
- •Preface
- •Acknowledgments
- •Contents
- •1.1 Introduction
- •1.2 Normal Embryology
- •1.3 Abnormalities of the Kidney
- •1.3.1 Renal Agenesis
- •1.3.2 Renal Hypoplasia
- •1.3.3 Supernumerary Kidneys
- •1.3.5 Polycystic Kidney Disease
- •1.3.6 Simple (Solitary) Renal Cyst
- •1.3.7 Renal Fusion and Renal Ectopia
- •1.3.8 Horseshoe Kidney
- •1.3.9 Crossed Fused Renal Ectopia
- •1.4 Abnormalities of the Ureter
- •1.5 Abnormalities of the Bladder
- •1.6 Abnormalities of the Penis and Urethra in Males
- •1.7 Abnormalities of Female External Genitalia
- •Further Reading
- •2.1 Introduction
- •2.2 Pathophysiology
- •2.3 Etiology of Hydronephrosis
- •2.5 Clinical Features
- •2.6 Investigations and Diagnosis
- •2.7 Treatment
- •2.8 Antenatal Hydronephrosis
- •Further Reading
- •3.1 Introduction
- •3.2 Embryology
- •3.3 Pathophysiology
- •3.4 Etiology of PUJ Obstruction
- •3.5 Clinical Features
- •3.6 Diagnosis and Investigations
- •3.7 Management of Newborns with PUJ Obstruction
- •3.8 Treatment
- •3.9 Post-operative Complications and Follow-Up
- •Further Reading
- •4: Renal Tumors in Children
- •4.1 Introduction
- •4.2 Wilms’ Tumor
- •4.2.1 Introduction
- •4.2.2 Etiology
- •4.2.3 Histopathology
- •4.2.4 Nephroblastomatosis
- •4.2.5 Clinical Features
- •4.2.6 Risk Factors for Wilms’ Tumor
- •4.2.7 Staging of Wilms Tumor
- •4.2.8 Investigations
- •4.2.9 Prognosis and Complications of Wilms Tumor
- •4.2.10 Surgical Considerations
- •4.2.11 Surgical Complications
- •4.2.12 Prognosis and Outcome
- •4.2.13 Extrarenal Wilms’ Tumors
- •4.3 Mesoblastic Nephroma
- •4.3.1 Introduction
- •4.3.3 Epidemiology
- •4.3.5 Clinical Features
- •4.3.6 Investigations
- •4.3.7 Treatment and Prognosis
- •4.4 Clear Cell Sarcoma of the Kidney (CCSK)
- •4.4.1 Introduction
- •4.4.2 Pathophysiology
- •4.4.3 Clinical Features
- •4.4.4 Investigations
- •4.4.5 Histopathology
- •4.4.6 Treatment
- •4.4.7 Prognosis
- •4.5 Malignant Rhabdoid Tumor of the Kidney
- •4.5.1 Introduction
- •4.5.2 Etiology and Pathophysiology
- •4.5.3 Histologic Findings
- •4.5.4 Clinical Features
- •4.5.5 Investigations and Diagnosis
- •4.5.6 Treatment and Outcome
- •4.5.7 Mortality/Morbidity
- •4.6 Renal Cell Carcinoma in Children
- •4.6.1 Introduction
- •4.6.2 Histopathology
- •4.6.4 Staging
- •4.6.5 Clinical Features
- •4.6.6 Investigations
- •4.6.7 Management
- •4.6.8 Prognosis
- •4.7 Angiomyolipoma of the Kidney
- •4.7.1 Introduction
- •4.7.2 Histopathology
- •4.7.4 Clinical Features
- •4.7.5 Investigations
- •4.7.6 Treatment and Prognosis
- •4.8 Renal Lymphoma
- •4.8.1 Introduction
- •4.8.2 Etiology and Pathogenesis
- •4.8.3 Diagnosis
- •4.8.4 Clinical Features
- •4.8.5 Treatment and Prognosis
- •4.9 Ossifying Renal Tumor of Infancy
- •4.10 Metanephric Adenoma
- •4.10.1 Introduction
- •4.10.2 Histopathology
- •4.10.3 Diagnosis
- •4.10.4 Clinical Features
- •4.10.5 Treatment
- •4.11 Multilocular Cystic Renal Tumor
- •Further Reading
- •Wilms’ Tumor
- •Mesoblastic Nephroma
- •Renal Cell Carcinoma in Children
- •Angiomyolipoma of the Kidney
- •Renal Lymphoma
- •Ossifying Renal Tumor of Infancy
- •Metanephric Adenoma
- •Multilocular Cystic Renal Tumor
- •5.1 Introduction
- •5.2 Embryology
- •5.4 Histologic Findings
