
- •Contents
- •Preface to the first edition
- •Flagella
- •Cell walls and mucilages
- •Plastids
- •Mitochondria and peroxisomes
- •Division of chloroplasts and mitochondria
- •Storage products
- •Contractile vacuoles
- •Nutrition
- •Gene sequencing and algal systematics
- •Classification
- •Algae and the fossil record
- •REFERENCES
- •CYANOPHYCEAE
- •Morphology
- •Cell wall and gliding
- •Pili and twitching
- •Sheaths
- •Protoplasmic structure
- •Gas vacuoles
- •Pigments and photosynthesis
- •Akinetes
- •Heterocysts
- •Nitrogen fixation
- •Asexual reproduction
- •Growth and metabolism
- •Lack of feedback control of enzyme biosynthesis
- •Symbiosis
- •Extracellular associations
- •Ecology of cyanobacteria
- •Freshwater environment
- •Terrestrial environment
- •Adaption to silting and salinity
- •Cyanotoxins
- •Cyanobacteria and the quality of drinking water
- •Utilization of cyanobacteria as food
- •Cyanophages
- •Secretion of antibiotics and siderophores
- •Calcium carbonate deposition and fossil record
- •Chroococcales
- •Classification
- •Oscillatoriales
- •Nostocales
- •REFERENCES
- •REFERENCES
- •REFERENCES
- •RHODOPHYCEAE
- •Cell structure
- •Cell walls
- •Chloroplasts and storage products
- •Pit connections
- •Calcification
- •Secretory cells
- •Iridescence
- •Epiphytes and parasites
- •Defense mechanisms of the red algae
- •Commercial utilization of red algal mucilages
- •Reproductive structures
- •Carpogonium
- •Spermatium
- •Fertilization
- •Meiosporangia and meiospores
- •Asexual spores
- •Spore motility
- •Classification
- •Cyanidiales
- •Porphyridiales
- •Bangiales
- •Acrochaetiales
- •Batrachospermales
- •Nemaliales
- •Corallinales
- •Gelidiales
- •Gracilariales
- •Ceramiales
- •REFERENCES
- •Cell structure
- •Phototaxis and eyespots
- •Asexual reproduction
- •Sexual reproduction
- •Classification
- •Position of flagella in cells
- •Flagellar roots
- •Multilayered structure
- •Occurrence of scales or a wall on the motile cells
- •Cell division
- •Superoxide dismutase
- •Prasinophyceae
- •Charophyceae
- •Classification
- •Klebsormidiales
- •Zygnematales
- •Coleochaetales
- •Charales
- •Ulvophyceae
- •Classification
- •Ulotrichales
- •Ulvales
- •Cladophorales
- •Dasycladales
- •Caulerpales
- •Siphonocladales
- •Chlorophyceae
- •Classification
- •Volvocales
- •Tetrasporales
- •Prasiolales
- •Chlorellales
- •Trebouxiales
- •Sphaeropleales
- •Chlorosarcinales
- •Chaetophorales
- •Oedogoniales
- •REFERENCES
- •REFERENCES
- •EUGLENOPHYCEAE
- •Nucleus and nuclear division
- •Eyespot, paraflagellar swelling, and phototaxis
- •Muciferous bodies and extracellular structures
- •Chloroplasts and storage products
- •Nutrition
- •Classification
- •Heteronematales
- •Eutreptiales
- •Euglenales
- •REFERENCES
- •DINOPHYCEAE
- •Cell structure
- •Theca
- •Scales
- •Flagella
- •Pusule
- •Chloroplasts and pigments
- •Phototaxis and eyespots
- •Nucleus
- •Projectiles
- •Accumulation body
- •Resting spores or cysts or hypnospores and fossil Dinophyceae
- •Toxins
- •Dinoflagellates and oil and coal deposits
- •Bioluminescence
- •Rhythms
- •Heterotrophic dinoflagellates
- •Direct engulfment of prey
- •Peduncle feeding
- •Symbiotic dinoflagellates
- •Classification
- •Prorocentrales
- •Dinophysiales
- •Peridiniales
- •Gymnodiniales
- •REFERENCES
- •REFERENCES
