
- •Contents
- •Preface to the first edition
- •Flagella
- •Cell walls and mucilages
- •Plastids
- •Mitochondria and peroxisomes
- •Division of chloroplasts and mitochondria
- •Storage products
- •Contractile vacuoles
- •Nutrition
- •Gene sequencing and algal systematics
- •Classification
- •Algae and the fossil record
- •REFERENCES
- •CYANOPHYCEAE
- •Morphology
- •Cell wall and gliding
- •Pili and twitching
- •Sheaths
- •Protoplasmic structure
- •Gas vacuoles
- •Pigments and photosynthesis
- •Akinetes
- •Heterocysts
- •Nitrogen fixation
- •Asexual reproduction
- •Growth and metabolism
- •Lack of feedback control of enzyme biosynthesis
- •Symbiosis
- •Extracellular associations
- •Ecology of cyanobacteria
- •Freshwater environment
- •Terrestrial environment
- •Adaption to silting and salinity
- •Cyanotoxins
- •Cyanobacteria and the quality of drinking water
- •Utilization of cyanobacteria as food
- •Cyanophages
- •Secretion of antibiotics and siderophores
- •Calcium carbonate deposition and fossil record
- •Chroococcales
- •Classification
- •Oscillatoriales
- •Nostocales
- •REFERENCES
- •REFERENCES
- •REFERENCES
- •RHODOPHYCEAE
- •Cell structure
- •Cell walls
- •Chloroplasts and storage products
- •Pit connections
- •Calcification
- •Secretory cells
- •Iridescence
- •Epiphytes and parasites
- •Defense mechanisms of the red algae
- •Commercial utilization of red algal mucilages
- •Reproductive structures
- •Carpogonium
- •Spermatium
- •Fertilization
- •Meiosporangia and meiospores
- •Asexual spores
- •Spore motility
- •Classification
- •Cyanidiales
- •Porphyridiales
- •Bangiales
- •Acrochaetiales
- •Batrachospermales
- •Nemaliales
- •Corallinales
- •Gelidiales
- •Gracilariales
- •Ceramiales
- •REFERENCES
- •Cell structure
- •Phototaxis and eyespots
- •Asexual reproduction
- •Sexual reproduction
- •Classification
- •Position of flagella in cells
- •Flagellar roots
- •Multilayered structure
- •Occurrence of scales or a wall on the motile cells
- •Cell division
- •Superoxide dismutase
- •Prasinophyceae
- •Charophyceae
- •Classification
- •Klebsormidiales
- •Zygnematales
- •Coleochaetales
- •Charales
- •Ulvophyceae
- •Classification
- •Ulotrichales
- •Ulvales
- •Cladophorales
- •Dasycladales
- •Caulerpales
- •Siphonocladales
- •Chlorophyceae
- •Classification
- •Volvocales
- •Tetrasporales
- •Prasiolales
- •Chlorellales
- •Trebouxiales
- •Sphaeropleales
- •Chlorosarcinales
- •Chaetophorales
- •Oedogoniales
- •REFERENCES
- •REFERENCES
- •EUGLENOPHYCEAE
- •Nucleus and nuclear division
- •Eyespot, paraflagellar swelling, and phototaxis
- •Muciferous bodies and extracellular structures
- •Chloroplasts and storage products
- •Nutrition
- •Classification
- •Heteronematales
- •Eutreptiales
- •Euglenales
- •REFERENCES
- •DINOPHYCEAE
- •Cell structure
- •Theca
- •Scales
- •Flagella
- •Pusule
- •Chloroplasts and pigments
- •Phototaxis and eyespots
- •Nucleus
- •Projectiles
- •Accumulation body
- •Resting spores or cysts or hypnospores and fossil Dinophyceae
- •Toxins
- •Dinoflagellates and oil and coal deposits
- •Bioluminescence
- •Rhythms
- •Heterotrophic dinoflagellates
- •Direct engulfment of prey
- •Peduncle feeding
- •Symbiotic dinoflagellates
- •Classification
- •Prorocentrales
- •Dinophysiales
- •Peridiniales
- •Gymnodiniales
- •REFERENCES
- •REFERENCES
- •Chlorarachniophyta
- •REFERENCES
- •CRYPTOPHYCEAE
- •Cell structure
- •Ecology
- •Symbiotic associations
- •Classification
- •Goniomonadales
- •Cryptomonadales