- •5.7 Associated Anomalies
- •5.8 Clinical Features
- •5.9 Investigations
- •5.10 Treatment
- •Further Reading
- •6: Congenital Ureteral Anomalies
- •6.1 Etiology
- •6.2 Clinical Features
- •6.3 Investigations and Diagnosis
- •6.4 Duplex (Duplicated) System
- •6.4.1 Introduction
- •6.4.3 Clinical Features
- •6.4.4 Investigations
- •6.4.5 Treatment and Prognosis
- •6.5 Ectopic Ureter
- •6.5.1 Introduction
- •6.5.3 Clinical Features
- •6.5.4 Diagnosis
- •6.5.5 Surgical Treatment
- •6.6 Ureterocele
- •6.6.1 Introduction
- •6.6.3 Clinical Features
- •6.6.4 Investigations and Diagnosis
- •6.6.5 Treatment
- •6.6.5.1 Surgical Interventions
- •6.8 Mega Ureter
- •Further Reading
- •7: Congenital Megaureter
- •7.1 Introduction
- •7.3 Etiology and Pathophysiology
- •7.4 Clinical Presentation
- •7.5 Investigations and Diagnosis
- •7.6 Treatment and Prognosis
- •7.7 Complications
- •Further Reading
- •8.1 Introduction
- •8.2 Pathophysiology
- •8.4 Etiology of VUR
- •8.5 Clinical Features
- •8.6 Investigations
- •8.7 Management
- •8.7.1 Medical Treatment of VUR
- •8.7.2 Antibiotics Used for Prophylaxis
- •8.7.3 Anticholinergics
- •8.7.4 Surveillance
- •8.8 Surgical Therapy of VUR
- •8.8.1 Indications for Surgical Interventions
- •8.8.2 Indications for Surgical Interventions Based on Age at Diagnosis and the Presence or Absence of Renal Lesions
- •8.8.3 Endoscopic Injection
- •8.8.4 Surgical Management
- •8.9 Mortality/Morbidity
- •Further Reading
- •9: Pediatric Urolithiasis
- •9.1 Introduction
- •9.2 Etiology
- •9.4 Clinical Features
- •9.5 Investigations
- •9.6 Complications of Urolithiasis
- •9.7 Management
- •Further Reading
- •10.1 Introduction
- •10.2 Embryology of Persistent Müllerian Duct Syndrome
- •10.3 Etiology and Inheritance of PMDS
- •10.5 Clinical Features
- •10.6 Treatment
- •10.7 Prognosis
- •Further Reading
- •11.1 Introduction
- •11.2 Physiology and Bladder Function
- •11.2.1 Micturition
- •11.3 Pathophysiological Changes of NBSD
- •11.4 Etiology and Clinical Features
- •11.5 Investigations and Diagnosis
- •11.7 Management
- •11.8 Clean Intermittent Catheterization
- •11.9 Anticholinergics
- •11.10 Botulinum Toxin Type A
- •11.11 Tricyclic Antidepressant Drugs
- •11.12 Surgical Management
- •Further Reading
- •12.1 Introduction
- •12.2 Etiology
- •12.3 Pathophysiology
- •12.4 Clinical Features
- •12.5 Investigations and Diagnosis
- •12.6 Management
- •Further Reading
- •13.1 Introduction
- •13.2 Embryology
- •13.3 Epispadias
- •13.3.1 Introduction
- •13.3.2 Etiology
- •13.3.4 Treatment
- •13.3.6 Female Epispadias
- •13.3.7 Surgical Repair of Female Epispadias
- •13.3.8 Prognosis
- •13.4 Bladder Exstrophy
- •13.4.1 Introduction
- •13.4.2 Associated Anomalies
- •13.4.3 Principles of Surgical Management of Bladder Exstrophy
- •13.4.4 Evaluation and Management
- •13.5 Cloacal Exstrophy
- •13.5.1 Introduction
- •13.5.2 Skeletal Changes in Cloacal Exstrophy
- •13.5.3 Etiology and Pathogenesis
- •13.5.4 Prenatal Diagnosis
- •13.5.5 Associated Anomalies
- •13.5.8 Surgical Reconstruction
- •13.5.9 Management of Urinary Incontinence
- •13.5.10 Prognosis
- •13.5.11 Complications
- •Further Reading
- •14.1 Introduction
- •14.2 Etiology
