
- •Contents
- •Preface to the first edition
- •Flagella
- •Cell walls and mucilages
- •Plastids
- •Mitochondria and peroxisomes
- •Division of chloroplasts and mitochondria
- •Storage products
- •Contractile vacuoles
- •Nutrition
- •Gene sequencing and algal systematics
- •Classification
- •Algae and the fossil record
- •REFERENCES
- •CYANOPHYCEAE
- •Morphology
- •Cell wall and gliding
- •Pili and twitching
- •Sheaths
- •Protoplasmic structure
- •Gas vacuoles
- •Pigments and photosynthesis
- •Akinetes
- •Heterocysts
- •Nitrogen fixation
- •Asexual reproduction
- •Growth and metabolism
- •Lack of feedback control of enzyme biosynthesis
- •Symbiosis
- •Extracellular associations
- •Ecology of cyanobacteria
- •Freshwater environment
- •Terrestrial environment
- •Adaption to silting and salinity
- •Cyanotoxins
- •Cyanobacteria and the quality of drinking water
- •Utilization of cyanobacteria as food
- •Cyanophages
- •Secretion of antibiotics and siderophores
- •Calcium carbonate deposition and fossil record
- •Chroococcales
- •Classification
- •Oscillatoriales
- •Nostocales
- •REFERENCES
- •REFERENCES
- •REFERENCES
- •RHODOPHYCEAE
- •Cell structure
- •Cell walls
- •Chloroplasts and storage products
- •Pit connections
- •Calcification
- •Secretory cells
- •Iridescence
- •Epiphytes and parasites
- •Defense mechanisms of the red algae
- •Commercial utilization of red algal mucilages
- •Reproductive structures
- •Carpogonium
- •Spermatium
- •Fertilization
- •Meiosporangia and meiospores
- •Asexual spores
- •Spore motility
- •Classification
- •Cyanidiales
- •Porphyridiales
- •Bangiales
- •Acrochaetiales
- •Batrachospermales
- •Nemaliales
- •Corallinales
- •Gelidiales
- •Gracilariales
- •Ceramiales
- •REFERENCES
- •Cell structure
- •Phototaxis and eyespots
- •Asexual reproduction
- •Sexual reproduction
- •Classification
- •Position of flagella in cells
- •Flagellar roots
- •Multilayered structure
- •Occurrence of scales or a wall on the motile cells
- •Cell division
- •Superoxide dismutase
- •Prasinophyceae
- •Charophyceae
- •Classification
- •Klebsormidiales
- •Zygnematales
- •Coleochaetales
- •Charales
- •Ulvophyceae
- •Classification
- •Ulotrichales
- •Ulvales
- •Cladophorales
- •Dasycladales
- •Caulerpales
- •Siphonocladales
- •Chlorophyceae
- •Classification
- •Volvocales
- •Tetrasporales
- •Prasiolales
- •Chlorellales
- •Trebouxiales
- •Sphaeropleales
- •Chlorosarcinales
- •Chaetophorales
- •Oedogoniales
- •REFERENCES
- •REFERENCES
- •EUGLENOPHYCEAE
- •Nucleus and nuclear division
- •Eyespot, paraflagellar swelling, and phototaxis
- •Muciferous bodies and extracellular structures
- •Chloroplasts and storage products
- •Nutrition
- •Classification
- •Heteronematales
- •Eutreptiales
- •Euglenales
- •REFERENCES
- •DINOPHYCEAE
- •Cell structure
- •Theca
- •Scales
- •Flagella
- •Pusule
- •Chloroplasts and pigments
- •Phototaxis and eyespots
- •Nucleus
- •Projectiles
- •Accumulation body
- •Resting spores or cysts or hypnospores and fossil Dinophyceae
- •Toxins
- •Dinoflagellates and oil and coal deposits