- •Chroomonadales
- •REFERENCES
- •CHRYSOPHYCEAE
- •Cell structure
- •Flagella and eyespot
- •Internal organelles
- •Extracellular deposits
- •Statospores
- •Nutrition
- •Ecology
- •Classification
- •Chromulinales
- •Parmales
- •Chrysomeridales
- •REFERENCES
- •SYNUROPHYCEAE
- •Classification
- •REFERENCES
- •EUSTIGMATOPHYCEAE
- •REFERENCES
- •PINGUIOPHYCEAE
- •REFERENCES
- •DICTYOCHOPHYCEAE
- •Classification
- •Rhizochromulinales
- •Pedinellales
- •Dictyocales
- •REFERENCES
- •PELAGOPHYCEAE
- •REFERENCES
- •BOLIDOPHYCEAE
- •REFERENCE
- •BACILLARIOPHYCEAE
- •Cell structure
- •Cell wall
- •Cell division and the formation of the new wall
- •Extracellular mucilage, biolfouling, and gliding
- •Motility
- •Plastids and storage products
- •Resting spores and resting cells
- •Auxospores
- •Rhythmic phenomena
- •Physiology
- •Chemical defense against predation
- •Ecology
- •Marine environment
- •Freshwater environment
- •Fossil diatoms
- •Classification
- •Biddulphiales
- •Bacillariales
- •REFERENCES
- •RAPHIDOPHYCEAE
- •REFERENCES
- •XANTHOPHYCEAE
- •Cell structure
- •Cell wall
- •Chloroplasts and food reserves
- •Asexual reproduction
- •Sexual reproduction
- •Mischococcales
- •Tribonematales
- •Botrydiales
- •Vaucheriales
- •REFERENCES
- •PHAEOTHAMNIOPHYCEAE
- •REFERENCES
- •PHAEOPHYCEAE
- •Cell structure
- •Cell walls
- •Flagella and eyespot
- •Chloroplasts and photosynthesis
- •Phlorotannins and physodes
- •Life history
- •Classification
- •Dictyotales
- •Sphacelariales
- •Cutleriales
- •Desmarestiales
- •Ectocarpales
- •Laminariales
- •Fucales
- •REFERENCES
- •PRYMNESIOPHYCEAE
- •Cell structure
- •Flagella
- •Haptonema
- •Chloroplasts
- •Other cytoplasmic structures
- •Scales and coccoliths
- •Toxins
- •Classification
- •Prymnesiales
- •Pavlovales
- •REFERENCES
- •Toxic algae
- •Toxic algae and the end-Permian extinction
- •Cooling of the Earth, cloud condensation nuclei, and DMSP
- •Chemical defense mechanisms of algae
- •The Antarctic and Southern Ocean
- •The grand experiment
- •Antarctic lakes as a model for life on the planet Mars or Jupiter’s moon Europa
- •Ultraviolet radiation, the ozone hole, and sunscreens produced by algae
- •Hydrogen fuel cells and hydrogen gas production by algae
- •REFERENCES
- •Glossary
- •Index

Chapter 23
Algae and the environment
It is possible to write whole books on the relationships between algae and the environment. In this chapter I have chosen a few subjects which have generated the most interest in the last decade.
Toxic algae
Algae can be harmful in two basic ways (Hallegraeff et al., 2003).
1Producing large populations in the aquatic environment Large growths of some algae (e.g., the diatom Chaetoceros (Figs. 17.44, 17.45) or the prymnesiophyte Chrysochromulina (Fig. 23.1(c))) can clog the gills of fish and can be particularly a problem in aquaculture systems. Anoxic conditions, resulting in fish kills, can occur at the end of blooms of other algae (e.g.,
green algae) as the algae die and decompose.
2Production of toxins Some algae produce toxins that sicken and kill other organisms that prey on these algae. Indeed, this probably was the reason that these algae were selected for in the evolutionary process since it reduced predation by grazers (Gilbert, 1996). Filter-feeding shellfish can accumulate large quantities of these toxins as they filter the algae out of the water. Consumption of the shellfish by man, birds, and animals results in sickness and death.