- •Chlorarachniophyta
- •REFERENCES
- •CRYPTOPHYCEAE
- •Cell structure
- •Ecology
- •Symbiotic associations
- •Classification
- •Goniomonadales
- •Cryptomonadales
- •Chroomonadales
- •REFERENCES
- •CHRYSOPHYCEAE
- •Cell structure
- •Flagella and eyespot
- •Internal organelles
- •Extracellular deposits
- •Statospores
- •Nutrition
- •Ecology
- •Classification
- •Chromulinales
- •Parmales
- •Chrysomeridales
- •REFERENCES
- •SYNUROPHYCEAE
- •Classification
- •REFERENCES
- •EUSTIGMATOPHYCEAE
- •REFERENCES
- •PINGUIOPHYCEAE
- •REFERENCES
- •DICTYOCHOPHYCEAE
- •Classification
- •Rhizochromulinales
- •Pedinellales
- •Dictyocales
- •REFERENCES
- •PELAGOPHYCEAE
- •REFERENCES
- •BOLIDOPHYCEAE
- •REFERENCE
- •BACILLARIOPHYCEAE
- •Cell structure
- •Cell wall
- •Cell division and the formation of the new wall
- •Extracellular mucilage, biolfouling, and gliding
- •Motility
- •Plastids and storage products
- •Resting spores and resting cells
- •Auxospores
- •Rhythmic phenomena
- •Physiology
- •Chemical defense against predation
- •Ecology
- •Marine environment
- •Freshwater environment
- •Fossil diatoms
- •Classification
- •Biddulphiales
- •Bacillariales
- •REFERENCES
- •RAPHIDOPHYCEAE
- •REFERENCES
- •XANTHOPHYCEAE
- •Cell structure
- •Cell wall
- •Chloroplasts and food reserves
- •Asexual reproduction
- •Sexual reproduction
- •Mischococcales
- •Tribonematales
- •Botrydiales
- •Vaucheriales
- •REFERENCES
- •PHAEOTHAMNIOPHYCEAE
- •REFERENCES
- •PHAEOPHYCEAE
- •Cell structure
- •Cell walls
- •Flagella and eyespot
- •Chloroplasts and photosynthesis
- •Phlorotannins and physodes
- •Life history
- •Classification
- •Dictyotales
- •Sphacelariales
- •Cutleriales
- •Desmarestiales
- •Ectocarpales
- •Laminariales
- •Fucales
- •REFERENCES
- •PRYMNESIOPHYCEAE
- •Cell structure
- •Flagella
- •Haptonema
- •Chloroplasts
- •Other cytoplasmic structures
- •Scales and coccoliths
- •Toxins
- •Classification
- •Prymnesiales
- •Pavlovales
- •REFERENCES
- •Toxic algae
- •Toxic algae and the end-Permian extinction
- •Cooling of the Earth, cloud condensation nuclei, and DMSP
- •Chemical defense mechanisms of algae
- •The Antarctic and Southern Ocean
- •The grand experiment
- •Antarctic lakes as a model for life on the planet Mars or Jupiter’s moon Europa
- •Ultraviolet radiation, the ozone hole, and sunscreens produced by algae
- •Hydrogen fuel cells and hydrogen gas production by algae
- •REFERENCES
- •Glossary
- •Index
HETEROKONTOPHYTA, PHAEOPHYCEAE |
427 |
|
|
they often form an important part of the salt marsh flora. These brackish water plants have almost totally lost the ability to reproduce sexually, and propagate by vegetative means only. Most of the Phaeophyceae grow in the intertidal belt and the upper littoral region. They dominate these regions in colder waters, particularly in the Northern Hemisphere, where the number of phaeophycean species is less than that of the Rhodophyceae, but the number of phaeophycean plants is much greater. In the tropics, the only place where large numbers of Phaeophyceae are found is the Sargasso Sea of the Atlantic.
The Phaeophyceae probably evolved from an organism in the Phaeothamniophyceae, which have motile cells similar to those in the Phaeophyceae, but lack the characteristic unilocular and plurilocular sporangia of the Phaeophyceae (Bailey et al., 1998).