- •Bioluminescence
- •Rhythms
- •Heterotrophic dinoflagellates
- •Direct engulfment of prey
- •Peduncle feeding
- •Symbiotic dinoflagellates
- •Classification
- •Prorocentrales
- •Dinophysiales
- •Peridiniales
- •Gymnodiniales
- •REFERENCES
- •REFERENCES
- •Chlorarachniophyta
- •REFERENCES
- •CRYPTOPHYCEAE
- •Cell structure
- •Ecology
- •Symbiotic associations
- •Classification
- •Goniomonadales
- •Cryptomonadales
- •Chroomonadales
- •REFERENCES
- •CHRYSOPHYCEAE
- •Cell structure
- •Flagella and eyespot
- •Internal organelles
- •Extracellular deposits
- •Statospores
- •Nutrition
- •Ecology
- •Classification
- •Chromulinales
- •Parmales
- •Chrysomeridales
- •REFERENCES
- •SYNUROPHYCEAE
- •Classification
- •REFERENCES
- •EUSTIGMATOPHYCEAE
- •REFERENCES
- •PINGUIOPHYCEAE
- •REFERENCES
- •DICTYOCHOPHYCEAE
- •Classification
- •Rhizochromulinales
- •Pedinellales
- •Dictyocales
- •REFERENCES
- •PELAGOPHYCEAE
- •REFERENCES
- •BOLIDOPHYCEAE
- •REFERENCE
- •BACILLARIOPHYCEAE
- •Cell structure
- •Cell wall
- •Cell division and the formation of the new wall
- •Extracellular mucilage, biolfouling, and gliding
- •Motility
- •Plastids and storage products
- •Resting spores and resting cells
- •Auxospores
- •Rhythmic phenomena
- •Physiology
- •Chemical defense against predation
- •Ecology
- •Marine environment
- •Freshwater environment
- •Fossil diatoms
- •Classification
- •Biddulphiales
- •Bacillariales
- •REFERENCES
- •RAPHIDOPHYCEAE
- •REFERENCES
- •XANTHOPHYCEAE
- •Cell structure
- •Cell wall
- •Chloroplasts and food reserves
- •Asexual reproduction
- •Sexual reproduction
- •Mischococcales
- •Tribonematales
- •Botrydiales
- •Vaucheriales
- •REFERENCES
- •PHAEOTHAMNIOPHYCEAE
- •REFERENCES
- •PHAEOPHYCEAE
- •Cell structure
- •Cell walls
- •Flagella and eyespot
- •Chloroplasts and photosynthesis
- •Phlorotannins and physodes
- •Life history
- •Classification
- •Dictyotales
- •Sphacelariales
- •Cutleriales
- •Desmarestiales
- •Ectocarpales
- •Laminariales
- •Fucales
- •REFERENCES
- •PRYMNESIOPHYCEAE
- •Cell structure
- •Flagella
- •Haptonema
- •Chloroplasts
- •Other cytoplasmic structures
- •Scales and coccoliths
- •Toxins
- •Classification
- •Prymnesiales
- •Pavlovales
- •REFERENCES
- •Toxic algae
- •Toxic algae and the end-Permian extinction
- •Cooling of the Earth, cloud condensation nuclei, and DMSP
- •Chemical defense mechanisms of algae
- •The Antarctic and Southern Ocean
- •The grand experiment
- •Antarctic lakes as a model for life on the planet Mars or Jupiter’s moon Europa
- •Ultraviolet radiation, the ozone hole, and sunscreens produced by algae
- •Hydrogen fuel cells and hydrogen gas production by algae
- •REFERENCES
- •Glossary
- •Index

Chapter 9
Cryptophyta
CRYPTOPHYCEAE |
and Lee, 1987). The number and shape of these |
|
plates are used to characterize genera taking into |
||
|
consideration that the haploid and diploid phases |
|
This group is composed primarily of flagellates |
of a single genus can have different plates (Hoef- |
|
that occur in both marine and freshwater envir- |
Emden and Melkonian, 2003). New periplast plates |