The algae that produce phycotoxins are:
Cyanophyceae (cyanobacteria)
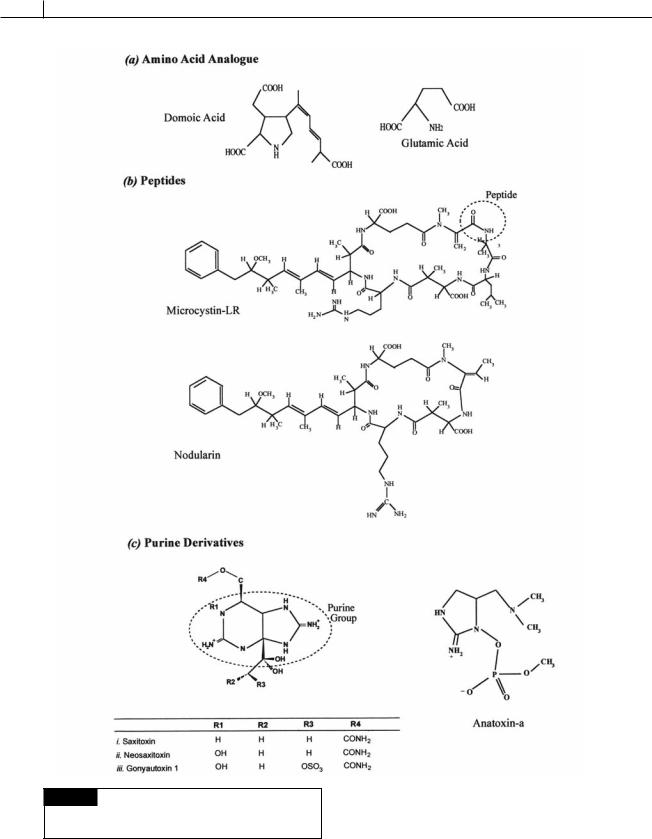
•Neurotoxins anatoxin (Fig. 23.2(c)) and saxitoxin
(Fig. 23.2(c)) that block the transmission of
signal from neuron to neuron. These alkaloids (nitrogen containing compounds) bind to voltage-activated Na -channels and block influx of Na , thereby preventing the generation of an action potential (Shimizu, 2000).
•Hepatotoxins microcystin (Fig. 23.2(b)) and nodularin (Fig. 23.2(b)) that are inhibitors of protein phosphatases 1 and 2A.
Dinophyceae (dinoflagellates)
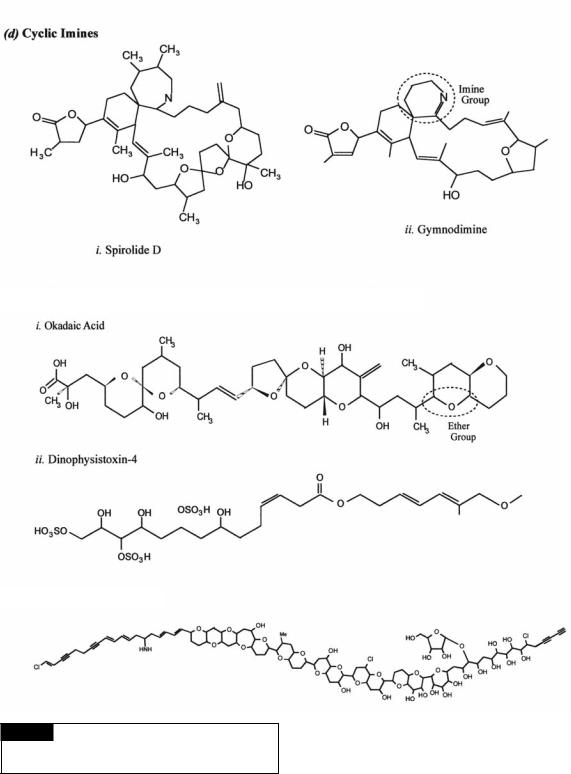
•Diarrhetic shellfish poisoning caused by okadaic acid (Fig. 23.3(e)), macrolide toxins, and yessotoxins (Fig. 23.4(f )). Okadaic acid and the related dinophysistoxins (Fig. 23.3(e)) are inhibitors of protein phosphatases, The free carboxyl groups bind to the catalytic site of the protein phosphatase. Yessotoxin may interfere with cyclic AMP in cells causing cytotoxicity (Cembella, 2003).
•Ciguatera fish poisoning caused by ciguatoxin
(Fig. 23.4(f )), maitotoxins (Fig. 23.4(f )), and brevetoxins (Fig. 23.4(f )). All of these compounds are ion-channel disrupters, increasing the permeability of the cell membrane to positive ions and causing membrane depolarization. Brevetoxins require a lactone ring for activity. Ciguatoxin and brevetoxin bind voltage-sensitive channels, increasing the permeability of the cell membrane and causing membrane depolarization (Cembella, 2003). Maitotoxin initiates Ca2 -channel activation, causing Ca2 -influx and activation of calmodulin (Igarashi et al., 1999). This is followed by

ALGAE AND THE ENVIRONMENT |
505 |
|
|
Fig. 23.1 Examples of toxic marine algae.
506 CHLOROPLAST E.R.: EVOLUTION OF TWO MEMBRANES
Fig. 23.2 Chemical structure of the phycotoxins
composed of amino acid analogues, peptides, and purine
derivatives.
ALGAE AND THE ENVIRONMENT |
507 |
|
|
(e) Non-nitrogen compounds containing linear and macrocyclic polyethers
iii. Prymnesin-2
Fig. 23.3 Chemical structure of phycotoxins composed of
cyclic imines and non-nitrogen compounds containing linear
and macrocyclic polyethers.

508 CHLOROPLAST E.R.: EVOLUTION OF TWO MEMBRANES
Fig. 23.4 Chemical structure of phycotoxins containing
non-nitrogen compounds with ladder-frame polyethers.
