
- •Contents
- •Preface to the first edition
- •Flagella
- •Cell walls and mucilages
- •Plastids
- •Mitochondria and peroxisomes
- •Division of chloroplasts and mitochondria
- •Storage products
- •Contractile vacuoles
- •Nutrition
- •Gene sequencing and algal systematics
- •Classification
- •Algae and the fossil record
- •REFERENCES
- •CYANOPHYCEAE
- •Morphology
- •Cell wall and gliding
- •Pili and twitching
- •Sheaths
- •Protoplasmic structure
- •Gas vacuoles
- •Pigments and photosynthesis
- •Akinetes
- •Heterocysts
- •Nitrogen fixation
- •Asexual reproduction
- •Growth and metabolism
- •Lack of feedback control of enzyme biosynthesis
- •Symbiosis
- •Extracellular associations
- •Ecology of cyanobacteria
- •Freshwater environment
- •Terrestrial environment
- •Adaption to silting and salinity
- •Cyanotoxins
- •Cyanobacteria and the quality of drinking water
- •Utilization of cyanobacteria as food
- •Cyanophages
- •Secretion of antibiotics and siderophores
- •Calcium carbonate deposition and fossil record
- •Chroococcales
- •Classification
- •Oscillatoriales
- •Nostocales
- •REFERENCES
- •REFERENCES
- •REFERENCES
- •RHODOPHYCEAE
- •Cell structure
- •Cell walls
- •Chloroplasts and storage products
- •Pit connections
- •Calcification
- •Secretory cells
- •Iridescence
- •Epiphytes and parasites
- •Defense mechanisms of the red algae
- •Commercial utilization of red algal mucilages
- •Reproductive structures
- •Carpogonium
- •Spermatium
- •Fertilization
- •Meiosporangia and meiospores
- •Asexual spores
- •Spore motility
- •Classification
- •Cyanidiales
- •Porphyridiales
- •Bangiales
- •Acrochaetiales
- •Batrachospermales
- •Nemaliales
- •Corallinales
- •Gelidiales
- •Gracilariales
- •Ceramiales
- •REFERENCES
- •Cell structure
- •Phototaxis and eyespots
- •Asexual reproduction
- •Sexual reproduction
- •Classification
- •Position of flagella in cells
- •Flagellar roots
- •Multilayered structure
- •Occurrence of scales or a wall on the motile cells
- •Cell division
- •Superoxide dismutase
- •Prasinophyceae
- •Charophyceae
- •Classification
- •Klebsormidiales
- •Zygnematales
- •Coleochaetales
- •Charales
- •Ulvophyceae
- •Classification
- •Ulotrichales
- •Ulvales
- •Cladophorales
- •Dasycladales
- •Caulerpales
- •Siphonocladales
- •Chlorophyceae
- •Classification
- •Volvocales
- •Tetrasporales
- •Prasiolales
- •Chlorellales
- •Trebouxiales
- •Sphaeropleales
- •Chlorosarcinales
- •Chaetophorales
- •Oedogoniales
- •REFERENCES
- •REFERENCES
- •EUGLENOPHYCEAE
- •Nucleus and nuclear division
- •Eyespot, paraflagellar swelling, and phototaxis
- •Muciferous bodies and extracellular structures
- •Chloroplasts and storage products
- •Nutrition
- •Classification
- •Heteronematales
- •Eutreptiales
- •Euglenales
- •REFERENCES
- •DINOPHYCEAE
- •Cell structure
- •Theca
- •Scales
- •Flagella
- •Pusule
- •Chloroplasts and pigments
- •Phototaxis and eyespots
- •Nucleus
- •Projectiles
- •Accumulation body
- •Resting spores or cysts or hypnospores and fossil Dinophyceae
- •Toxins
- •Dinoflagellates and oil and coal deposits
- •Bioluminescence
- •Rhythms
- •Heterotrophic dinoflagellates
- •Direct engulfment of prey
- •Peduncle feeding
- •Symbiotic dinoflagellates
- •Classification
- •Prorocentrales
- •Dinophysiales
- •Peridiniales
- •Gymnodiniales
- •REFERENCES
- •REFERENCES
- •Chlorarachniophyta
