
- •Contents
- •Preface to the first edition
- •Flagella
- •Cell walls and mucilages
- •Plastids
- •Mitochondria and peroxisomes
- •Division of chloroplasts and mitochondria
- •Storage products
- •Contractile vacuoles
- •Nutrition
- •Gene sequencing and algal systematics
- •Classification
- •Algae and the fossil record
- •REFERENCES
- •CYANOPHYCEAE
- •Morphology
- •Cell wall and gliding
- •Pili and twitching
- •Sheaths
- •Protoplasmic structure
- •Gas vacuoles
- •Pigments and photosynthesis
- •Akinetes
- •Heterocysts
- •Nitrogen fixation
- •Asexual reproduction
- •Growth and metabolism
- •Lack of feedback control of enzyme biosynthesis
- •Symbiosis
- •Extracellular associations
- •Ecology of cyanobacteria
- •Freshwater environment
- •Terrestrial environment
- •Adaption to silting and salinity
- •Cyanotoxins
- •Cyanobacteria and the quality of drinking water
- •Utilization of cyanobacteria as food
- •Cyanophages
- •Secretion of antibiotics and siderophores
- •Calcium carbonate deposition and fossil record
- •Chroococcales
- •Classification
- •Oscillatoriales
- •Nostocales
- •REFERENCES
- •REFERENCES
- •REFERENCES
- •RHODOPHYCEAE
- •Cell structure
- •Cell walls
- •Chloroplasts and storage products
- •Pit connections
- •Calcification
- •Secretory cells
- •Iridescence
- •Epiphytes and parasites
- •Defense mechanisms of the red algae
- •Commercial utilization of red algal mucilages
- •Reproductive structures
- •Carpogonium
- •Spermatium
- •Fertilization
- •Meiosporangia and meiospores
- •Asexual spores
- •Spore motility
- •Classification
- •Cyanidiales
- •Porphyridiales
- •Bangiales
- •Acrochaetiales
- •Batrachospermales
- •Nemaliales
- •Corallinales
- •Gelidiales
- •Gracilariales
- •Ceramiales
- •REFERENCES
- •Cell structure
- •Phototaxis and eyespots
- •Asexual reproduction
- •Sexual reproduction
- •Classification
- •Position of flagella in cells
- •Flagellar roots
- •Multilayered structure
- •Occurrence of scales or a wall on the motile cells
- •Cell division
- •Superoxide dismutase
- •Prasinophyceae
- •Charophyceae
- •Classification
- •Klebsormidiales
- •Zygnematales
- •Coleochaetales
- •Charales
- •Ulvophyceae
- •Classification
- •Ulotrichales
- •Ulvales
- •Cladophorales
- •Dasycladales
- •Caulerpales
- •Siphonocladales
- •Chlorophyceae
- •Classification
- •Volvocales
- •Tetrasporales
- •Prasiolales
- •Chlorellales
- •Trebouxiales
- •Sphaeropleales
- •Chlorosarcinales
- •Chaetophorales
- •Oedogoniales
- •REFERENCES
- •REFERENCES
- •EUGLENOPHYCEAE
- •Nucleus and nuclear division
- •Eyespot, paraflagellar swelling, and phototaxis
- •Muciferous bodies and extracellular structures
- •Chloroplasts and storage products
- •Nutrition
- •Classification
- •Heteronematales
- •Eutreptiales
- •Euglenales
- •REFERENCES
- •DINOPHYCEAE
- •Cell structure
- •Theca
- •Scales
- •Flagella
- •Pusule
- •Chloroplasts and pigments
- •Phototaxis and eyespots
- •Nucleus
- •Projectiles
- •Accumulation body