- •14.3 Clinical Features
- •14.4 Associated Anomalies
- •14.5 Diagnosis
- •14.6 Treatment and Prognosis
- •Further Reading
- •15: Cloacal Anomalies
- •15.1 Introduction
- •15.2 Associated Anomalies
- •15.4 Clinical Features
- •15.5 Investigations
- •Further Reading
- •16: Urachal Remnants
- •16.1 Introduction
- •16.2 Embryology
- •16.4 Clinical Features
- •16.5 Tumors and Urachal Remnants
- •16.6 Management
- •Further Reading
- •17: Inguinal Hernias and Hydroceles
- •17.1 Introduction
- •17.2 Inguinal Hernia
- •17.2.1 Incidence
- •17.2.2 Etiology
- •17.2.3 Clinical Features
- •17.2.4 Variants of Hernia
- •17.2.6 Treatment
- •17.2.7 Complications of Inguinal Herniotomy
- •17.3 Hydrocele
- •17.3.1 Embryology
- •17.3.3 Treatment
- •Further Reading
- •18: Cloacal Exstrophy
- •18.1 Introduction
- •18.2 Etiology and Pathogenesis
- •18.3 Associated Anomalies
- •18.4 Clinical Features and Management
- •Further Reading
- •19: Posterior Urethral Valve
- •19.1 Introduction
- •19.2 Embryology
- •19.3 Pathophysiology
- •19.5 Clinical Features
- •19.6 Investigations and Diagnosis
- •19.7 Management
- •19.8 Medications Used in Patients with PUV
- •19.10 Long-Term Outcomes
- •19.10.3 Bladder Dysfunction
- •19.10.4 Renal Transplantation
- •19.10.5 Fertility
- •Further Reading
- •20.1 Introduction
- •20.2 Embryology
- •20.4 Clinical Features
- •20.5 Investigations
- •20.6 Treatment
- •20.7 The Müllerian Duct Cyst
- •Further Reading
- •21: Hypospadias
- •21.1 Introduction
- •21.2 Effects of Hypospadias
- •21.3 Embryology
- •21.4 Etiology of Hypospadias
- •21.5 Associated Anomalies
- •21.7 Clinical Features of Hypospadias
- •21.8 Treatment
- •21.9 Urinary Diversion
- •21.10 Postoperative Complications
- •Further Reading
- •22: Male Circumcision
- •22.1 Introduction
- •22.2 Anatomy and Pathophysiology
- •22.3 History of Circumcision
- •22.4 Pain Management
- •22.5 Indications for Circumcision
- •22.6 Contraindications to Circumcision
- •22.7 Surgical Procedure
- •22.8 Complications of Circumcision
- •Further Reading
- •23: Priapism in Children
- •23.1 Introduction
- •23.2 Pathophysiology
- •23.3 Etiology
- •23.5 Clinical Features
- •23.6 Investigations
- •23.7 Management
- •23.8 Prognosis
- •23.9 Priapism and Sickle Cell Disease
- •23.9.1 Introduction
- •23.9.2 Epidemiology
- •23.9.4 Pathophysiology
- •23.9.5 Clinical Features
- •23.9.6 Treatment
- •23.9.7 Prevention of Stuttering Priapism
- •23.9.8 Complications of Priapism and Prognosis
- •Further Reading
- •24.1 Introduction
- •24.2 Embryology and Normal Testicular Development and Descent
- •24.4 Causes of Undescended Testes and Risk Factors
- •24.5 Histopathology
- •24.7 Clinical Features and Diagnosis
- •24.8 Treatment
- •24.8.1 Success of Surgical Treatment
- •24.9 Complications of Orchidopexy
- •24.10 Infertility and Undescended Testes
- •24.11 Undescended Testes and the Risk of Cancer
- •Further Reading
- •25: Varicocele
- •25.1 Introduction
- •25.2 Etiology
- •25.3 Pathophysiology
- •25.4 Grading of Varicoceles
- •25.5 Clinical Features
- •25.6 Diagnosis
- •25.7 Treatment
- •25.8 Postoperative Complications
- •25.9 Prognosis
- •Further Reading
- •26.1 Introduction
- •26.2 Etiology and Risk Factors
- •26.3 Diagnosis