ALGAE AND THE ENVIRONMENT |
509 |
|
|
promotion of phospholipase A2 activity and ultimately cell membrane disruption.
•Paralytic shellfish poisoning caused by saxitoxin (Fig. 23.2(c)) and approximately two dozen naturally occurring analogues (Fig. 23.2(c)). The saxitonins bind to voltageactivated Na -channels, blocking influx of Na and preventing the generation of an action potential. Saxitoxins are retained primarily inside cells with very little excretion or leakage from cells. These highly water-soluble, but stable, compounds must be released during senescence or cell lysis, which coincides with the decline of a bloom (Cembella, 2003).
•Spirolide poisoning. This group of cyclic imines include the spirolides (Fig. 23.3(d )), gymnodimine (Fig. 23.3(d )), pteriatoxin and pinnatoxins. The imine ring gives the molecules their toxic properties. They are “fast-acting toxins”, causing death of mice within minutes after oral application.
Bacillariophyceae (diatoms)
•Amnesic shellfish poisoning caused by the neurotoxin domoic acid (Fig. 23.2(a)). Domoic acid is an amino acid that functions as a glutamate agonist (compound that has an affinity for a cell receptor), and in humans causes extensive depolarization in areas rich in glutamate receptors (e.g., the hippocampus). The biosynthesis of domoic acid is inducible and sustained only under stress conditions (Pan et al., 1998).
Raphidophyceae (chloromonads)
•“Red tide” poisoning. Members of the Raphidophyceae produce brevetoxins (Fig. 23.4 (f )), neurotoxins that bind to voltage-activated Na -channels causing rapid influx of Na -ions and membrane depolarization.
Prymnesiophyceae (haptophytes)
•The phycotoxin prymnesin (Fig. 23.3(e)) produces hemolysis of red blood cells by disrupting the pores in the cell membrane, causing influx of positive ions and membrane depolarization.
Another way of classifying phycotoxins is by their chemical structure (Cembella, 2003):
1Amino acid analogues. Domoic acid (Fig. 23.2(a)). This is a glutamate receptor agonist produced
by the diatom Pseudo-nitzchia spp. (Figs. 17.27, 17.29, 23.1(d )).
2Peptides. Microcystins (Figs. 23.2(b)) and nodularin (Fig. 23.2(b)). These phycotoxins are produced by cyanobacteria. Microcystin is formed by species of Microcystis (Figs. 2.48, 2.56(b)), Anabaena (Figs. 2.16, 2.18(d )), Nostoc (Figs. 2.46(b), (c), (d), 2.57(a)), Nodularia (Fig. 2.42(a)) and Oscillatoria (Figs. 2.19(a), (b), 2.34(a)), while nodularins are produced by species of
Nodularia (Fig. 2.42(a)).
3Purine derivatives. Saxitoxins (Fig. 23.2(c )), gonyautoxins (Fig. 23.2(c)), and anatoxin
(Fig. 23.2(c )). There are about two dozen analogues of these molecules with their potency varying over several orders of
magnitude. Saxitoxin has a LD50 of 10 g kg 1. The decarbamoyl derivates have intermediate
toxicity (decarbamoyl saxitoxin has a LD50 of 20 g kg 1) while the N-sulphocarbamoyl
forms have a low toxicity (N-sulphocarbamoyl
(B-toxin)) has a LD50 of 163 g kg 1). Saxitoxins are produced by some
cyanobacteria and by the dinoflagellates Alexandrium spp. (Figs. 7.35, 7.36, 23.1(f )),
Pyrodinium bahamense (Fig. 7.34), and Gymnodinium catenatum. Anatoxin is produced by the cyanobacteria Anabaena (Figs. 2.16, 2.18(d )), Aphanizomenon (Fig. 2.18(b)), Oscillatoria
(Figs. 2.19(a), (b), 2.34(a)), and Trichodesmium
(Fig. 2.56(g)).
4Cyclic imines. Spirolides (Fig. 23.3(d )) and gymnodimine (Fig. 23.3(d )). The spirolides are
produced by the marine dinoflagellate
Alexandrium ostenfeldii while gymnodimine is produced by the dinoflagellate Karenia selliformis.
5Non-nitrogen compounds containing linear and macrocyclic polyethers. Okadaic acid (Fig. 23.3(e)), dinophysistoxins (Fig. 23.3(e)), and prymnesium (Fig. 23.3(e)). Okadaic acid and the related dinophysistoxins are produced by the benthic dinoflagellate Prorocentrum (Figs. 7.30(c), 7.55) and the planktonic forms of Dinophysis (Figs. 7.30(a), (b)). Prymnesium is formed by species of the haptophyte Prymnesium (Fig. 22.7).