- •Resting spores or cysts or hypnospores and fossil Dinophyceae
- •Toxins
- •Dinoflagellates and oil and coal deposits
- •Bioluminescence
- •Rhythms
- •Heterotrophic dinoflagellates
- •Direct engulfment of prey
- •Peduncle feeding
- •Symbiotic dinoflagellates
- •Classification
- •Prorocentrales
- •Dinophysiales
- •Peridiniales
- •Gymnodiniales
- •REFERENCES
- •REFERENCES
- •Chlorarachniophyta
- •REFERENCES
- •CRYPTOPHYCEAE
- •Cell structure
- •Ecology
- •Symbiotic associations
- •Classification
- •Goniomonadales
- •Cryptomonadales
- •Chroomonadales
- •REFERENCES
- •CHRYSOPHYCEAE
- •Cell structure
- •Flagella and eyespot
- •Internal organelles
- •Extracellular deposits
- •Statospores
- •Nutrition
- •Ecology
- •Classification
- •Chromulinales
- •Parmales
- •Chrysomeridales
- •REFERENCES
- •SYNUROPHYCEAE
- •Classification
- •REFERENCES
- •EUSTIGMATOPHYCEAE
- •REFERENCES
- •PINGUIOPHYCEAE
- •REFERENCES
- •DICTYOCHOPHYCEAE
- •Classification
- •Rhizochromulinales
- •Pedinellales
- •Dictyocales
- •REFERENCES
- •PELAGOPHYCEAE
- •REFERENCES
- •BOLIDOPHYCEAE
- •REFERENCE
- •BACILLARIOPHYCEAE
- •Cell structure
- •Cell wall
- •Cell division and the formation of the new wall
- •Extracellular mucilage, biolfouling, and gliding
- •Motility
- •Plastids and storage products
- •Resting spores and resting cells
- •Auxospores
- •Rhythmic phenomena
- •Physiology
- •Chemical defense against predation
- •Ecology
- •Marine environment
- •Freshwater environment
- •Fossil diatoms
- •Classification
- •Biddulphiales
- •Bacillariales
- •REFERENCES
- •RAPHIDOPHYCEAE
- •REFERENCES
- •XANTHOPHYCEAE
- •Cell structure
- •Cell wall
- •Chloroplasts and food reserves
- •Asexual reproduction
- •Sexual reproduction
- •Mischococcales
- •Tribonematales
- •Botrydiales
- •Vaucheriales
- •REFERENCES
- •PHAEOTHAMNIOPHYCEAE
- •REFERENCES
- •PHAEOPHYCEAE
- •Cell structure
- •Cell walls
- •Flagella and eyespot
- •Chloroplasts and photosynthesis
- •Phlorotannins and physodes
- •Life history
- •Classification
- •Dictyotales
- •Sphacelariales
- •Cutleriales
- •Desmarestiales
- •Ectocarpales
- •Laminariales
- •Fucales
- •REFERENCES
- •PRYMNESIOPHYCEAE
- •Cell structure
- •Flagella
- •Haptonema
- •Chloroplasts
- •Other cytoplasmic structures
- •Scales and coccoliths
- •Toxins
- •Classification
- •Prymnesiales
- •Pavlovales
- •REFERENCES
- •Toxic algae
- •Toxic algae and the end-Permian extinction
- •Cooling of the Earth, cloud condensation nuclei, and DMSP
- •Chemical defense mechanisms of algae
- •The Antarctic and Southern Ocean
- •The grand experiment
- •Antarctic lakes as a model for life on the planet Mars or Jupiter’s moon Europa
- •Ultraviolet radiation, the ozone hole, and sunscreens produced by algae
- •Hydrogen fuel cells and hydrogen gas production by algae
- •REFERENCES
- •Glossary
- •Index
386 CHLOROPLAST E.R.: EVOLUTION OF TWO MEMBRANES
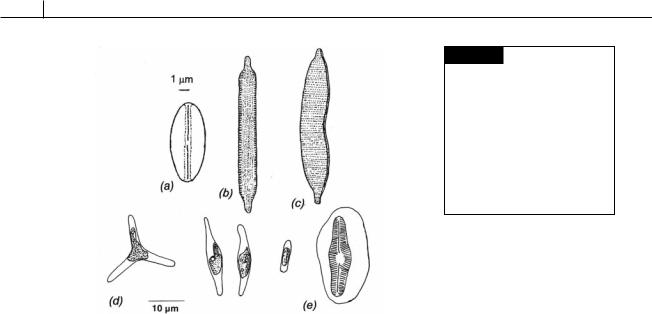
Fig. 17.26 (a) Navicula pelliculosa.