- •REFERENCES
- •CRYPTOPHYCEAE
- •Cell structure
- •Ecology
- •Symbiotic associations
- •Classification
- •Goniomonadales
- •Cryptomonadales
- •Chroomonadales
- •REFERENCES
- •CHRYSOPHYCEAE
- •Cell structure
- •Flagella and eyespot
- •Internal organelles
- •Extracellular deposits
- •Statospores
- •Nutrition
- •Ecology
- •Classification
- •Chromulinales
- •Parmales
- •Chrysomeridales
- •REFERENCES
- •SYNUROPHYCEAE
- •Classification
- •REFERENCES
- •EUSTIGMATOPHYCEAE
- •REFERENCES
- •PINGUIOPHYCEAE
- •REFERENCES
- •DICTYOCHOPHYCEAE
- •Classification
- •Rhizochromulinales
- •Pedinellales
- •Dictyocales
- •REFERENCES
- •PELAGOPHYCEAE
- •REFERENCES
- •BOLIDOPHYCEAE
- •REFERENCE
- •BACILLARIOPHYCEAE
- •Cell structure
- •Cell wall
- •Cell division and the formation of the new wall
- •Extracellular mucilage, biolfouling, and gliding
- •Motility
- •Plastids and storage products
- •Resting spores and resting cells
- •Auxospores
- •Rhythmic phenomena
- •Physiology
- •Chemical defense against predation
- •Ecology
- •Marine environment
- •Freshwater environment
- •Fossil diatoms
- •Classification
- •Biddulphiales
- •Bacillariales
- •REFERENCES
- •RAPHIDOPHYCEAE
- •REFERENCES
- •XANTHOPHYCEAE
- •Cell structure
- •Cell wall
- •Chloroplasts and food reserves
- •Asexual reproduction
- •Sexual reproduction
- •Mischococcales
- •Tribonematales
- •Botrydiales
- •Vaucheriales
- •REFERENCES
- •PHAEOTHAMNIOPHYCEAE
- •REFERENCES
- •PHAEOPHYCEAE
- •Cell structure
- •Cell walls
- •Flagella and eyespot
- •Chloroplasts and photosynthesis
- •Phlorotannins and physodes
- •Life history
- •Classification
- •Dictyotales
- •Sphacelariales
- •Cutleriales
- •Desmarestiales
- •Ectocarpales
- •Laminariales
- •Fucales
- •REFERENCES
- •PRYMNESIOPHYCEAE
- •Cell structure
- •Flagella
- •Haptonema
- •Chloroplasts
- •Other cytoplasmic structures
- •Scales and coccoliths
- •Toxins
- •Classification
- •Prymnesiales
- •Pavlovales
- •REFERENCES
- •Toxic algae
- •Toxic algae and the end-Permian extinction
- •Cooling of the Earth, cloud condensation nuclei, and DMSP
- •Chemical defense mechanisms of algae
- •The Antarctic and Southern Ocean
- •The grand experiment
- •Antarctic lakes as a model for life on the planet Mars or Jupiter’s moon Europa
- •Ultraviolet radiation, the ozone hole, and sunscreens produced by algae
- •Hydrogen fuel cells and hydrogen gas production by algae
- •REFERENCES
- •Glossary
- •Index
Chapter 18
Heterokontophyta
RAPHIDOPHYCEAE
The Raphidophyceae, or chloromonads, have chlorophylls a and c, and two membranes of chloroplast endoplasmic reticulum. The anterior flagellum is commonly tinsel, whereas the posterior flagellum is naked (Figs. 18.1, 18.2, 18.3). The freshwater species of the Raphidophyceae are green, whereas the marine forms are yellowish and contain the carotenoid fucoxanthin (Vesk and
Moestrup, 1987). The closest relatives of the Raphidophyceae are the Eustigmatophyceae and the Chrysophyceae (Cavalier-Smith and Chao, 1996).
Marine species are euryhaline and eurythermic (tolerant of a wide salinity and temperature range) and occur in temperate and subtropical waters worldwide. Marine genera are Chattonella (Fig. 18.3), Fibrocapsa (Fig. 18.1(b)), and Heterosigma (Fig. 18.1(a)). Many of the marine species produce neurotoxic compounds that are similar to brevetoxin (Fig.18.2).
