
- •Preface
- •Acknowledgments
- •Contents
- •1.1 Introduction
- •1.2 Normal Embryology
- •1.3 Abnormalities of the Kidney
- •1.3.1 Renal Agenesis
- •1.3.2 Renal Hypoplasia
- •1.3.3 Supernumerary Kidneys
- •1.3.5 Polycystic Kidney Disease
- •1.3.6 Simple (Solitary) Renal Cyst
- •1.3.7 Renal Fusion and Renal Ectopia
- •1.3.8 Horseshoe Kidney
- •1.3.9 Crossed Fused Renal Ectopia
- •1.4 Abnormalities of the Ureter
- •1.5 Abnormalities of the Bladder
- •1.6 Abnormalities of the Penis and Urethra in Males
- •1.7 Abnormalities of Female External Genitalia
- •Further Reading
- •2.1 Introduction
- •2.2 Pathophysiology
- •2.3 Etiology of Hydronephrosis
- •2.5 Clinical Features
- •2.6 Investigations and Diagnosis
- •2.7 Treatment
- •2.8 Antenatal Hydronephrosis
- •Further Reading
- •3.1 Introduction
- •3.2 Embryology
- •3.3 Pathophysiology
- •3.4 Etiology of PUJ Obstruction
- •3.5 Clinical Features
- •3.6 Diagnosis and Investigations
- •3.7 Management of Newborns with PUJ Obstruction
- •3.8 Treatment
- •3.9 Post-operative Complications and Follow-Up
- •Further Reading
- •4: Renal Tumors in Children
- •4.1 Introduction
- •4.2 Wilms’ Tumor
- •4.2.1 Introduction
- •4.2.2 Etiology
- •4.2.3 Histopathology
- •4.2.4 Nephroblastomatosis
- •4.2.5 Clinical Features
- •4.2.6 Risk Factors for Wilms’ Tumor
- •4.2.7 Staging of Wilms Tumor
- •4.2.8 Investigations
- •4.2.9 Prognosis and Complications of Wilms Tumor
- •4.2.10 Surgical Considerations
- •4.2.11 Surgical Complications
- •4.2.12 Prognosis and Outcome
- •4.2.13 Extrarenal Wilms’ Tumors
- •4.3 Mesoblastic Nephroma
- •4.3.1 Introduction
- •4.3.3 Epidemiology
- •4.3.5 Clinical Features
- •4.3.6 Investigations
- •4.3.7 Treatment and Prognosis
- •4.4 Clear Cell Sarcoma of the Kidney (CCSK)
- •4.4.1 Introduction
- •4.4.2 Pathophysiology
- •4.4.3 Clinical Features
- •4.4.4 Investigations
- •4.4.5 Histopathology
- •4.4.6 Treatment
- •4.4.7 Prognosis
- •4.5 Malignant Rhabdoid Tumor of the Kidney
- •4.5.1 Introduction
- •4.5.2 Etiology and Pathophysiology
- •4.5.3 Histologic Findings
- •4.5.4 Clinical Features
- •4.5.5 Investigations and Diagnosis
- •4.5.6 Treatment and Outcome
- •4.5.7 Mortality/Morbidity
- •4.6 Renal Cell Carcinoma in Children
- •4.6.1 Introduction
- •4.6.2 Histopathology
- •4.6.4 Staging
- •4.6.5 Clinical Features
- •4.6.6 Investigations
- •4.6.7 Management
- •4.6.8 Prognosis
- •4.7 Angiomyolipoma of the Kidney
- •4.7.1 Introduction
- •4.7.2 Histopathology
- •4.7.4 Clinical Features
- •4.7.5 Investigations
- •4.7.6 Treatment and Prognosis
- •4.8 Renal Lymphoma
- •4.8.1 Introduction
- •4.8.2 Etiology and Pathogenesis
- •4.8.3 Diagnosis
- •4.8.4 Clinical Features
- •4.8.5 Treatment and Prognosis
- •4.9 Ossifying Renal Tumor of Infancy
- •4.10 Metanephric Adenoma
- •4.10.1 Introduction
- •4.10.2 Histopathology
- •4.10.3 Diagnosis
- •4.10.4 Clinical Features
- •4.10.5 Treatment
- •4.11 Multilocular Cystic Renal Tumor
- •Further Reading
- •Wilms’ Tumor
- •Mesoblastic Nephroma
- •Renal Cell Carcinoma in Children
- •Angiomyolipoma of the Kidney
- •Renal Lymphoma
- •Ossifying Renal Tumor of Infancy
- •Metanephric Adenoma
- •Multilocular Cystic Renal Tumor
