
- •Contents
- •Preface to the first edition
- •Flagella
- •Cell walls and mucilages
- •Plastids
- •Mitochondria and peroxisomes
- •Division of chloroplasts and mitochondria
- •Storage products
- •Contractile vacuoles
- •Nutrition
- •Gene sequencing and algal systematics
- •Classification
- •Algae and the fossil record
- •REFERENCES
- •CYANOPHYCEAE
- •Morphology
- •Cell wall and gliding
- •Pili and twitching
- •Sheaths
- •Protoplasmic structure
- •Gas vacuoles
- •Pigments and photosynthesis
- •Akinetes
- •Heterocysts
- •Nitrogen fixation
- •Asexual reproduction
- •Growth and metabolism
- •Lack of feedback control of enzyme biosynthesis
- •Symbiosis
- •Extracellular associations
- •Ecology of cyanobacteria
- •Freshwater environment
- •Terrestrial environment
- •Adaption to silting and salinity
- •Cyanotoxins
- •Cyanobacteria and the quality of drinking water
- •Utilization of cyanobacteria as food
- •Cyanophages
- •Secretion of antibiotics and siderophores
- •Calcium carbonate deposition and fossil record
- •Chroococcales
- •Classification
- •Oscillatoriales
- •Nostocales
- •REFERENCES
- •REFERENCES
- •REFERENCES
- •RHODOPHYCEAE
- •Cell structure
- •Cell walls
- •Chloroplasts and storage products
- •Pit connections
- •Calcification
- •Secretory cells
- •Iridescence
- •Epiphytes and parasites
- •Defense mechanisms of the red algae
- •Commercial utilization of red algal mucilages
- •Reproductive structures
- •Carpogonium
- •Spermatium
- •Fertilization
- •Meiosporangia and meiospores
- •Asexual spores
- •Spore motility
- •Classification
- •Cyanidiales
- •Porphyridiales
- •Bangiales
- •Acrochaetiales
- •Batrachospermales
- •Nemaliales
- •Corallinales
- •Gelidiales
- •Gracilariales
- •Ceramiales
- •REFERENCES
- •Cell structure
- •Phototaxis and eyespots
- •Asexual reproduction
- •Sexual reproduction
- •Classification
- •Position of flagella in cells
- •Flagellar roots
- •Multilayered structure
- •Occurrence of scales or a wall on the motile cells
- •Cell division
- •Superoxide dismutase
- •Prasinophyceae
- •Charophyceae
- •Classification
- •Klebsormidiales
- •Zygnematales
- •Coleochaetales
- •Charales
- •Ulvophyceae
- •Classification
- •Ulotrichales
- •Ulvales
- •Cladophorales
- •Dasycladales
- •Caulerpales
- •Siphonocladales
- •Chlorophyceae
- •Classification
- •Volvocales
- •Tetrasporales
- •Prasiolales
- •Chlorellales
- •Trebouxiales
- •Sphaeropleales
- •Chlorosarcinales
- •Chaetophorales
- •Oedogoniales
- •REFERENCES
- •REFERENCES
- •EUGLENOPHYCEAE
- •Nucleus and nuclear division
- •Eyespot, paraflagellar swelling, and phototaxis
- •Muciferous bodies and extracellular structures
- •Chloroplasts and storage products
- •Nutrition
- •Classification
- •Heteronematales
- •Eutreptiales
- •Euglenales
- •REFERENCES
- •DINOPHYCEAE
- •Cell structure
- •Theca
- •Scales
- •Flagella
- •Pusule
- •Chloroplasts and pigments
- •Phototaxis and eyespots
- •Nucleus
- •Projectiles
- •Accumulation body
- •Resting spores or cysts or hypnospores and fossil Dinophyceae
- •Toxins
- •Dinoflagellates and oil and coal deposits
- •Bioluminescence