- •26.4 Intermittent Testicular Torsion
- •26.6 Effects of Testicular Torsion
- •26.7 Clinical Features
- •26.8 Treatment
- •26.9.1 Introduction
- •26.9.2 Etiology of Extravaginal Torsion
- •26.9.3 Clinical Features
- •26.9.4 Treatment
- •26.10 Torsion of the Testicular or Epididymal Appendage
- •26.10.1 Introduction
- •26.10.2 Embryology
- •26.10.3 Clinical Features
- •26.10.4 Investigations and Treatment
- •Further Reading
- •27: Testicular Tumors in Children
- •27.1 Introduction
- •27.4 Etiology of Testicular Tumors
- •27.5 Clinical Features
- •27.6 Staging
- •27.6.1 Regional Lymph Node Staging
- •27.7 Investigations
- •27.8 Treatment
- •27.9 Yolk Sac Tumor
- •27.10 Teratoma
- •27.11 Mixed Germ Cell Tumor
- •27.12 Stromal Tumors
- •27.13 Simple Testicular Cyst
- •27.14 Epidermoid Cysts
- •27.15 Testicular Microlithiasis (TM)
- •27.16 Gonadoblastoma
- •27.17 Cystic Dysplasia of the Testes
- •27.18 Leukemia and Lymphoma
- •27.19 Paratesticular Rhabdomyosarcoma
- •27.20 Prognosis and Outcome
- •Further Reading
- •28: Splenogonadal Fusion
- •28.1 Introduction
- •28.2 Etiology
- •28.4 Associated Anomalies
- •28.5 Clinical Features
- •28.6 Investigations
- •28.7 Treatment
- •Further Reading
- •29: Acute Scrotum
- •29.1 Introduction
- •29.2 Torsion of Testes
- •29.2.1 Introduction
- •29.2.3 Etiology
- •29.2.4 Clinical Features
- •29.2.5 Effects of Torsion of Testes
- •29.2.6 Investigations
- •29.2.7 Treatment
- •29.3 Torsion of the Testicular or Epididymal Appendage
- •29.3.1 Introduction
- •29.3.2 Embryology
- •29.3.3 Clinical Features
- •29.3.4 Investigations and Treatment
- •29.4.1 Introduction
- •29.4.2 Etiology
- •29.4.3 Clinical Features
- •29.4.4 Investigations and Treatment
- •29.5 Idiopathic Scrotal Edema
- •29.6 Testicular Trauma
- •29.7 Other Causes of Acute Scrotum
- •29.8 Splenogonadal Fusion
- •Further Reading
- •30.1 Introduction
- •30.2 Imperforate Hymen
- •30.3 Vaginal Atresia
- •30.5 Associated Anomalies
- •30.6 Embryology
- •30.7 Clinical Features
- •30.8 Investigations
- •30.9 Management
- •Further Reading
- •31: Disorders of Sexual Development
- •31.1 Introduction
- •31.2 Embryology
- •31.3 Sexual and Gonadal Differentiation
- •31.5 Evaluation of a Newborn with DSD
- •31.6 Diagnosis and Investigations
- •31.7 Management of Patients with DSD
- •31.8 Surgical Corrections of DSD
- •31.9 Congenital Adrenal Hyperplasia (CAH)
- •31.10 Androgen Insensitivity Syndrome (Testicular Feminization Syndrome)
- •31.13 Gonadal Dysgenesis
- •31.15 Ovotestis Disorders of Sexual Development
- •31.16 Other Rare Disorders of Sexual Development
- •Further Reading
- •Index
4.2 Wilms’ Tumor |
123 |
|
|
–Renal function can be impaired in those with bilateral Wilms tumor.
–The risk factors for renal damage include:
•Stromal predominant histology
•Intralobar rests
• Age at diagnosis of younger than
24 months
•Metachronous bilateral Wilms tumor
•WT1 mutation etiology.
•Hepatic complications:
–Hepatic damage in patients with Wilms tumor can be caused by radiation therapy and the use of cytotoxic drugs particularly dactinomycin and vincristine.
–The dactinomycin hepatotoxicity is dose-related.
–Radiotherapy is the main cause of hepatic toxicity but this rare nowadays.