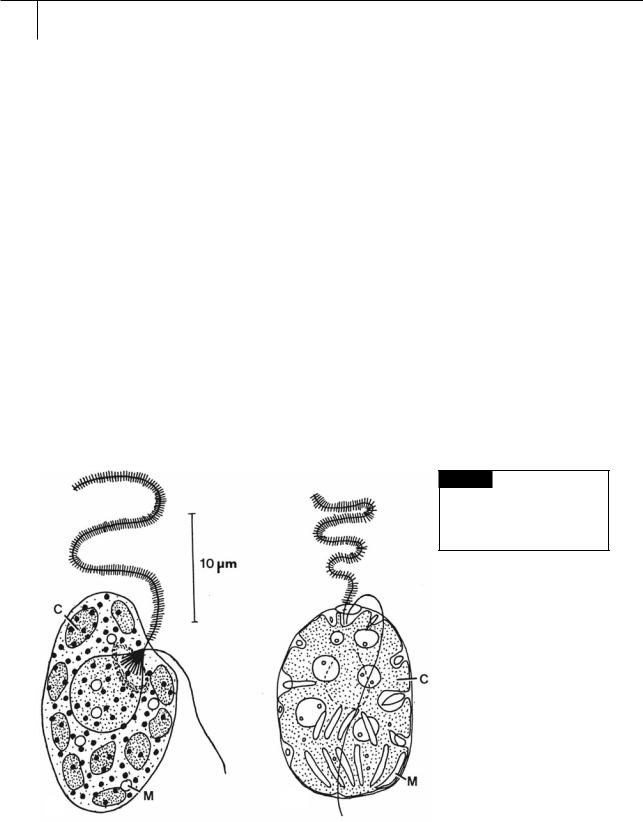
Fig. 18.1 (a) Heterosigma carterae. (b) Fibrocapsa japonica.
(C) Chloroplast; (M) mucocyst. ((a) after Leadbeater, 1969;
(b) after Hara and Chihara, 1985.)
(b)
(a)
410 CHLOROPLAST E.R.: EVOLUTION OF TWO MEMBRANES
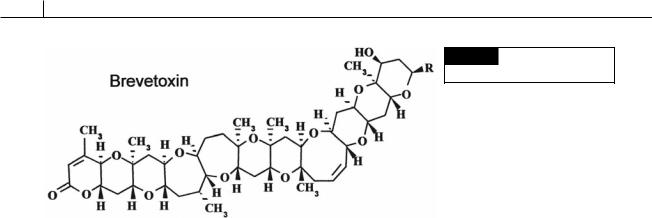
Fig. 18.2 The chemical structure
of brevetoxin.
Uptake of the toxin by fish results in depolarization of nerves supplying the heart. This reduces the heart rate, thereby lowering blood pressure, which in turn affects the transfer of oxygen to the gill lamellae, creating hypoxic conditions that lead to fish mortality (Tyrrell et al., 2001).
Toxic red-tide blooms of the marine Chattonella antiqua and Heterosigma carterae (Taylor, 1992) have occurred in the Seto Inland Sea in Japan (Watanabe et al., 1988). These red tides occurred in the summer when a salinity and temperature stratification occurred at a depth of 5–10 meters. There was little mixing of waters above and below the stratified layer resulting in the upper layer being deficient in nutrients while the bottom layer was anaerobic. Heterosigma carterae flourishes under these conditions because it has a daily vertical migration of 10 to 15 meters and this allows the alga to move between the stratified layers. This migration enables H. carterae to use the nutrients in the lower layers, and light and oxygen in the upper layer, resulting in the red-tide blooms of the organism. The migration is correlated with the production and degradation of cytoplasmic fat particles (Wada et al., 1987). Heterosigma carterae has a wide salinity tolerance in culture (3 to 50%) and loses its motility at temperatures below 10 °C. At 5 to 10 °C, the alga forms non-motile masses capable of surviving in continuous darkness for up to 15 weeks (Han et al., 2002). This is a far longer dark period than the motile cells can tolerate and still live. In Narragansett Bay, Rhode Island, blooms of H. carterae occur during a period of low nitrogen concentration, but at a time when phosphate levels are near a yearly maximum (Tomas, 1979).