- •Rhythms
- •Heterotrophic dinoflagellates
- •Direct engulfment of prey
- •Peduncle feeding
- •Symbiotic dinoflagellates
- •Classification
- •Prorocentrales
- •Dinophysiales
- •Peridiniales
- •Gymnodiniales
- •REFERENCES
- •REFERENCES
- •Chlorarachniophyta
- •REFERENCES
- •CRYPTOPHYCEAE
- •Cell structure
- •Ecology
- •Symbiotic associations
- •Classification
- •Goniomonadales
- •Cryptomonadales
- •Chroomonadales
- •REFERENCES
- •CHRYSOPHYCEAE
- •Cell structure
- •Flagella and eyespot
- •Internal organelles
- •Extracellular deposits
- •Statospores
- •Nutrition
- •Ecology
- •Classification
- •Chromulinales
- •Parmales
- •Chrysomeridales
- •REFERENCES
- •SYNUROPHYCEAE
- •Classification
- •REFERENCES
- •EUSTIGMATOPHYCEAE
- •REFERENCES
- •PINGUIOPHYCEAE
- •REFERENCES
- •DICTYOCHOPHYCEAE
- •Classification
- •Rhizochromulinales
- •Pedinellales
- •Dictyocales
- •REFERENCES
- •PELAGOPHYCEAE
- •REFERENCES
- •BOLIDOPHYCEAE
- •REFERENCE
- •BACILLARIOPHYCEAE
- •Cell structure
- •Cell wall
- •Cell division and the formation of the new wall
- •Extracellular mucilage, biolfouling, and gliding
- •Motility
- •Plastids and storage products
- •Resting spores and resting cells
- •Auxospores
- •Rhythmic phenomena
- •Physiology
- •Chemical defense against predation
- •Ecology
- •Marine environment
- •Freshwater environment
- •Fossil diatoms
- •Classification
- •Biddulphiales
- •Bacillariales
- •REFERENCES
- •RAPHIDOPHYCEAE
- •REFERENCES
- •XANTHOPHYCEAE
- •Cell structure
- •Cell wall
- •Chloroplasts and food reserves
- •Asexual reproduction
- •Sexual reproduction
- •Mischococcales
- •Tribonematales
- •Botrydiales
- •Vaucheriales
- •REFERENCES
- •PHAEOTHAMNIOPHYCEAE
- •REFERENCES
- •PHAEOPHYCEAE
- •Cell structure
- •Cell walls
- •Flagella and eyespot
- •Chloroplasts and photosynthesis
- •Phlorotannins and physodes
- •Life history
- •Classification
- •Dictyotales
- •Sphacelariales
- •Cutleriales
- •Desmarestiales
- •Ectocarpales
- •Laminariales
- •Fucales
- •REFERENCES
- •PRYMNESIOPHYCEAE
- •Cell structure
- •Flagella
- •Haptonema
- •Chloroplasts
- •Other cytoplasmic structures
- •Scales and coccoliths
- •Toxins
- •Classification
- •Prymnesiales
- •Pavlovales
- •REFERENCES
- •Toxic algae
- •Toxic algae and the end-Permian extinction
- •Cooling of the Earth, cloud condensation nuclei, and DMSP
- •Chemical defense mechanisms of algae
- •The Antarctic and Southern Ocean
- •The grand experiment
- •Antarctic lakes as a model for life on the planet Mars or Jupiter’s moon Europa
- •Ultraviolet radiation, the ozone hole, and sunscreens produced by algae
- •Hydrogen fuel cells and hydrogen gas production by algae
- •REFERENCES
- •Glossary
- •Index
382 CHLOROPLAST E.R.: EVOLUTION OF TWO MEMBRANES
(c)
(d)
(b)
(a)
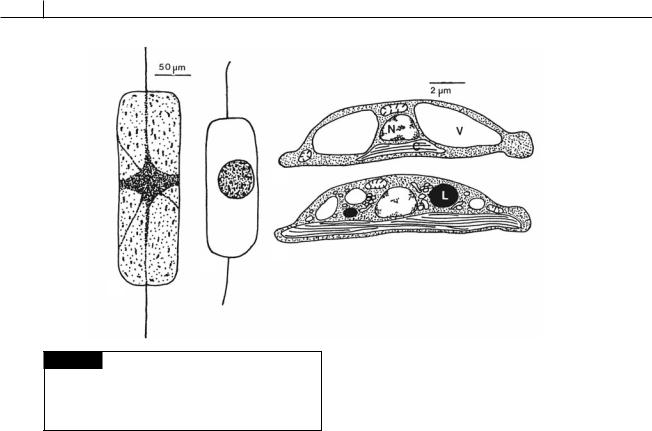
Fig. 17.22 (a), (b) Ditylum brightwelli. (a) Vegetative cell. (b) Resting spore. (c), (d) Amphora coffaeformis. Drawings of
the ultrastructure of a vegetative cell (c) and a resting cell (d).
(C) Chloroplast; (L) lipid; (N) nucleus; (V) vacuole. ((a), (b) after Gross, 1940; (c), (d) after Anderson, 1975.)