- •5.1 Introduction
- •5.2 Embryology
- •5.4 Histologic Findings
- •5.7 Associated Anomalies
- •5.8 Clinical Features
- •5.9 Investigations
- •5.10 Treatment
- •Further Reading
- •6: Congenital Ureteral Anomalies
- •6.1 Etiology
- •6.2 Clinical Features
- •6.3 Investigations and Diagnosis
- •6.4 Duplex (Duplicated) System
- •6.4.1 Introduction
- •6.4.3 Clinical Features
- •6.4.4 Investigations
- •6.4.5 Treatment and Prognosis
- •6.5 Ectopic Ureter
- •6.5.1 Introduction
- •6.5.3 Clinical Features
- •6.5.4 Diagnosis
- •6.5.5 Surgical Treatment
- •6.6 Ureterocele
- •6.6.1 Introduction
- •6.6.3 Clinical Features
- •6.6.4 Investigations and Diagnosis
- •6.6.5 Treatment
- •6.6.5.1 Surgical Interventions
- •6.8 Mega Ureter
- •Further Reading
- •7: Congenital Megaureter
- •7.1 Introduction
- •7.3 Etiology and Pathophysiology
- •7.4 Clinical Presentation
- •7.5 Investigations and Diagnosis
- •7.6 Treatment and Prognosis
- •7.7 Complications
- •Further Reading
- •8.1 Introduction
- •8.2 Pathophysiology
- •8.4 Etiology of VUR
- •8.5 Clinical Features
- •8.6 Investigations
- •8.7 Management
- •8.7.1 Medical Treatment of VUR
- •8.7.2 Antibiotics Used for Prophylaxis
- •8.7.3 Anticholinergics
- •8.7.4 Surveillance
- •8.8 Surgical Therapy of VUR
- •8.8.1 Indications for Surgical Interventions
- •8.8.2 Indications for Surgical Interventions Based on Age at Diagnosis and the Presence or Absence of Renal Lesions
- •8.8.3 Endoscopic Injection
- •8.8.4 Surgical Management
- •8.9 Mortality/Morbidity
- •Further Reading
- •9: Pediatric Urolithiasis
- •9.1 Introduction
- •9.2 Etiology
- •9.4 Clinical Features
- •9.5 Investigations
- •9.6 Complications of Urolithiasis
- •9.7 Management
- •Further Reading
- •10.1 Introduction
- •10.2 Embryology of Persistent Müllerian Duct Syndrome
- •10.3 Etiology and Inheritance of PMDS
- •10.5 Clinical Features
- •10.6 Treatment
- •10.7 Prognosis
- •Further Reading
- •11.1 Introduction
- •11.2 Physiology and Bladder Function
- •11.2.1 Micturition
- •11.3 Pathophysiological Changes of NBSD
- •11.4 Etiology and Clinical Features
- •11.5 Investigations and Diagnosis
- •11.7 Management
- •11.8 Clean Intermittent Catheterization
- •11.9 Anticholinergics
- •11.10 Botulinum Toxin Type A
- •11.11 Tricyclic Antidepressant Drugs
- •11.12 Surgical Management
- •Further Reading
- •12.1 Introduction
- •12.2 Etiology
- •12.3 Pathophysiology
- •12.4 Clinical Features
- •12.5 Investigations and Diagnosis
- •12.6 Management
- •Further Reading
- •13.1 Introduction
- •13.2 Embryology
- •13.3 Epispadias
- •13.3.1 Introduction
- •13.3.2 Etiology
- •13.3.4 Treatment
- •13.3.6 Female Epispadias
- •13.3.7 Surgical Repair of Female Epispadias
- •13.3.8 Prognosis
- •13.4 Bladder Exstrophy
- •13.4.1 Introduction
- •13.4.2 Associated Anomalies
- •13.4.3 Principles of Surgical Management of Bladder Exstrophy
- •13.4.4 Evaluation and Management
- •13.5 Cloacal Exstrophy
- •13.5.1 Introduction
- •13.5.2 Skeletal Changes in Cloacal Exstrophy
- •13.5.3 Etiology and Pathogenesis
- •13.5.4 Prenatal Diagnosis
- •13.5.5 Associated Anomalies
- •13.5.8 Surgical Reconstruction
- •13.5.9 Management of Urinary Incontinence
- •13.5.10 Prognosis