(b) Nitzschia palea. (c) Hantzschia amphioxys. (d), (e) Phaeodactylum tricornutum. (d) Three forms, triradiate, fusiform, and oval. The alga is pleomorphic, ovate cells form on agar or solutions low in calcium while fusiform and triradiate cells occur in liquid medium. (e) An oval cell embedding in mucilage with a single valve. (After Wilson, 1946.)
equal halves. The auxospore produces bands that mold the cell into the tubular perizonium. The new pennate frustule is formed within the perizonium of the mature auxospore.
Sexual reproduction in diatoms can occur only after two general conditions have been met (Edlund and Stoermer, 1997). First, cells must reach a minimum size range, typically 30–40% of their maximum size. Second, there must be the presence of correct environmental conditions. These include combinations of temperature, light, nutrients, trace metals, organic growth factors, and osmolarity (Potapova and Snoeijs, 1997). Contrary to most other algal groups, sexuality is primarily a means of size restoration, and is not normally a factor in dormancy or dispersal (Edlund and Stoermer, 1997).
Rhythmic phenomena
It is possible to synchronize the division of diatom cells in a culture in a couple of different ways. Lewin (1966) has shown that removal of silicon from cultures of Navicula pelliculosa (Fig. 17.26(a)) stops growth of the cells at a stage prior to cytokinesis. When silica is added to the culture,
all of the cells then divide synchronously. Another way of obtaining synchronized cell divisions is by keeping the diatoms in the dark for a long period followed by exposure to light. In Nitzschia palea (Fig. 17.26(b)) the shortest time that can be obtained between cell divisions is 16 hours (von Denffer, 1949). If the cells are grown on an 8 hour light : 8 hour dark cycle, synchronously dividing cells are obtained. If the cycle is shortened to 6 hours light : 6 hours dark, then cell division occurs every second dark period because there is insufficient time for the diatom to prepare itself for the next division.
Fauré-Fremiet (1951) was the first to observe that the diatom Hantzschia amphioxys (Fig. 17.26(c)) accumulates on mud flats in certain areas during low tides, causing brown spots on the mud. The cells can glide rapidly at a rate of half their length in a second and can reverse their phototactic response. During low tide the cells are positively phototactic, so that they come to the surface of the mud, and at high tide they are negatively phototactic, so that they descend into the mud. At high tide they also exude a mucilaginous material, which causes them to stick to one another and to sand grains. This rhythmic phenomenon prevents the cells from being washed away by the tide.
HETEROKONTOPHYTA, BACILLARIOPHYCEAE |
387 |
|
|
Physiology
For growth of Navicula pelliculosa (Fig. 17.26(a)), silicon cannot be replaced by any of the elements similar to it in physical and chemical properties or in atomic radius, such as Ge, C, Sn, Pb, As, P, B, Al, Mg, or Fe (Lewin, 1962). Lewin (1966) has shown that with a mixture of algae and other organisms, concentrations of germanium dioxide (GeO2) above 1.5 mg liter 1 will specifically suppress the growth of diatoms. In cultures of many types of algae, diatom contaminants frequently grow so fast that they obscure the growth of the algae the investigator is working with. The finding that GeO2 specifically inhibits diatom growth was a welcome one for phycologists working on algal cultures. In experiments with 14 pure cultures of diatoms, 1 mg liter 1 of GeO2 reduced the growth rate significantly; 10 mg liter 1 was even more inhibitory to growth and in a few cases killed the cells. Phaeodactylum tricornutum (Fig. 17.46(d)), a diatom with little or no silicified wall, was the least sensitive to GeO2 of the diatoms tested. By increasing the amount of SiO2 in solution, it is possible to reverse the inhibitory effect of GeO2 on growth. The results indicate that GeO2 is a specific inhibitor of silicate utilization because concentrations of GeO2 as high as 400 mg liter 1 have no effect on respiration. The minimum inhibitory concentrations of GeO2 depends to a certain extent on the pH and concentration of the nutrient medium. Such inhibitory concentrations are rarely, if ever, reached in natural waters, the highest concentration reported being that of a mineral spring in Niigata Prefecture, Japan, which contained only 0.03 mg liter 1 of Ge, with normal concentrations in seawater being about 5 10 5 mg liter 1.