Resting spores and resting cells
Some diatom cells form thick, ornamented walls at different times in their life cycle and become resting spores (Fig. 17.22) (McQuoid and Hobson, 1996). If such cells are planktonic, they fall to the bottom where, presumably, they await more favorable conditions. In Ditylum brightwelli (Gross, 1940), the resting spore is formed by the protoplasm shrinking and the plasmalemma drawing away from the cell wall (Fig. 17.22(a), (b)). The resting spore then formed has a much smaller volume than the original cell, the main decrease being due to the loss of vacuoles and their contents. In germination, the resting spore produces a number of fine protoplasmic strands, which radiate in all directions. Chloroplasts are gradually released from the central mass and move along the processes while the whole protoplast expands. After 2 days, the protoplast has assumed a spherical shape, resembling an auxospore. This protoplast elongates and becomes cylindrical, with a valve appearing at one end within 24 hours and a
second valve appearing at the other end after 48 hours. The new cells are much wider than the parent cells. As noted above, the normal method of reproduction, by binary fission, results in a decreased size in the daughter cells. In some species a certain size is reached when sexually or asexually produced auxospores may reestablish the maximum cell size (Nagai et al., 1995). It is, however, known that auxospore formation is generally infrequent, probably taking place once in two or more years in some species. Resting spore formation is therefore a much more frequent method of reestablishment of cell size (Fig. 17.44).
Resting spores are formed after the diatom cells have been subjected to a stress shock. In the ocean, light, temperature, and salinity are relatively stable, with sudden changes rarely occurring, even though there are seasonal changes. In the marine environment, it is usually stress shocks from nutrient depletion that trigger resting spore formation (Davis et al., 1980). Because of the exponential nature of phytoplankton growth, the limiting nutrient is reduced from a considerable level to near zero during the last doubling of the phytoplankton at the end of a bloom (in 1 day or less for many diatoms). Thus a sudden nutrient shock is a typical feature at the end of a diatom bloom.
Marine centric diatoms commonly form morphologically different resting spores, whereas
HETEROKONTOPHYTA, BACILLARIOPHYCEAE |
383 |
|
|
there are only a few pennate diatoms that do. Eunotia soleirolii is one of the few freshwater pennate diatoms that form resting spores (von Stosch and Fecher, 1979). In the freshwater environment, diatoms are subjected to more environmental shocks than in the marine environment. Eunotia soleirolii will form resting spores when subjected to (1) high temperatures (24 °C); (2) high or low pH; or (3) iron, silica, phosphate, or nitrate deficiencies. In many other diatoms, resting spores germinate when placed in fresh medium. However, the resting spores of E. soleirolii require dormancy to be broken by a dark period at –2 °C to 15 °C for a minimum of 4 to 5 weeks. Thus E. soleirolii thrives in colder seasons in northern Europe and is induced to form resting spores by rising temperatures and perhaps concomitant nutrient deficiencies. The resting spores are viable for up to 3 years, but would normally last the summer before dormancy would be broken by the cold temperature of autumn, resulting in germination of the resting spores.
Resting cells have the same morphology as vegetative cells and do not form a protective layer, thereby differing from resting spores. Growing vegetative cells of Amphora coffaeformis require 4 weeks in the dark to form resting cells (Anderson, 1975). Formation of resting cells initially involves autophagic activity, with the breakdown of existing structures. Large vacuoles decrease in size, and many small ones develop; the mitochondria become fewer; and large lipid bodies are formed (Fig. 17.22). The resting cells contain as much chlorophyll as the vegetative cells, and the whole cell appears to be a parsimonious assemblage of organelles prepared to resume metabolism and growth upon return to favorable conditions. During the summer, diatom cells sink into deep water below the euphotic zone, where they form resting cells and become dormant. Such cells remain viable for at least 2 months in such an environment (Anderson, 1976). Their respiratory rate is 20% that of normal cells, and their photosynthetic capability is very low. Vertical mixing brings these cells and nutrient-rich water to the surface, and within 2 days the cells become active and begin to reproduce. Viable resting cells of diatoms have been collected at 6150-m water depth in the North Atlantic.
Auxospores
Auxospore formation is a second mechanism (in addition to resting spores) for reestablishing the original size of the cell. The auxospores are formed by the fusion of two gametes. In the centric and gonoid diatoms, the male gamete is motile (Fig. 17.45), whereas the female gamete (egg) is nonmotile (Figs. 17.42, 17.44). In the pennate and trellisoid diatoms, both gametes are non-flagellated (Figs. 17.29, 17.46) (Chepurnov et al., 2004).