- •13.5.11 Complications
- •Further Reading
- •14.1 Introduction
- •14.2 Etiology
- •14.3 Clinical Features
- •14.4 Associated Anomalies
- •14.5 Diagnosis
- •14.6 Treatment and Prognosis
- •Further Reading
- •15: Cloacal Anomalies
- •15.1 Introduction
- •15.2 Associated Anomalies
- •15.4 Clinical Features
- •15.5 Investigations
- •Further Reading
- •16: Urachal Remnants
- •16.1 Introduction
- •16.2 Embryology
- •16.4 Clinical Features
- •16.5 Tumors and Urachal Remnants
- •16.6 Management
- •Further Reading
- •17: Inguinal Hernias and Hydroceles
- •17.1 Introduction
- •17.2 Inguinal Hernia
- •17.2.1 Incidence
- •17.2.2 Etiology
- •17.2.3 Clinical Features
- •17.2.4 Variants of Hernia
- •17.2.6 Treatment
- •17.2.7 Complications of Inguinal Herniotomy
- •17.3 Hydrocele
- •17.3.1 Embryology
- •17.3.3 Treatment
- •Further Reading
- •18: Cloacal Exstrophy
- •18.1 Introduction
- •18.2 Etiology and Pathogenesis
- •18.3 Associated Anomalies
- •18.4 Clinical Features and Management
- •Further Reading
- •19: Posterior Urethral Valve
- •19.1 Introduction
- •19.2 Embryology
- •19.3 Pathophysiology
- •19.5 Clinical Features
- •19.6 Investigations and Diagnosis
- •19.7 Management
- •19.8 Medications Used in Patients with PUV
- •19.10 Long-Term Outcomes
- •19.10.3 Bladder Dysfunction
- •19.10.4 Renal Transplantation
- •19.10.5 Fertility
- •Further Reading
- •20.1 Introduction
- •20.2 Embryology
- •20.4 Clinical Features
- •20.5 Investigations
- •20.6 Treatment
- •20.7 The Müllerian Duct Cyst
- •Further Reading
- •21: Hypospadias
- •21.1 Introduction
- •21.2 Effects of Hypospadias
- •21.3 Embryology
- •21.4 Etiology of Hypospadias
- •21.5 Associated Anomalies
- •21.7 Clinical Features of Hypospadias
- •21.8 Treatment
- •21.9 Urinary Diversion
- •21.10 Postoperative Complications
- •Further Reading
- •22: Male Circumcision
- •22.1 Introduction
- •22.2 Anatomy and Pathophysiology
- •22.3 History of Circumcision
- •22.4 Pain Management
- •22.5 Indications for Circumcision
- •22.6 Contraindications to Circumcision
- •22.7 Surgical Procedure
- •22.8 Complications of Circumcision
- •Further Reading
- •23: Priapism in Children
- •23.1 Introduction
- •23.2 Pathophysiology
- •23.3 Etiology
- •23.5 Clinical Features
- •23.6 Investigations
- •23.7 Management
- •23.8 Prognosis
- •23.9 Priapism and Sickle Cell Disease
- •23.9.1 Introduction
- •23.9.2 Epidemiology
- •23.9.4 Pathophysiology
- •23.9.5 Clinical Features
- •23.9.6 Treatment
- •23.9.7 Prevention of Stuttering Priapism
- •23.9.8 Complications of Priapism and Prognosis
- •Further Reading
- •24.1 Introduction
- •24.2 Embryology and Normal Testicular Development and Descent
- •24.4 Causes of Undescended Testes and Risk Factors
- •24.5 Histopathology
- •24.7 Clinical Features and Diagnosis
- •24.8 Treatment
- •24.8.1 Success of Surgical Treatment
- •24.9 Complications of Orchidopexy
- •24.10 Infertility and Undescended Testes
- •24.11 Undescended Testes and the Risk of Cancer
- •Further Reading
- •25: Varicocele
- •25.1 Introduction
- •25.2 Etiology
- •25.3 Pathophysiology
- •25.4 Grading of Varicoceles
- •25.5 Clinical Features
- •25.6 Diagnosis
- •25.7 Treatment