|
onments. The cells contain chlorophylls a and c2 |
are added in an area adjacent to the vestibulum |
|
and phycobiliproteins that occur inside the thy- |
(Brett and Wetherbee, 1996). Sulfated fucose-rich |
|
lakoids of the chloroplast. The cell body is asym- |
polysaccharides can be excreted outside of the cell |
|
metric with a clearly defined dorsi-ventral/ |
(Giroldo and Vieira, 2002). |
|
right-left sides (Figs. 9.1, 9.9, 9.10). The asymmetric |
The chloroplast most likely evolved from a |
|
cell shape results in a peculiar swaying motion |
symbiosis between an organism similar to the |
|
during swimming. Most cryptophytes have a |
phagocytic cryptomonad Goniomonas and a red |
|
single lobed chloroplast with a central pyrenoid. |
alga (Kugrens and Lee, 1991; Liaud et al., 1997; |
|
|
McFadden et al., 1994). The chloroplast is sur- |
|
Cell structure |
rounded by two membranes of chloroplast endo- |
|
plasmic reticulum and the two membranes of the |
||
|
||
|
chloroplast envelope (Fig. 9.1). Between the outer |
|
There are two apically or laterally attached |
membrane and the inner membrane of the chloro- |
|
flagella at the base of a depression. Each flagel- |
plast endoplasmic reticulum are starch grains and |
|
lum is approximately the same length as the body |
a nucleomorph (Figs. 9.1, 9.4). The nucleomorph |
|
of the cell (Figs. 9.1, 9.8, 9.9, 9.10). Depending on |
contains three minute paired-chromosomes with |
|
the species, there are one or two rows of micro- |
531 genes (humans have at least 31 000 genes) that |
|
tubular hairs attached to the flagellum. In |
encode 30 proteins targeted into the chloroplast |
|
Cryptomonas sp., the hairs on one flagellum are 2.5 |
(Douglas et al., 2001; Cavalier-Smith, 2002). The |
|
m long and in two rows whereas the hairs on the |
nucleomorph is probably the remnant of the |
|
other flagellum are only 1 m long and arranged |
nucleus of the endosymbiont in the event that led |
|
in a single row (Heath et al., 1970; Kugrens et al., |
to chloroplast E.R. The nucleomorph is sur- |
|
1987). Small, 150-nm-diameter organic scales (Fig. |
rounded by an envelope that has pores similar to |
|
9.2) are common on the flagellar surface and |
those in a nuclear envelope. The nucleomorph |
|
sometimes on the cell body (Lee and Kugrens, |
exhibits a rudimentary type of division utilizing |
|
1986). |
microtubules (Morrall and Greenwood, 1982). |
|
The outer portion of the cell, or periplast |
The nucleomorph divides in preprophase of the |
|
(Gantt, 1971), is composed of the plasma mem- |
main nucleus following basal body replication, |
|
brane and a plate, or series of plates, directly under |
but before division of the chloroplast and the |
|
the plasma membrane (Figs. 9.1, 9.10) (Kugrens |
chloroplast endoplasmic reticulum (McKerracher |
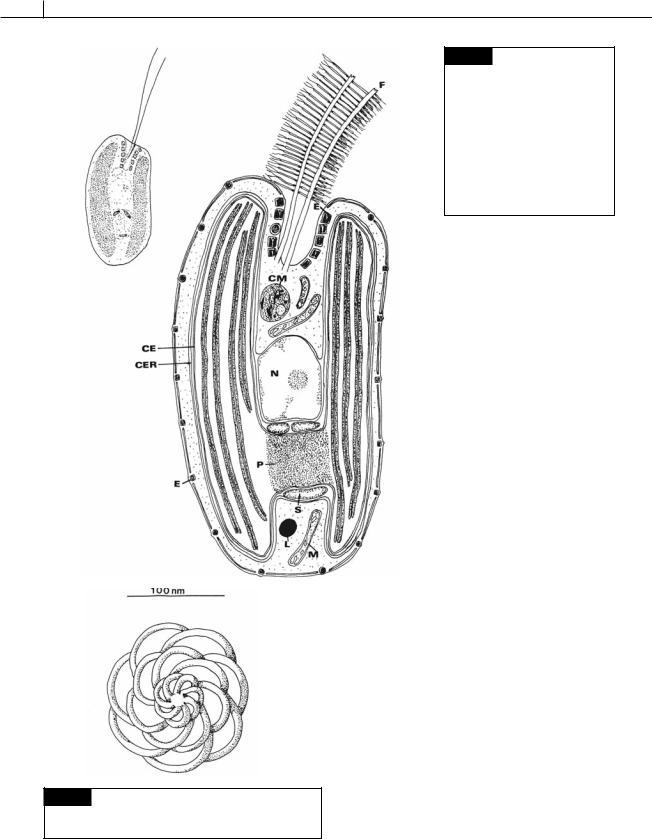
322 CHLOROPLAST E.R.: EVOLUTION OF TWO MEMBRANES
Fig. 9.2 Drawing of the most common type of flagellar
scale found in freshwater cryptophytes. (From Lee and
Kugrens, 1986.)