In addition to responding adversely to germanium in solution, diatoms are sensitive to copper. Erickson (1972) has shown that Thalassiosira pseudonana has its growth inhibited at concentrations of copper as low as 5 g liter 1. The presence of detritus affects the toxicity to a certain extent. Concentrations of 0.25 ppm copper as CuSO4 · 5H2O are normally used to control algal blooms without affecting fish in freshwater lakes. Copper toxicity and silicon metabolism are linked in
several marine diatoms. Skeletonema costatum
(Fig. 17.8(e), 17.31(a)) and Thalassiosira pseudonana exhibit slower growth in the presence of copper. Inhibition of growth by copper is alleviated by increasing the silicic acid concentration in the media (Morel et al., 1978; Rueter et al., 1981). It is probable that copper interferes with the silicic acid transport site across the plasma membrane and slows uptake of silicic acid (Rueter et al., 1981; Rueter, 1983).
The effects of heavy metals on diatoms can be divided into three groups (Thomas et al., 1980): (1) Cu, Zi, and Ge affect the biochemical pathway of silicon metabolism; (2) Hg, Cd, and Pb interfere with cell division and cause morphologically distorted cells to be produced; and (3) Cr, Ni, Se, and Sb have no effects up to a concentration of 1 M, well above the concentrations that show effects with other toxic metals.
Some normally photosynthetic diatoms are able to grow under heterotrophic conditions (Kroger, 2001). In two studies (Lewin, 1953; Lewin and Lewin, 1960), a number of cultures of photosynthetic diatoms were found that would grow heterotrophically, usually only with glucose as a carbon source. One of these diatoms, Cyclotella cryptica (Hellebust, 1971), can grow in the dark in an organic medium with glucose (but not lactate or tryptone) as the sole carbon source. When the organism is growing in the light, it does not have the mechanism for the utilization of glucose in the medium. It requires about 24 hours in the dark in a glucose medium before it is able to use the glucose as a carbon source. This lag period indicates that the lack of light induces an uptake and/or assimilation system for the glucose. White (1974) suggested that such facultative heterotrophy enables these diatoms to settle into bottom deposits, live heterotrophically for long periods, then rise and begin photosynthesis again. Although the above diatoms still have functional chloroplasts, there are some apochlorotic diatoms lacking functional chloroplasts (Lewin and Lewin, 1967). The latter, which are species of Nitzschia (Fig. 17.26(b)), are able to grow with lactate or succinate as the sole organic carbon source.
Amnesic shellfish poisoning occurs when shellfish filter diatoms from the genera Nitzschia (Figs. 17.26(b), 17.35), Pseudo-nitzschia (Figs. 17.27,
388 CHLOROPLAST E.R.: EVOLUTION OF TWO MEMBRANES
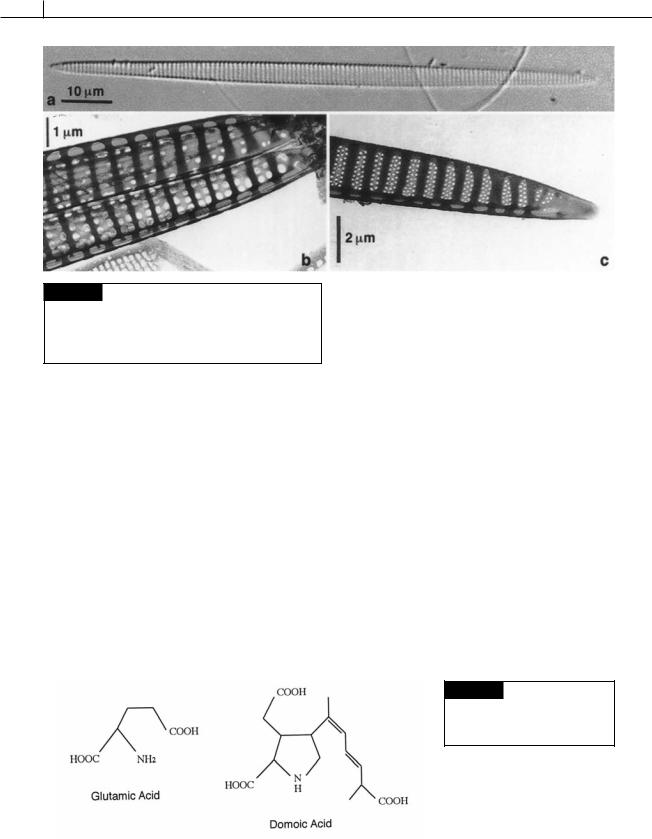
Fig. 17.27 Pseudo-nitzschia, a diatom responsible for amnesic shellfish poisoning. (a) Light micrograph of one valve. (b), (c) Transmission electron micrographs of the ends of cleaned valves showing the ribs and poroids. (From Hasle, 1995.)