- •25.8 Postoperative Complications
- •25.9 Prognosis
- •Further Reading
- •26.1 Introduction
- •26.2 Etiology and Risk Factors
- •26.3 Diagnosis
- •26.4 Intermittent Testicular Torsion
- •26.6 Effects of Testicular Torsion
- •26.7 Clinical Features
- •26.8 Treatment
- •26.9.1 Introduction
- •26.9.2 Etiology of Extravaginal Torsion
- •26.9.3 Clinical Features
- •26.9.4 Treatment
- •26.10 Torsion of the Testicular or Epididymal Appendage
- •26.10.1 Introduction
- •26.10.2 Embryology
- •26.10.3 Clinical Features
- •26.10.4 Investigations and Treatment
- •Further Reading
- •27: Testicular Tumors in Children
- •27.1 Introduction
- •27.4 Etiology of Testicular Tumors
- •27.5 Clinical Features
- •27.6 Staging
- •27.6.1 Regional Lymph Node Staging
- •27.7 Investigations
- •27.8 Treatment
- •27.9 Yolk Sac Tumor
- •27.10 Teratoma
- •27.11 Mixed Germ Cell Tumor
- •27.12 Stromal Tumors
- •27.13 Simple Testicular Cyst
- •27.14 Epidermoid Cysts
- •27.15 Testicular Microlithiasis (TM)
- •27.16 Gonadoblastoma
- •27.17 Cystic Dysplasia of the Testes
- •27.18 Leukemia and Lymphoma
- •27.19 Paratesticular Rhabdomyosarcoma
- •27.20 Prognosis and Outcome
- •Further Reading
- •28: Splenogonadal Fusion
- •28.1 Introduction
- •28.2 Etiology
- •28.4 Associated Anomalies
- •28.5 Clinical Features
- •28.6 Investigations
- •28.7 Treatment
- •Further Reading
- •29: Acute Scrotum
- •29.1 Introduction
- •29.2 Torsion of Testes
- •29.2.1 Introduction
- •29.2.3 Etiology
- •29.2.4 Clinical Features
- •29.2.5 Effects of Torsion of Testes
- •29.2.6 Investigations
- •29.2.7 Treatment
- •29.3 Torsion of the Testicular or Epididymal Appendage
- •29.3.1 Introduction
- •29.3.2 Embryology
- •29.3.3 Clinical Features
- •29.3.4 Investigations and Treatment
- •29.4.1 Introduction
- •29.4.2 Etiology
- •29.4.3 Clinical Features
- •29.4.4 Investigations and Treatment
- •29.5 Idiopathic Scrotal Edema
- •29.6 Testicular Trauma
- •29.7 Other Causes of Acute Scrotum
- •29.8 Splenogonadal Fusion
- •Further Reading
- •30.1 Introduction
- •30.2 Imperforate Hymen
- •30.3 Vaginal Atresia
- •30.5 Associated Anomalies
- •30.6 Embryology
- •30.7 Clinical Features
- •30.8 Investigations
- •30.9 Management
- •Further Reading
- •31: Disorders of Sexual Development
- •31.1 Introduction
- •31.2 Embryology
- •31.3 Sexual and Gonadal Differentiation
- •31.5 Evaluation of a Newborn with DSD
- •31.6 Diagnosis and Investigations
- •31.7 Management of Patients with DSD
- •31.8 Surgical Corrections of DSD
- •31.9 Congenital Adrenal Hyperplasia (CAH)
- •31.10 Androgen Insensitivity Syndrome (Testicular Feminization Syndrome)
- •31.13 Gonadal Dysgenesis
- •31.15 Ovotestis Disorders of Sexual Development
- •31.16 Other Rare Disorders of Sexual Development
- •Further Reading
- •Index

582 |
27 Testicular Tumors in Children |
|
|
•Stage grouping:
–Stage IA – T1 N0 M0 S0
–Stage IB – T2, 3, 4 N0 M0 S0
–Stage IS – Any T N0 M0 S1, 2, 3
–Stage IIA – Any T N1 M0 S0, 1
–Stage IIB – Any T N2 M0 S0, 1
–Stage IIC – Any T N3 M0 S0, 1
–Stage IIIA – Any T Any N M1a S0, 1
–Stage IIIB – Any T Any N M0, 1a S2
–Stage IIIC – Any T Any N M1a, 1b S3
27.7Investigations
•CBC and differential.