–Hepatic toxicity was reported in 2.8–14.3% of children who did not receive radiotherapy.
–Some patients with Wilms tumor have developed hepatic veno-occlusive disease.
•Hepatic veno-occlusive disease is characterized by hepatomegaly or pain in the right upper quadrant, jaundice, ascites, and unexplained weight gain.
•It is seen in children with Wilms tumor undergoing nephrectomy first and in those receiving combination chemotherapy before surgery.
•Other complications include:
–Congestive heart failure is a well-known complication of the administration of anthracyclines (e.g. doxorubicin).
–Radiation-induced pulmonary complications. This is more commonly seen in those who receive bilateral pulmonary irradiation.
–Women who received whole-abdomen irradiation in childhood can develop ovarian failure.
–Male patients are at risk for testicular failure after whole-abdomen radiation therapy or certain types of chemotherapy, most notably that involving alkylating agents.
–Radiation therapy may affect the growth of any given bone but the spine is most notably affected. The effect is dose related and can lead to scoliosis.
–There is an increased risk for second malignant neoplasm following chemotherapy and or radiotherapy.
•Most secondary malignant neoplasms reported (e.g., bone tumors, breast and thyroid cancers) have occurred in irradiated areas.
•Certain chemotherapeutic agents, including doxorubicin, dactinomycin, and vincristine, may contribute to an increased risk for secondary malignancies.
•A recent interesting finding was that female survivors of Wilms tumor have a 9.1-fold increased risk of developing invasive breast cancer and had an estimated cumulative risk of invasive breast cancer at age 40 of 4.5 %. The risk was highest among children who had been treated with chest radiotherapy.
4.2.10 Surgical Considerations
•According to Children’s Oncology Group (COG), the treatment of Wilms tumor is nephrectomy followed by chemotherapy, with or without postoperative radiotherapy.
•Certain children with stage I disease and favorable histology do well with nephrectomy alone.
•Radiotherapy:
–Postoperative radiotherapy is started within 14 days of nephrectomy.
–The current dose for radiation therapy for favorable histology Wilms tumor is approximately 1,080 cGy for the abdomen and 1,200 cGy for the lung.
–Patients with stage IV favorable histology Wilms tumor and lung metastases whose pulmonary lesions do not disappear after 6 weeks of chemotherapy receive wholelung radiation therapy.
•In North America:
–Patients with suspected Wilms’ tumor undergo nephrectomy immediately.
–During this procedure, the contralateral kidney is explored to ensure that Wilms’ tumor is unilateral.
124 |
4 Renal Tumors in Children |
|
|
–Currently, may surgeons will not explore the contralateral kidney and will depend on preoperative CT-scan or MRI evaluation.
–Lymph node biopsy samples are obtained for staging purposes.
–Immediate nephrectomy is not performed in patients with bilateral Wilms’ tumor or those with very large unresectable Wilms tumor.
•In most European centers:
–A presumptive diagnosis of Wilms’ tumor is made based on imaging findings alone.
–Administer chemotherapy before nephrectomy.
•Transcutaneous biopsy is not usually recommended and may in fact complicate treatment by causing preoperative tumor spill, requiring whole abdominal radiotherapy.
•Aspiration cytology is a valuable investigation to diagnose Wilms’ tumor but it requires a good pathologist to read it (Fig. 4.51).
•The usual approach in most patients is nephrectomy followed by chemotherapy, with or without postoperative radiotherapy.
•Children found to have loss of heterozygosity at 1p and 16q receive more aggressive chemotherapy because they have a worse prognosis than do children without this heterozygosity loss.
•Children younger than age 12 months diagnosed with perilobar nephrogenic rests have a markedly increased risk of developing a contralateral Wilms’ tumor.
•The National Wilms’ Tumor Study Group (NWTSG) and the International Society of Pediatric Oncology (SIOP) have identified several chemotherapeutic agents through their clinical trials.
•At present, survival rates of children with Wilms’ tumor are approximately 80–90 %.
•Chemotherapy without initial surgical resection can be used in the following situations:
–Inoperable tumors (Figs. 4.52, 4.53, 4.54, 4.55, 4.56, and 4.57):
•Large tumors that involve vital structures make resection difficult; the complication rate is high, and the incidence of tumor rupture and spill is also high.
–Intracaval tumor extension:
•This occurs in 5 % of cases of Wilms’ tumor.
•This is associated with a 40 % rate of surgical complications.