Cell structure
The cell structure (Figs. 21.2, 21.3) is in many ways similar to that of the Chrysophyceae,
Prymnesiophyceae, Bacillariophyceae, and Xanthophyceae, which are closely related to the Phaeophyceae. The main difference lies in the large amounts of extracellular polysaccharides surrounding the protoplast.
Cell walls
Phaeophycean cell walls are generally composed of at least two layers, with cellulose making up the main structural skeleton (Kloareg and Quatrano, 1988). The amorphous component of the cell wall is made up of alginic acid and fucoidin, whereas the mucilage and cuticle are composed primarily of alginic acid (Evans and Holligan, 1972a; Vreeland, 1972). Alginic acid is basically made up of -1,4 linked mannuronic acid units that have a variable amount of guluronic acid units attached through C-1 and C-4 linkages (Fig. 1.7). Fucoidin is composed primarily of -1,2 linked sulfated-fucose units, with a lesser amount of -1,4 linked sulfated-fucose units (Fig. 1.7). The relative quantities of alginic acid and fucoidin vary between species, different parts of the plant, and different environments.
Calcification of the wall occurs only in some
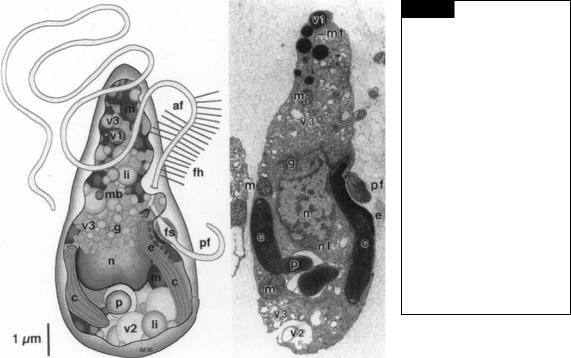
Fig. 21.2 Left: Diagrammatic representation of a male gamete of
Ectocarpus siliculosus showing the distribution of cellular organelles.
Right: Transmission electron micrograph of a thin section of a male gamete of E. siliculosus. (af) Anterior flagellum; (c) chloroplast;
(e) eyespot; (fh) flagellar hairs (present along entire length, for clarity only shown on part of the flagellum); (fs) proximal swelling of the posterior flagellum; (g) Golgi apparatus; (li) lipid body;
(m)mitochondrion; (mb) microbody; (mt) microtubules;
(n)nucleus; (p) pyrenoid; (pf) posterior flagellum; (v1) physode; (v2) storage granule; (v3) vesicles with cell wall or adhesive material. (From Maier, 1997a.)
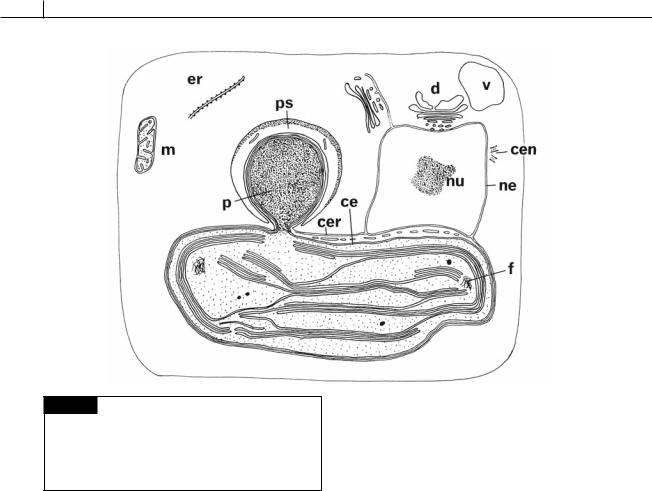
428 CHLOROPLAST E.R.: EVOLUTION OF TWO MEMBRANES
Fig. 21.3 Diagram of a hypothetical brown algal cell.
(ce) Chloroplast envelope; (cen) centrioles; (cer) chloroplast endoplasmic reticulum; (d) dictyosome; (er) endoplasmic reticulum; (f) DNA fibrils; (m) mitochondrion; (ne) nuclear envelope; (nu) nucleolus; (p) pyrenoid; (ps) pyrenoid sac;
(v) vacuole. (From Bouck, 1965.)
species of Padina where calcium carbonate is deposited as needle-shaped crystals of aragonite in concentric bands on the surface of the fan-like thallus (Borowitzka et al., 1974).