•Liver function tests, electrolytes, BUN and creatinine.
•A chest x-ray or chest CT will identify pulmonary metastases.
•20 % of yolk-sac tumors may present with metastases to the lung.
•Measurements of alpha-fetoprotein (AFP), human chorionic gonadotropin (beta-hCG), and lactate dehydrogenase (LDH) are important in the management of patients with testicular tumors such as seminomas.
•Tumor marker levels are used to assess response to treatment and to predict the likelihood of complete remission.
•Lactate dehydrogenase: The LDH level is an independent prognostic factor in patients with germ cell tumors (including seminoma). It is thought to reflect tumor burden.
•Tumor markers:
–These are important in the evaluation of testis tumors in children and adolescents.
–Both human chorionic gonadotropin (HCG) and alpha-fetoprotein (AFP) are important tumor markers.
–Yolk sac tumors do not elaborate HCG, and AFP is the only important tumor marker in prepubertal patients.
–AFP is a glycoprotein typically associated with the human fetus. AFP is found in nonseminomatous germ cell tumors, as well as in hepatocellular carcinomas, cirrhosis, hepatitis, and pregnancy.
–The half-life of AFP is approximately 5 days.
–Elevated AFP levels are rare in pure seminomas and indicate that nonseminomatous elements are also present (i.e., mixed tumor).
–AFP is elaborated by 90 % of yolk sac tumors in children.
–It is important to note that serum AFP levels can normally be as high as 50,000 ng/ mL in the newborn dropping to approximately 300 ng/mL by 2 months of age. AFP levels do not achieve “normal” values until nearly 1 year of age.
–Therefore, while an elevated AFP in a child over 1 year old with a testis tumor almost always reflects the presence of a yolk sac tumor, an “elevated” level in infants can occur in the setting of a benign tumor.
–The post-operative value of tumor markers is also important.
–Tumor marker serum levels should fall at a predictable rate based on the biological
half-life of each marker (approximately 5 days for AFP and 48 h for HCG).
–Failure of the markers to decline at the expected rate reflects the likely persistence of residual tumor.
–Persistently elevated AFP levels after surgery suggest tumor metastases or recurrence.
–Liver dysfunction can also cause falsepositive elevations of AFP levels.
–Beta-hCG is a glycoprotein typically produced by the placenta.
–Elevations in beta-hCG levels are found in the serum of approximately 15 % of patients with seminoma.
–The half-life of beta-hCG is approximately 22 h.
–Serum testosterone levels may be elevated in Leydig-cell tumors.
–Gonadoblastoma may elevate levels of beta-HCG.
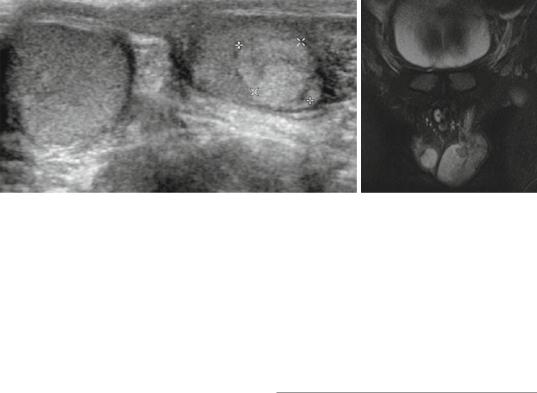
•Scrotal, abdominal and pelvic ultrasound (Figs. 27.11 and 27.12):
–Imaging of the primary tumor almost always begins with ultrasonography.