•Chemotherapy after staging and biopsy is beneficial in reducing the tumor and thrombus size.
–Bilateral Wilms’ tumor (Figs. 4.58, 4.59, 4.60, 4.61, 4.62, and 4.63).
–SIOP advocates chemotherapy without previous laparotomy and biopsy. The NWTSG suggests that this approach results in a 1–5 % risk of treating a benign disease.
–Chemotherapy without proper surgical staging (e.g., staging by means of imaging
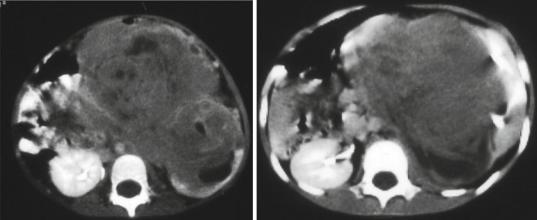
Figs. 4.52 and 4.53 CT-can showing large Wilms Tumors that appear inoperable
4.2 Wilms’ Tumor |
125 |
|
|
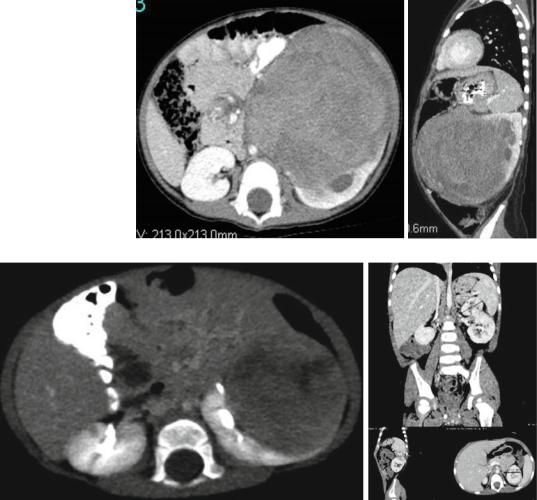
Figs. 4.54 and
4.55 CT-scan showing large left sided Wilms tumor
Figs. 4.56 and 4.57 Radiological evaluation of a large left Wilms tumor that was treated with preoperative chemotherapy showing an excellent response
studies only) may alter the actual initial stage of the disease by the time of surgery and may subsequently alter decisions regarding the adjuvant chemotherapy and radiation therapy, which is based on the surgical staging.
•Postoperative chemotherapy and radiotherapy protocols are based on the surgical staging and follow the guidelines of the NWTSG.
•Stage I:
–Nephrectomy ±18 weeks of chemotherapy depending on age of the patient and weight of tumor.
–A child less than 2 years old and a tumor less than 550 g only requires Nephrectomy and observation.
•Stage II:
–Nephrectomy + abdominal radiation + 24 weeks of chemotherapy.
•Stage III:
–Abdominal radiation + 24 weeks of chemotherapy + nephrectomy after tumor shrinkage.
•Stage IV:
–Nephrectomy + abdominal radiation + 24 weeks of chemotherapy + radiation of metastatic site as appropriate.
126 |
4 Renal Tumors in Children |
|
|
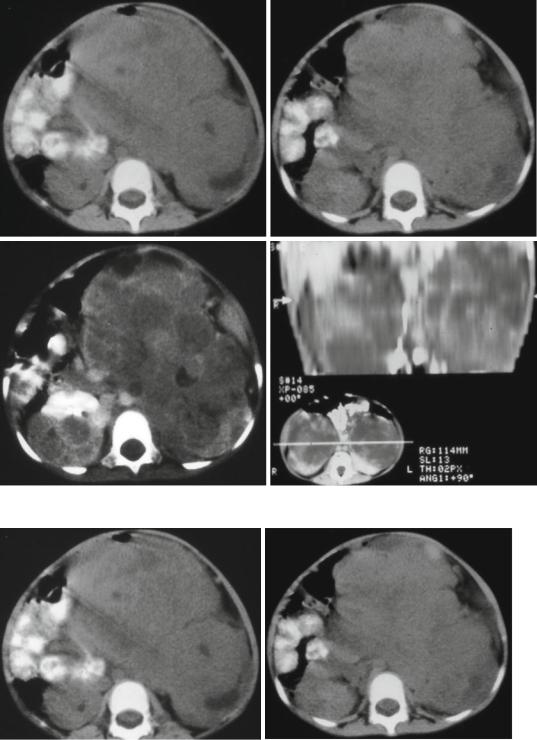
Figs. 4.58, 4.59, 4.60, and 4.61 Abdominal CT-scans showing bilateral Wilms tumors
Figs. 4.62 and 4.63 Abdominal CT-scans showing bilateral Wilms tumor before and after chemotherapy. Note the excellent response to chemotherapy with complete disap-
pearance of Wilms tumor on one side and marked reduction of the tumor on the other side
4.2 Wilms’ Tumor |
127 |
|
|
•Stage V:
–Individualized therapy based on tumor burden.