The parenchymatous Phaeophyceae have plasmodesmata or pores between most of the cells. These pores are bounded by the plasmalemma, and protoplasm is continuous from one cell to the next through them. In the Laminariales, Fucales, and Dictyotales, the pores are grouped in primary pit areas, whereas in the more primitive parenchymatous Phaeophyceae the plasmodesmata are scattered in the cell wall (Bisalputra, 1966; Bourne and Cole, 1968; Cole, 1970).
Flagella and eyespot
Generally the motile cells of the Phaeophyceae (always zoospores or gametes, as there are no motile vegetative cells) have a long anterior tinsel flagellum with tripartite hairs and a shorter pos-
teriorly directed whiplash flagellum (Fig. 21.2) (Bouck, 1969; Loiseaux and West, 1970). The Fucales (Fig. 21.44) are an exception to this, with the posterior flagellum of the spermatozoid being longer than the anterior flagellum. The posterior flagellum usually has a swelling near the base, and this swelling fits into a depression of the cell immediately above the eyespot. The eyespot consists of 40 to 80 lipid globules arranged in a single layer between the outermost band of the thylakoids and the chloroplast envelope. The eyespot (stigma) acts as a concave mirror focusing light onto the flagellar swelling, which is the photoreceptor site for phototaxis in brown-algal flagellate cells (Kawai et al., 1996). Light at 420 and 460 nm is most effective in phototaxis in the brown algae, and is probably detected by a flavin-like substance in the flagellar swelling of the posterior flagellum (Kawai et al., 1991).
Chloroplasts and photosynthesis
The chloroplasts of the Phaeophyceae have three thylakoids per band and are surrounded by the chloroplast envelope and two membranes of chloroplast E.R. (Figs. 21.3, 21.4). The outer membrane of the chloroplast E.R. is generally continu-
HETEROKONTOPHYTA, PHAEOPHYCEAE |
429 |
|
|
ous with the outer membrane of the nuclear envelope in the Ectocarpales but appears to be discontinuous in the Dictyotales, Laminariales, and Fucales. Membrane-bounded tubules are common in the area between the chloroplast E.R. and chloroplast envelope where the latter two are not closely appressed (Bouck, 1965; Evans, 1968). Microfibrils of DNA occur in the plastids, and in Sphacelaria sp. there is a ring-shaped genophore inside the outermost band of thylakoids (Bisalputra and Burton, 1969). The DNA microfibrils are both linear and circular and are attached to the thylakoid membranes. The plastids contain chlorophylls a, c1, and c2, with the major carotenoid being fucoxanthin.
All the phaeophycean orders have representatives with pyrenoids (Figs. 21.3, 21.4) (Chi, 1971), but their presence, even in one species, can vary according to the stage of the plant. If the species is one that has pyrenoids only in some stages, then a pyrenoid is usually present in the eggs and/or sporelings but absent in the macroscopic phase,
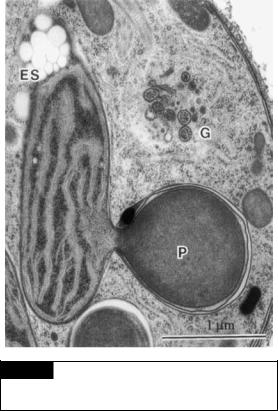
Fig. 21.4 A transmission electron micrograph of a portion of a cell of Scytosiphon lomentaria. (ES) Eyespot; (g) Golgi;
(P) pyrenoid. (From Nagasato and Motomura, 2002.)
spermatozoids, and/or zoospores (Bourne and Cole, 1968; Evans, 1968; Bisalputra et al., 1971). A pyrenoid in the Phaeophyceae is usually a stalklike structure set off from the main body of the chloroplast and containing a granular substance not traversed by thylakoids. Surrounding the pyrenoid but outside the chloroplast E.R. is a membrane-bounded sac that presumably contains the reserve product. The long-term storage product is laminarin (Fig. 1.7), a -1,3 linked glucan. The sugar alcohol, D-mannitol (Fig. 1.7) is, however, the accumulation product (up to 25% of the dry weight of some Laminaria species in the autumn) of photosynthesis.