–Ultrasound distinguishes testicular from extratesticular masses and the ultrasono-
27.8 Treatment |
583 |
|
|
Figs. 27.11 and 27.12 Scrotal ultrasound and MRI showing left testicular tumor. Histological evaluation showed this to be Leydig cell tumor
graphic appearance of specific testis tumors has been described.
–The ultrasonographic features, while suggestive, are not diagnostic.
–When the clinical findings and ultrasound are suggestive of malignancy, a computerized tomography (CT) scan of the abdomen and pelvis is obtained to identify retroperitoneal involvement.
–Benign tumors tend to be well-circumscribed with sharp borders and decreased blood flow on Doppler studies.
–Epidermoid cysts usually demonstrate echogenic debris within the well-defined cyst.
–Yolk sac tumors tend to be more solid in appearance.
–The typical testicular tumor is intratesticular and may produce one or more discrete hypoechoic masses or diffuse abnormalities with microcalcifications that can be detected.
–Calcifications are more frequent in seminoma than in nonseminomatous tumors.
–When the clinical findings and ultrasound are suggestive of a benign tumor, no further evaluation is undertaken.
•CT scan and MRI of the abdomen and pelvis (Figs. 27.11 and 27.12):
–This is important in determining the extent of the tumor.
–It is also valuable in identifying the presence and extent of retroperitoneal lymphadenopathy.
–Retroperitoneal lymph nodes measuring 1–2 cm are confirmed to be pathologically involved with metastatic tumor in approximately 70 % of cases.
•Patients with rhabdomyosarcomas require chest radiography, abdominal-pelvic CT scanning, bone scanning, and bone-marrow aspiration.
27.8Treatment
•A solid scrotal mass should be considered malignant until proved otherwise.
•Any suspicion of the testicular tumor warrants an inguinal approach to prevent scrotal violation by the tumor.
•High inguinal orchiectomy has been a standard surgical treatment.
•Current trends emphasize that testis-sparing surgery should be performed for benign lesions such as teratoma, leydig cell tumor, and epidermoid cyst based on frozen biopsy findings.
•The three basic types of treatment are surgery, radiation therapy, and chemotherapy.
•In most patients with testicular cancer, the disease is cured readily with minimal long-term morbidity.
•Stage 1 tumors: Orchiectomy followed by surveillance.
•Surveillance includes:
–Frequent physical examinations.
–Radiographic evaluation of the chest and retroperitoneum.
584 |
27 Testicular Tumors in Children |
|
|
–Serum tumor marker measurement.
–Patients who developed metastatic disease are treated with two to four courses of multiagent platinum-based chemotherapy.
–Patients who present with locally advanced disease, metastases, or persistently elevated serum tumor markers are similarly treated with multiagent platinum-based chemotherapy.
•An elevated AFP level in a child over 1 year of age virtually always reflects the presence of a yolk sac tumor and precludes a testis-sparing approach.
•However, in all infants, and in older children with a normal AFP, the likelihood of a benign tumor is considerable.
•This is also true in boys presenting with androgenization.
•However, in adolescents with normal tumor markers and an ultrasound appearance highly suggestive of a benign lesion, such as an epidermoid cyst, testis-sparing may be considered.
•A testis-sparing approach:
–Through an inguinal approach.
–The testis is delivered into the inguinal incision.
–The cord is occluded with a non-crushing clamp or vessel loop.
–The tunica vaginalis is opened.
–The tumor is excised or enucleated and sent for frozen section.
–If a benign histology is confirmed, then the testicular defect is closed with absorbable suture and the testis is returned to the scrotum.
–If a malignancy is detected, or the frozen section is nondiagnostic, then an orchiectomy is performed.
–Reports from small series suggest that this approach is safe and is effective in preserving testicular tissue.
–The lesions successfully treated with tumor enucleation included:
•Teratomas
•Epidermoid cysts
•Sertoli cell tumors
•Leydig cell tumors
•While treatment success depends on the stage, the average survival rate after 5 years is around 95 %, and stage 1 cancers cases if monitored properly have essentially a 100 % survival rate.
•Inguinal orchiectomy:
–The initial treatment for testicular cancer is surgery to remove the affected testicle.
–This is to be done through an inguinal approach and never through the scrotum.
–The lymphatic drainage of the scrotum is into the lower legs, while the lymphatic drainage of the testicles is into the abdomen and retroperitoneal lymph nodes.