Fig. 9.1 Drawing of a cell of the Cryptophyceae as seen in the light and electron microscope. (CE) Chloroplast envelope; (CER) chloroplast endoplasmic
reticulum; (CM) Corps de Maupas;
(D) dorsal; (E) ejectisome; (L) lipid;
(M) mitochondrion; (N) nucleus; (NM) nucleomorph; (P) pyrenoid; (PP) periplast plate; (S) starch;
(V) ventral.
and Gibbs, 1982). The only cryptophyte that is known to lack a nucleomorph is Goniomonas (Figs. 9.8, 9.9(c)), a colorless cryptophyte that lacks a plastid. A second colorless cryptophyte, Chilomonas (Fig. 9.9(b)), is a reduced form of a photosynthetic cryptophyte and contains a leucoplast and a nucleomorph (McKerracher and Gibbs, 1982).
In the chloroplast, the thylakoids are grouped in pairs (Fig. 9.3), and there are no connections between adjacent thylakoids. The Cryptophyta is the only group to have this arrangement of thylakoids. Chlorophylls a and c2 are present. The major carotenoid present is -carotene, and the major xanthophyll, diatoxanthin. There are three spectral types of phycoerythrin and three spectral
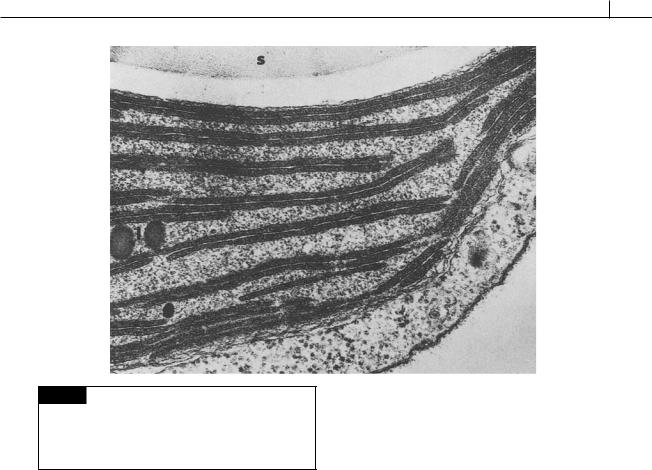
CRYPTOPHYTA 323
Fig. 9.3 Transmission electron micrograph of part of a chloroplast of Chroomonas mesostigmatica. The thylakoids are grouped in pairs. The dense contents of the thylakoids represent the phycobilisomes. Also present are lipid droplets
(l) and a large starch grain (s). 50 000. (From Dodge, 1969.)
types of phycocyanin, all of which are different from the phycobiliproteins found in the cyanobacteria and red algae (Hill and Rowan, 1989). The phycobiliproteins are in the intrathylakoid space (inside the thylakoids (Fig. 9.3) (Gantt et al., 1971; Spear-Bernstein and Miller, 1984), and are not on the stromal side of the thylakoids in phycobilisomes as occurs in the cyanobacteria and red algae. Each photosynthetic cryptophyte has only one species of phycobiliprotein – either a phycoerythrin or a phycocyanin – but never both. No allophycocyanin is present (Gantt, 1979). Allophycocyanin acts as a bridge in the transfer of light energy from phycoerythrin and phycocyanin to chlorophyll a of the reaction center in red algae and cyanobacteria. The presence of allophycocyanin may not be necessary in the cryptophytes because the greater absorption range of cryptophycean phycobiliproteins in conjunction with chlorophyll c overlaps the chlorophyll a absorption spectrum. There is a variation in
the amount of pigments under different lightintensity conditions. Cells of Cryptomonas grown under low light-intensity conditions (10 E m 2 s 1) contain twice as much of chlorophylls a and c2, and six times as much phycoerythrin per cell, as those grown under high light-intensity conditions (260 E m 2 s 1) (Thinh, 1983). Under low light-intensity conditions there is a higher concentration of phycoerythrin and the thylakoids are thicker.