•The management of bilateral Wilms’ tumor must be individualized according to the extent of tumor present in both kidneys with a goal to preserving adequate kidney tissue to avoid kidney failure.
–The initial procedure should be biopsies of both kidneys to establish the diagnosis and histological types in both kidneys.
–Approximately 4 % of cases have different types between the two kidneys.
–The patient is treated with chemotherapy and restudied by abdominal CT or MRI to evaluate tumor response and determine whether a surgical procedure would be beneficial.
–If considerable tumor persists in both kidneys, additional chemotherapy is administered, and surgery is delayed.
–Radiation therapy is withheld if possible in these cases to reduce the risk of radiation injury to the remaining kidney tissue.
–In some patients, the tumor persists in both kidneys, and resection of the tumor with preservation of functioning kidney tissue is not possible. The only remaining option for these rare patients ultimately is removal of both kidneys.
•Stage I–IV Anaplasia:
–Children with stage I anaplastic tumors can be managed with the same regimen given
to stage I favorable histology patients.
–Children with stage II through stage IV diffuse anaplasia, however, represent a higherrisk group.
–These tumors are more resistant to the chemotherapy traditionally used in children with Wilms’ tumor (favorable histology), and require more aggressive regimens.
•About 5–10 % of patients with Wilms tumor present with acquired von Willebrand disease at the time of diagnosis.
•The reason for this acquired von Willebrand disease is not known ad several hypothesis have been postulated including:
–Absorption of the von Willebrand factor (vWF) by tumor cells.
–Hyperviscosity caused by elevated serum levels of hyaluronic acid.
–An immunoglobulin G (IgG)–type antibody that prevents aggregation of normal platelet cells.
•The presence of acquired von Willebrand disease in these patients will lead to excessive bleeding during surgery and should be treated preoperatively.
•Desmopressin (DDAVP), a drug that promotes the release of vWF from storage sites, is recommended.
•If DDAVP is ineffective, cryoprecipitator (a specific vWF concentrate) should be administered.
•Management of lung metastasis (Figs. 4.64, 4.65, and 4.66):
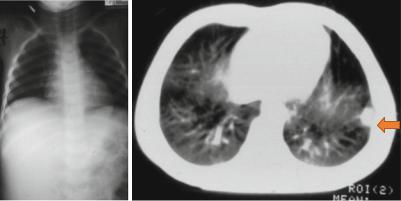
Figs. 4.64 and 4.65 Abdominal and chest x-ray in a patient with right Wilms tumor and normal looking chest x-ray and chest CT-scan showing a single secondary in the chest
128 |
4 Renal Tumors in Children |
|
|
–A normal chest x-ray during initial evaluation does not exclude the presence of pulmonary metastasis and a chest CT-scan should be done as part of their evaluation.
–Children with normal looking chest radiography and positive findings on chest CT-scan require tissue diagnosis of the lung nodules.
–It is important to have tissue diagnosis of the lung nodules because several conditions (e.g. histoplasmosis, atelectasis, pseudotumor, intrapulmonary lymph node, pneumonia) can mimic pulmonary metastases.
–These secondaries can be single or multiple.
–A biopsy can be performed percutaneously or thoracoscopically.
–Single secondaries can be excised totally while multiple lesions are biopsied.
–Patients with favorable histology Wilms’ tumor with lung metastasis and no other sites
Fig. 4.66 Chest CT-scan showing multiple bilateral pulmonary secondaries in a child with Wilms tumor
of distant spread or presence of 1p and 16q deletion are treated with 6 weeks of actinomy- cin-D, doxorubicin, and vincristine.
4.2.10.1Surgical Management
(Figs. 4.67 and 4.68)
•The first step in the treatment of Wilms’ tumor is surgical staging followed by radical nephrectomy, if possible.
•Begin the abdominal exploration through a transverse incision.