17.29), and Amphora (Figs. 17.22(c), (d)), 17.35) from marine waters (Bates, 2000; Lundholm and Moestrup, 2000). Subsequent ingestion of the shellfish by man and birds results in memory loss (amnesia), abdominal cramps, vomiting, disorientation, and even death. Amnesic shellfish poisoning was first recognized in 1987 in Prince Edward Island, Canada, where it caused 3 deaths and 105 other affected individuals after blue mussels were eaten (Subba Rao et al., 1988). The diatom produces domoic acid, a derivative of the neuroexcitatory amino acid L-glutamic acid (Fig. 17.28). Domoic acid is especially prevalent in moribund cells of the diatom and can be induced by depriving the cells of nutrients, particularly silicate and phosphate (Pan et al., 1996).
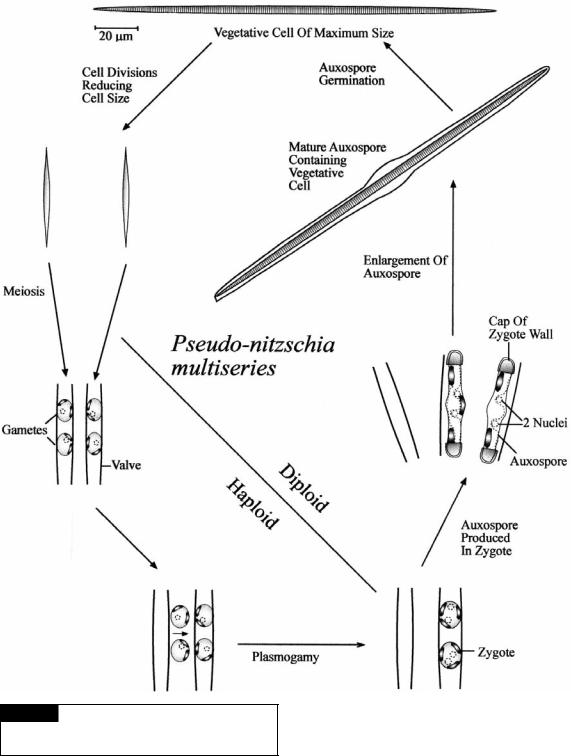
Sexual reproduction in Pseudo-nitzschia multiseries is on an internal clock controlled by the size of the cells (Davidovich and Bates, 1998). A maximum cell size is attained after sexual reproduction by auxospore germination producing vegetative cells with a length of 120–170 m (Fig. 17.29). Sexual reproduction is induced in nature about three years later after successive cell divisions have decreased the cell size by 30–40%. Mating in P. multiseries begins when cells of different strains contact each other, valve to valve in parallel. Gametogenesis occurs by cells dividing meiotically to form morphologically similar nonflagellated gametes. The valves of the parent frustules separate and gametes from one cell (male) travel by amoeboid movement to the passive gametes in the other cell (female). Within a couple of minutes, the gametes fuse to form a spherical zygote that expands to produce auxospores still attached to one of the parent valves. The fully expanded auxospore still has unfused
Fig. 17.28 The structure of domoic acid, the causative chemical of amnesic shellfish poisoning, and its analog, L-glutamic acid.
HETEROKONTOPHYTA, BACILLARIOPHYCEAE |
389 |
|
|
Fig. 17.29 The life cycle of the toxic diatom Pseudo-
nitzschia multiseries. (Modified from Davidovich and Bates,
1998.)