The reserve product (similar in appearance to starch grains) is appressed to the pyrenoid area outside of the chloroplast envelope but inside the chloroplast E.R. The cryptophytes are the only algae that form their storage product in this area. The starch is an -1,4-glucan composed of about 30% amylose and amylopectin. Cryptophycean starch is similar to potato starch and starch found in the green algae and dinoflagellates (Antia et al., 1979).
Some of the Cryptophyceae have eyespots. The eyespots that have been reported consist of lipid granules inside the chloroplast envelope. In
Chroomonas mesostigmatica, the red eyespot is in the center of the cell (Fig. 9.4) and is an extension of the chloroplast beyond the pyrenoid (Dodge, 1969). In Cryptomonas rostella, the eyespot is
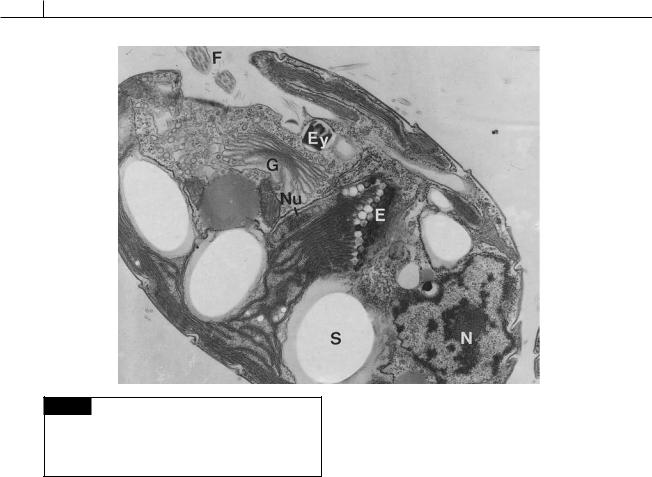
324 CHLOROPLAST E.R.: EVOLUTION OF TWO MEMBRANES
Fig. 9.4 Transmission electron micrograph of a cell of
Chroomonas mesostigmatica showing the eyespot (E) present in the chloroplast. (Ey) Ejectisome; (F) flagellum; (G) Golgi;
(N) nucleus; (Nu) nucleomorph; (S) starch. (Micrograph provided by Paul Kugrens.)
beneath the chloroplast membrane near the depression. Some of the Cryptophyceae exhibit positive phototaxis, with maximum sensitivity of the colorless Chilomonas being in the blue at 366 nm (Halldal, 1958).
The Cryptophyceae have projectiles called ejectisomes, which are of different structure from the trichocysts of the Dinophyceae and which are probably closely related to the R-bodies of the kappa particles of the ciliates (Hovasse et al., 1967; Kugrens et al., 1994). Within a cell there are usually large ejectisomes near the anterior depression and smaller ejectisomes around the cell periphery (Figs. 9.1, 9.4, 9.8). Both sizes of ejectisomes have the same structure; they are made up of two unequal-sized bodies enclosed within a single membrane (Fig. 9.5). Each of these bodies is a long tape curled up on a very tight spiral. The tape is tapered, with the greatest width being on the outside of the ejectisome. The
smaller body is joined to the first and sits at an angle within the V-shaped portion of the larger body. The two bodies actually constitute one long tape with two spirals. The bodies are always arranged so that the smaller body is near the surface. The ejectisomes discharge when the organism is irritated (Fig. 9.5), the discharged ejectisome being a long tubular structure with a short portion at an angle to the long portion. The discharged small ejectisome from the cell periphery is 4 m long, whereas that of a larger ejectisome from under the anterior depression is about 20 m long. The discharge of the ejectisome results in a movement of the organism in the opposite direction. The discharge of the ejectisome could function as an escape mechanism, or it could be a direct defense mechanism causing damage to an offending organism. Ejectisomes originate in vesicles in the area of Golgi bodies.
The Corps de Maupas is a large vesicular structure in the anterior portion of the cell (Fig. 9.1). Its main function is probably that of disposing of unwanted protoplasmic structures by digestion (Lucas, 1970a,b).