•The kidney is explored by mobilizing the ipsilateral colon and opening the Gerota fascia.
•Exploration of the contralateral kidney is currently not recommended because of the improvement in imaging techniques (computed tomography [CT] scanning, magnetic resonance imaging [MRI]).
•If the tumor is unresectable, biopsies are performed and the nephrectomy is deferred until after chemotherapy, which, in most cases, will shrink the tumor.
•Radical nephrectomy is the treatment of choice.
•If bilateral disease is diagnosed, nephrectomy is not performed, but biopsy specimens are obtained.
•New protocols in the management of bilateral Wilms’ tumor are being explored. If the disease is unilateral, radical nephrectomy and regional lymph node dissection or sampling are performed.
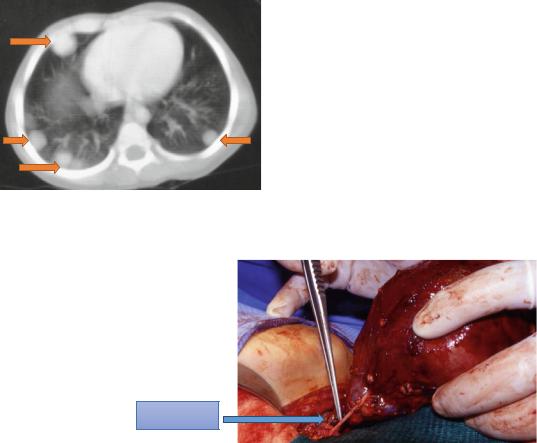
Fig. 4.67 A clinical intraoperative photograph showing radical nephrectomy for Wilms tumor. Note also
the ureter which should URETER be excised as low as
possible
4.2 Wilms’ Tumor |
129 |
|
|
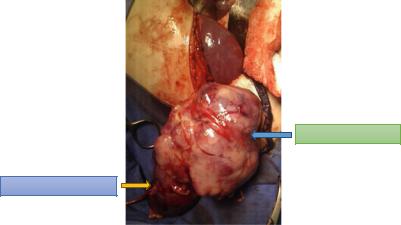
Fig. 4.68 A clinical intraoperative photograph showing radical nephrectomy for Wilms tumor
WILMS’ TUMOR
NORMAL KIDNEY
Stage and histology |
Surgery |
Chemotherapy |
Radiotherapy |
Stage I or II favorable |
Nephrectomy |
Vincristine, dactinomycin |
No |
histology without loss of |
|
|
|
heterozygosity (LOH) 1p |
|
|
|
and 16q |
|
|
|
Stage I or II favorable |
Nephrectomy |
Vincristine, dactinomycin, |
No |
histology with LOH 1p |
|
doxorubicin |
|
and 16q |
|
|
|
Stage III and IV favorable |
Nephrectomy |
Vincristine, dactinomycin, |
Yes |
histology without LOH 1p |
|
doxorubicin |
|
and 16q |
|
|
|
Stage III and IV favorable |
Nephrectomy |
Doxorubicin, |
Yes |
histology with LOH 1p |
|
cyclophosphamide, etoposide |
|
and 16q |
|
|
|
•Partial nephrectomy:
–The role of partial nephrectomy remains controversial.
–Partial nephrectomy may be feasible in only 10–15 % of patients, as most tumors are too large at initial diagnosis.
–The main concern regarding a nephronsparing procedure is that of local recurrence.
–The NWTS-4 study showed an 8 % local recurrence rate following partial nephrectomy for patients with bilateral disease.
–In the presence of bilateral Wilms’ tumors, solitary kidney, or renal insufficiency, partial nephrectomy is a reasonable consideration.
•If inferior vena cava (IVC) thrombus is present, preoperative chemotherapy will reduce the cavotomy rate by 50 %.
Bilateral Wilms’ tumor:
–With bilateral Wilms’ tumor (6 % of cases), surgical exploration, biopsy of both sides, and accurate surgical staging (including lymph node biopsy of both sides) are performed.
–This is followed by 6 weeks of chemotherapy that is appropriate to the stage and histology of the tumor.
–Reassessment is then performed using imaging studies, followed by definitive surgery which can be any of the followings:
•Unilateral radical nephrectomy and partial nephrectomy on the contralateral side.
•Bilateral partial nephrectomy.
•Unilateral nephrectomy only, if the response was complete on the opposite side. This approach dramatically