In a number of brown algae, the mannitol concentration in the cell increases or decreases as the salinity of the surrounding medium increases or decreases (Reed et al., 1985). This osmoregulatory mechanism prevents the cells from bursting in hypotonic media or shrinking in hypertonic media. The increase in mannitol concentration occurs in the dark as well as in the light, showing that photosynthesis is not involved in the process.
The brown algae are unique among the algae in having uptake of inorganic carbon, and therefore photosynthetic carbon fixation, stimulated by blue light (Forster and Dring, 1994). Most of the Phaeophyceae live in the littoral zone where they receive a generous amount of light. Photosynthesis is usually limited by the supply of inorganic carbon in this environment. The Phaeophyceae have evolved a mechanism to increase the amount of inorganic carbon uptake, but only when the cells are illuminated by blue light, thereby conserving the energy required for the process when the cells are in the dark. The only brown algae that do not have this mechanism are the fucoids, which appear to have evolved a separated carbon-concentrating mechanism.
Phlorotannins and physodes
Phlorotannins (phaeophycean tannins) are stored in physodes (Fig. 21.5) in the cytoplasm of many brown algae. Phlorotannins are formed by Golgi in the perinuclear area of the cell by polymerization of phloroglucinol (1,3,5-tri-hydroxy- benzene) (Fig. 21.6) through the acetate-malonate pathway (Schoenwaelder and Clayton, 2000; Pavia

430 CHLOROPLAST E.R.: EVOLUTION OF TWO MEMBRANES
Fig. 21.5 Physodes in zygotes of Hormosira banksii. (a) Bright-field microscopy of sectioned zygotes showing accumulation of physodes around the nucleus (arrow). (b) Transmission electron micrograph showing physodes
around the nucleus. (From Schoenwaelder and Clayton, 2000.)
Fig. 21.6 Chemical structure of phloroglucinal, the basic building block of polyphenols and the chemical structure of the polyphenol procyanidin.
et al., 2003). The phlorotannins in the physodes appear as a colorless, highly refractive acidic fluid that stains red with vanillin and hydrochloric acid. The tannins are non-glycosidic (do not contain sugars), bind proteins, have strong reducing action, and are astringent to the taste. They are readily oxidized in air, resulting in the formation of a black pigment, phycophaein, giving dried brown algae their characteristic black color.
The phlorotannin content of brown algae varies from 1% to 15% of dry mass. Phlorotannins occur
at high levels in brown algae of the temperate and tropical Atlantic, whereas levels are low (less than 2% of dry mass) in the tropical Pacific and Indo-Pacific (Targett and Arnold, 1998). In temperate areas, the fucoids (Fucales) have high concentrations of phlorotannins whereas kelps (Laminariales) have low concentrations of phlorotannins. The same species of brown alga will have a higher phlorotannin content when deprived of nitrogen (Van Alstyne and Pelletreau, 2000).
Phlorotannins have been postulated to function in (1) deterring grazing by herbivores, (2) absorbing ultraviolet radiation, and (3) serving as a component of cell walls (Henry and Van Alstyne, 2004). The effect of phlorotannins as a chemical defense against herbivores has been best studied. Phlorotannins deter feeding by inhibiting the glycosidases of gastropods (Shibata et al., 2002). Phlorotannins of high molecular weight inhibit herbivory more than phlorotannins of low molecular weight (Target and Arnold, 1998; Kubanek et al., 2004). There is variation among herbivores in the effectiveness of phlorotannins against grazing. Herbivores with digestive systems containing surfactants (wetting agents) or high pH are able to tolerate phlorotannins better. Phlorotannins are not normally secreted outside the cell (Shibata et al., 2002). It is necessary for the cells to be damaged before the phlorotannins are released.
Phlorotannins are always present in brown algae and thus are a “constitutive” chemical defense. Stimulation of phlorotannin synthesis by