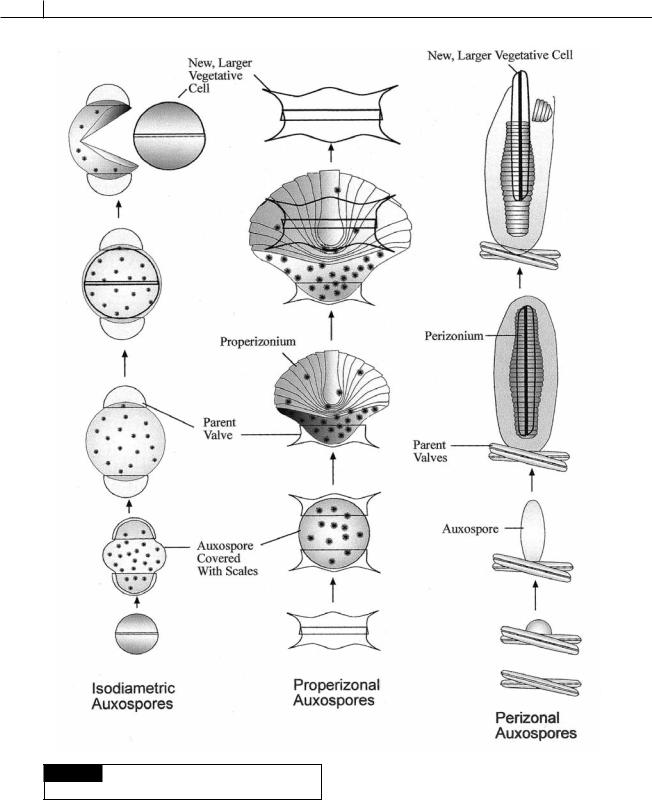
Depending on the species, auxospores develop in one of three different ways (Fig. 17.23) (von Stosch, 1982; Medlin and Kaczmarska, 2004).
1Isodiametric auxospores. Centric diatoms such as
Melosira (Figs. 17.35, 17.40, 17.41, 17.42, 17.43), Coscinodiscus (Figs. 17.2(a), 17.6(b), 17.15, 17.38(a), (b)), and Stephanopyxis (Figs. 17.33(a), 17.38(c), (d)) produce this type of auxospore. The globular auxospore more or less maintains its shape during development. The auxospore usually is attached to the parent valves during maturation. The auxospore has a wall with embedded scales (Fig. 17.24), inside of which are produced the roughly dome-
shaped valves of the mature vegetative cells.
2Properizonial auxospores. Centric diatoms such as Chaetoceros (Figs. 17.6(a), 17.32, 17.44, 17.45) produce non-isodiametric (non-spherical) mature auxospores. The immature globular auxospore splits and bands are produced from an asymmetrical auxospore. The bands are collectively called the properizonium. In Chaetoceros, a fan-like assortment of bands is formed on the dorsal surface of the auxospore while the ventral surface is composed of a primary envelope containing scales. The mature auxospore produces a flattened
frustule similar in shape to the vegetative cell.
3Perizonial auxospores. Pennate diatoms such as Navicula (Figs. 17.2(d), 17.16, 17.25, 17.26(a), 17.33(b), 17.35) and Pseudo-nitzschia (Figs. 17.27, 17.29) form this type of non-isodiametric mature auxospore (Sato et al., 2004). The immature globular auxospore has scales embedded in a primary wall. The primary auxosore wall splits and separates into two
384 CHLOROPLAST E.R.: EVOLUTION OF TWO MEMBRANES
Fig. 17.23 Diagrammatic summary of the three types of
sexual auxospores in diatoms.
HETEROKONTOPHYTA, BACILLARIOPHYCEAE |
385 |
|
|
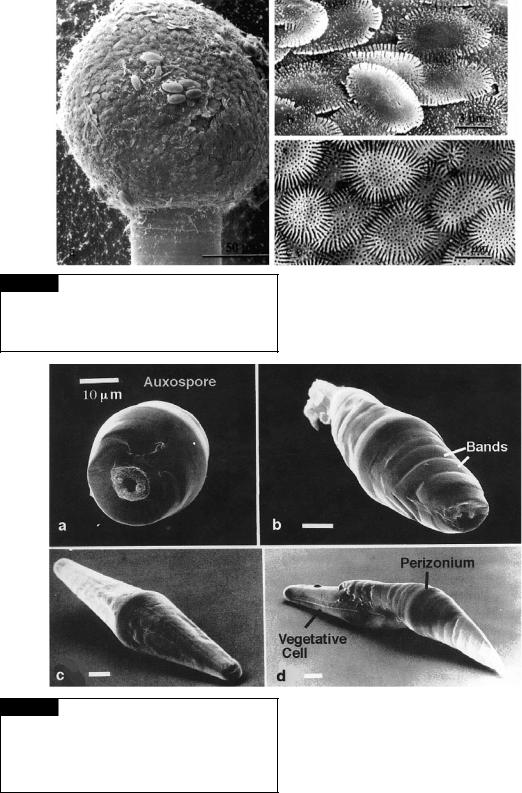
Fig. 17.24 Ellerbeckia arenaria. Scanning electron micrographs of auxospores. (Left) Scale-covered auxospore perched in the epitheca. (Top right) External view of scales. (Bottom right) Internal view of scales. (From Schmid and Crawford, 2001.)
Fig 17.25 Scanning electron micrographs of sexual stages of Navicula cuspidata. (a) Immature auxospore. (b) Auxospore midway through elongation. The underlying perizonal bands are evident. (c) Mature auxospore showing biconical shape of the perizonium. (d) Vegetative cell emerging from the perizonium. (From Cohn et al., 1989.)