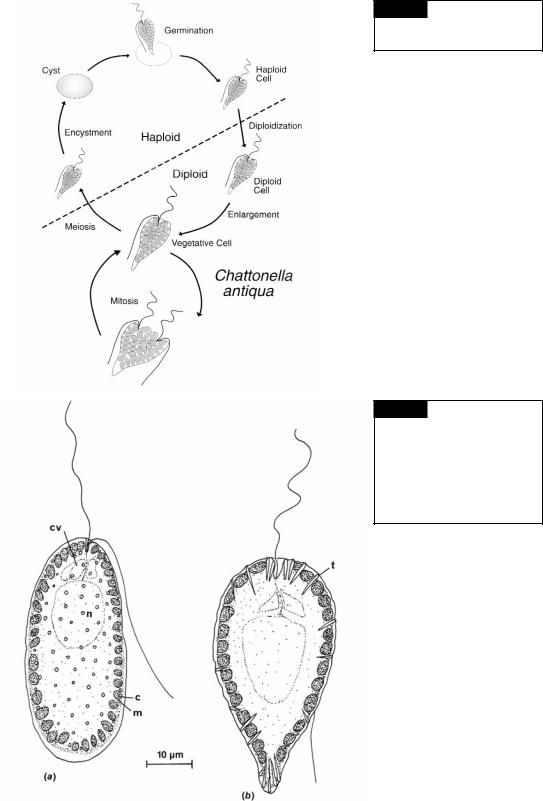
Chattonella antiqua is a marine raphidophyte that produces blooms in coastal Japan. In 1972, a bloom killed 500 million dollars worth of caged yellow-tail fish in the Seto Inland Sea (Okaichi, 1989). C. antiqua has the highest toxicity during the early to mid-logarithmic phase. After reaching the stationary phase, the toxicity decreases markedly (Khan et al., 1996). The life cycle of Chattonella antiqua involves a vegetative propagation phase and a non-motile dormant phase (Fig. 18.3) (Yamaguchi and Imai, 1994). The vegetative diploid cells grow by binary fission under normal growth conditions. Small haploid cells are produced when the nutrients are depleted in the medium. These haploid cells change into cysts under low-light conditions and spend several months dormant in bottom sediments. The period of dormancy usually lasts from the end of summer to the following spring and is enforced by low temperatures (Imai and Itoh, 1987). Swarmers germinate from the cysts and somehow become diploid, although how diploidization occurs is not known. The resulting diploid vegetative cells complete the life history.
In freshwater, Raphidophyceae are associated with mud bottoms or ponds with abundant macrophytes. Vacuolaria contains chlorophylls a and c as well as the carotenoid -carotene and the xanthophylls lutein epoxide and antheraxanthin (Chapman and Haxo, 1966; Guillard and Lorenzen, 1972). The numerous chloroplasts have three thylakoids per band, and there are two membranes of chloroplast E.R. around the chloroplasts (Koch and Schnepf, 1967; Mignot, 1967). Vacuolaria (Fig. 18.4(a)) can be free-swimming or in a palmelloid state. In the palmelloid condition,
HETEROKONTOPHYTA, RAPHIDOPHYCEAE |
411 |
|
|
Fig. 18.3 The life cycle of
Chattonella antiqua. (Adapted from
Yamaguchi and Imai, 1994.)
Fig. 18.4 (a) Vacuolaria virescens, showing chloroplasts (c), mucilaginous bodies (m), flagella, contractile vacuoles (cv), and the nucleus (n); (b) Gonyostomum semen with chloroplasts, trichocysts (t), contractile vacuoles, and the nucleus. (After Mignot, 1967.)

412 CHLOROPLAST E.R.: EVOLUTION OF TWO MEMBRANES
the cells are more or less spherical, 35 to 50 m in diameter, yellow-green with each cell surrounded by a thick gelatinous matrix. In the freeswimming state, the organisms vary considerably in shape from broadly ovate to elongate. The flagella emerge from a simple notch slightly to one side of the anterior ventral region. The anteriorly directed flagellum has microtubular hairs, whereas the posteriorly directed flagellum is whiplash. There is no stigma, but the organism is positively phototactic. Vacuolaria divides only in the palmelloid state. Mitosis is typically eukaryotic, with the nuclear membrane remaining intact (Heywood and Godward, 1972). No sexual reproduction has been reported in Vacuolaria. Occasionally the cells form cysts with the resistant sheaths surrounding the cells (Spencer, 1971).
