innervation of the sphincter with normal sacral reflexes
–An increase in sphincter activity during filling and complete silencing during emptying.
•An upper motor neuron lesion is present when there is:
–Detrusor overactivity
–And/or hyperactive EMG responses to sacral reflexes
–And/or a failure of the sphincter muscle, on EMG, to relax (either partially or completely) with a bladder contraction or leaking at capacity.
•A lower motor neuron lesion is present when there are:
–No contractions of the detrusor muscle
–And/or there is a degree of denervation, either partial or complete, in the sphincter muscle, with characteristic EMG changes in the motor units or no motor unit activity at all, respectively
–And little or no response in the sphincter to sacral reflexes and/or bladder filling or emptying.
11.6Classification of Neurogenic Bladder
•There are various systems of classification of neurogenic bladder.
•Urodynamic and functional classifications have been more practical for defining the extent of the pathology and planning treatment in children.
•The main classification of neurogenic bladder is that based on urodynamic findings.
•The bladder and sphincter are two units working in harmony to make a single functional unit.
•The initial approach should be to evaluate the state of each unit and define the pattern of bladder dysfunction.
•This depends on the nature of the neurological deficit:
–The bladder may be overactive with increased contractions, low capacity and compliance or inactive with no effective contractions.
–The outlet sphincter may be independently overactive causing functional obstruction or paralyzed with no resistance to urinary flow.
–These conditions may present in different combinations.
•This is important to plan a rational treatment for each individual patient.
•Urodynamic Studies are important to evaluate the lower urinary tract function and its deviations from normal.
•Since the treatment plan mainly depends upon a good understanding of the underlying problem in the lower urinary tract, a well performed urodynamic study is mandatory in the evaluation of each child with neurogenic bladder.
•A urodynamic study is also important to assess the response of the vesicourethral unit to therapy.
•In meningomyelocoele, most patients will present with hyperreflexive detrusor and dyssynergic sphincter, which is a dangerous combination as pressure is built up and the upper urinary tract is threatened.