–For this reason, transscrotal testicular biopsy is not recommended.
•Retroperitoneal Lymph Node Dissection:
–In the case of nonseminomas that appear to be stage I, retroperitonea lymph node dissection may be done to accurately determine whether the cancer is in stage I or stage II and to reduce the risk of metastasis to the retroperitonal lymph nodes.
–This approach, while standard in many places, it is not the standard approach due to costs and the high level of expertise required to perform successful surgery.
•Adjuvant treatment:
–Perhaps the greatest advance in the management of testicular cancer was the introduction of platinum-based chemotherapy.
–Survival for both prepubertal and adult tumors has increased dramatically since its introduction.
–Indeed, multiagent chemotherapy has become the standard therapy for virtually all metastatic prepubertal testicular malignancies.
–Following excision of the primary tumor, universally benign tumors require no further evaluation or treatment.
–Treatment options for potentially malignant tumors include surveillance, chemotherapy, retroperitoneal lymph node dissection, and radiation therapy.
–Since testicular cancers can spread, patients are usually offered adjuvant treatment in the form of chemotherapy or radiotherapy.

27.9 Yolk Sac Tumor |
585 |
|
|
–The type of adjuvant therapy depends largely on the histology of the tumor and the stage of the tumor at the time of surgery.
–If the cancer is not advanced, patients may be offered careful surveillance by periodic CT scans and blood tests, in place of adjuvant treatment.
–For many patients with stage I cancer, adjuvant therapy following surgery may not be appropriate and patients will undergo surveillance instead.
–This approach ensures that chemotherapy and or radiotherapy is only given to the patients that need it
–Virtually all germ cell tumors are sensitive to platinum-based multiagent chemotherapy which plays a major role in their management.
–Retroperitoneal lymph node dissection plays an important staging and therapeutic role for mixed germ cell tumors in adolescents, but is rarely employed in cases of prepubertal yolk sac tumor.
–Radiation therapy is primarily used in treating seminoma – a very rare tumor in the pediatric population.
–The specific adjuvant therapy for a given patient is dependent on tumor histology and stage.
–The appropriate surgical treatment for any patient with suspected testicular tumor is radical inguinal orchiectomy with high ligation of the spermatic cord.
–Transscrotal biopsy or transscrotal orchiectomy is inappropriate and should be avoided.
–Adjuvant moderate-dose pelvic and/or paraaortic radiotherapy remains the standard treatment for patients with early-stage seminoma (stage I, IIA, or IIB) after orchiectomy.
–Patients who are found to have more advanced disease (stage IIC, III, IV) have a high risk of systemic relapse if treated with surgery and radiation alone, and the standard treatment for these patients is combination chemotherapy.
•Radiation therapy:
–Radiation may be used to treat stage 2 seminoma cancers, or as adjuvant therapy in the case of stage 1 seminomas, to minimize the likelihood of tumor spread.
–Radiation therapy is ineffective and is not used to treat nonseminoma tumors.
•Chemotherapy:
–Non-seminoma:
•Chemotherapy is the standard treatment for non-seminoma when the cancer has spread to other parts of the body (that is, stage 2B or 3).
•The standard chemotherapy protocol is three, or sometimes four, cycles of Bleomycin-Etoposide-Cisplatin.
•An alternative, equally effective treatment involves the use of four cycles of Etoposide-Cisplatin.
•Retroperitoneal lymph node dissection may also be performed after chemotherapy to remove masses left behind (stage 2B or more advanced), particularly in the cases of large nonseminomas.
–Seminoma:
•As an adjuvant treatment, the use of chemotherapy as an alternative to radiation therapy in the treatment of seminoma is increasing.
•Radiation therapy appears to have more significant long-term side effects.
•Two doses, or occasionally a single dose
of carboplatin, typically delivered 3 weeks apart, is proving to be a successful adjuvant treatment.
•Seminomas can recur decades after the primary tumor is removed. This calls for a close and long-term follow-up.
27.9Yolk Sac Tumor
•Pure yolk sac tumors occur almost exclusively in infants and very young children.
•Histologic evaluation of the yolk-sac tumor demonstrates eosinophilic periodic acidSchiff (PAS)–positive inclusions in the