Vacuolaria has muciferous bodies (mucocysts) in the peripheral cytoplasm (Fig. 18.4(a)), which are similar in structure to those in the Prymnesiophyceae and Chrysophyceae. The genus Gonyostomum (Fig. 18.4(b)) has trichocysts similar to those in the Dinophyceae (Mignot, 1967).
REFERENCES
Cavalier-Smith, T., and Chao, E. E. (1996). 18S rRNA sequence of Heterosigma carterae (Raphidophyceae), and the phylogeny of heterokont algae (Ochrophyta).
Phycologia 35:500–10.
Chapman, D. J., and Haxo, F. T. (1966). Chloroplast pigments of the Chloromonadophyceae. J. Phycol. 2:89–91.
Guillard, R. R. L., and Lorenzen, C. J. (1972). Yellowgreen algae with chlorophyllide c. J. Phycol. 8:10–14.
Han, M.-S., Kim, Y.-P. and Cattolico, R. A. (2002). Heterosigma akashiwo (Raphidophyceae) resting cell formation in batch culture: strain identity versus physiological response. J. Phycol. 38:304–17.
Hara, Y., and Chihara, M. (1985). Ultrastructure and taxonomy of Fibrocapsa japonica (Class Raphidophyceae). Arch. Protistenk. 130:133–41.
Heywood, P., and Godward, M. B. E. (1972). Centromeric organization in the chloromonadophycean alga
Vacuolaria virescens. Chromosoma 39:333–9.
Imai, I., and Itoh, K. (1987). Annual life cycle of Chattonella spp., causative flagellates of noxious red tides in the Inland Sea of Japan. Marine Biology 94:287–92.
Khan, S., Arakawa, O., and Onoue, Y. (1996). A toxicological study of the marine phytoflagellate,
Chattonella antiqua (Raphidophyceae). Phycologia
35:239–44.
Koch, W., and Schnepf, E. (1967). Einige elektronenmikroskopische Beobachtungen an Vacuolaria virescens Ciénk. Arch. Mikrobiol. 57:196–8.
Leadbeater, B. S. C. (1969). A fine structural study of
Olisthodiscus luteus Carter. Br. Phycol. J. 4:3–17. Mignot, J. -P. (1967). Structure et ultrastructure de
quelques Chloromonadines. Protistologia 3:5–24. Okaichi, T. (1989). Red tide problems in the Seto Inland
Sea, Japan. In Red Tides: Biology, Environmental Science and Toxicology, ed. T. Okaichi, D. M. Anderson, and T. Nemoto, pp. 137–42. New York: Elsevier Science Publishing.
Spencer, L. B. (1971). A study of Vacuolaria virescens Cienkowski. J. Phycol. 7:274–9.
Taylor, F. J. R. (1992). The taxonomy of harmful marine phytoplankton. G. Bot. Ital. 126:209–19.
Tomas, C. R. (1979). Olisthodiscus luteus (Chrysophyceae). III. Uptake and utilization of nitrogen and phosphorus. J. Phycol. 15:5–12.
Tyrrell, J. V., Bergquist, P. R., Bergquist, P. L., and Scholin, C. A. (2001). Detection and enumeration of
Heterosigma akashiwo and Fibrocapsa japonica
(Raphidophyceae) using rRNA-targeted oligonucleotide probes. Phycologia 40:457–67.
Vesk, M., and Moestrup, Ø. (1987). The flagellar root system in Heterosigma akashiwo (Raphidophyceae).
Protoplasma 137:15–28.
Wada, M., Hara, Y., Kato, M., Yamada, M., and Fujii, T. (1987). Diurnal appearance, fine structure and chemical composition of fatty particles in Heterosigma akashiwo (Raphidophyceae). Protoplasma 137:134–9.
Watanabe, M., Kohata, K., and Kunugi, M. (1988). Phosphate accumulation and metabolism by Heterosigma akashiwo (Raphidophyceae) during diel vertical migration in a stratified microsm. J. Phycol. 24:22–8.
Yamaguchi, M., and Imai, I. (1994). A microfluorometric analysis of nuclear DNA at different stages in the life history of Chattonella antiqua and Chattonella marina (Raphidophyceae). Phycologia 33:163–70.
