
- •Contents
- •Preface to the first edition
- •Flagella
- •Cell walls and mucilages
- •Plastids
- •Mitochondria and peroxisomes
- •Division of chloroplasts and mitochondria
- •Storage products
- •Contractile vacuoles
- •Nutrition
- •Gene sequencing and algal systematics
- •Classification
- •Algae and the fossil record
- •REFERENCES
- •CYANOPHYCEAE
- •Morphology
- •Cell wall and gliding
- •Pili and twitching
- •Sheaths
- •Protoplasmic structure
- •Gas vacuoles
- •Pigments and photosynthesis
- •Akinetes
- •Heterocysts
- •Nitrogen fixation
- •Asexual reproduction
- •Growth and metabolism
- •Lack of feedback control of enzyme biosynthesis
- •Symbiosis
- •Extracellular associations
- •Ecology of cyanobacteria
- •Freshwater environment
- •Terrestrial environment
- •Adaption to silting and salinity
- •Cyanotoxins
- •Cyanobacteria and the quality of drinking water
- •Utilization of cyanobacteria as food
- •Cyanophages
- •Secretion of antibiotics and siderophores
- •Calcium carbonate deposition and fossil record
- •Chroococcales
- •Classification
- •Oscillatoriales
- •Nostocales
- •REFERENCES
- •REFERENCES
- •REFERENCES
- •RHODOPHYCEAE
- •Cell structure
- •Cell walls
- •Chloroplasts and storage products
- •Pit connections
- •Calcification
- •Secretory cells
- •Iridescence
- •Epiphytes and parasites
- •Defense mechanisms of the red algae
- •Commercial utilization of red algal mucilages
- •Reproductive structures
- •Carpogonium
- •Spermatium
- •Fertilization
- •Meiosporangia and meiospores
- •Asexual spores
- •Spore motility
- •Classification
- •Cyanidiales
- •Porphyridiales
- •Bangiales
- •Acrochaetiales
- •Batrachospermales
- •Nemaliales
- •Corallinales
- •Gelidiales
- •Gracilariales
- •Ceramiales
- •REFERENCES
- •Cell structure
- •Phototaxis and eyespots
- •Asexual reproduction
- •Sexual reproduction
- •Classification
- •Position of flagella in cells
- •Flagellar roots
- •Multilayered structure
- •Occurrence of scales or a wall on the motile cells
- •Cell division
- •Superoxide dismutase
- •Prasinophyceae
- •Charophyceae
- •Classification
- •Klebsormidiales
- •Zygnematales
- •Coleochaetales
- •Charales
- •Ulvophyceae
- •Classification
- •Ulotrichales
- •Ulvales
- •Cladophorales
- •Dasycladales
- •Caulerpales
- •Siphonocladales
- •Chlorophyceae
- •Classification
- •Volvocales
- •Tetrasporales
- •Prasiolales
- •Chlorellales
- •Trebouxiales
- •Sphaeropleales
- •Chlorosarcinales
- •Chaetophorales
- •Oedogoniales
- •REFERENCES
- •REFERENCES
- •EUGLENOPHYCEAE
- •Nucleus and nuclear division
- •Eyespot, paraflagellar swelling, and phototaxis
- •Muciferous bodies and extracellular structures
- •Chloroplasts and storage products
- •Nutrition
- •Classification
- •Heteronematales
- •Eutreptiales
- •Euglenales
- •REFERENCES
- •DINOPHYCEAE
- •Cell structure
- •Theca
- •Scales
- •Flagella
- •Pusule
- •Chloroplasts and pigments
- •Phototaxis and eyespots
- •Nucleus
- •Projectiles
- •Accumulation body
- •Resting spores or cysts or hypnospores and fossil Dinophyceae
- •Toxins
- •Dinoflagellates and oil and coal deposits
- •Bioluminescence
- •Rhythms
- •Heterotrophic dinoflagellates
- •Direct engulfment of prey
- •Peduncle feeding
- •Symbiotic dinoflagellates
- •Classification
- •Prorocentrales
- •Dinophysiales
- •Peridiniales
- •Gymnodiniales
- •REFERENCES
- •REFERENCES
- •Chlorarachniophyta
- •REFERENCES
- •CRYPTOPHYCEAE
- •Cell structure
- •Ecology
- •Symbiotic associations
- •Classification
- •Goniomonadales
- •Cryptomonadales
- •Chroomonadales
- •REFERENCES
- •CHRYSOPHYCEAE
- •Cell structure
- •Flagella and eyespot
- •Internal organelles
- •Extracellular deposits
- •Statospores
- •Nutrition
- •Ecology
- •Classification
- •Chromulinales
- •Parmales
- •Chrysomeridales
- •REFERENCES
- •SYNUROPHYCEAE
- •Classification
- •REFERENCES
- •EUSTIGMATOPHYCEAE
- •REFERENCES
- •PINGUIOPHYCEAE
- •REFERENCES
- •DICTYOCHOPHYCEAE
- •Classification
- •Rhizochromulinales
- •Pedinellales
- •Dictyocales
- •REFERENCES
- •PELAGOPHYCEAE
- •REFERENCES
- •BOLIDOPHYCEAE
- •REFERENCE
- •BACILLARIOPHYCEAE
- •Cell structure
- •Cell wall
- •Cell division and the formation of the new wall
- •Extracellular mucilage, biolfouling, and gliding
- •Motility
- •Plastids and storage products
- •Resting spores and resting cells
- •Auxospores
- •Rhythmic phenomena
- •Physiology
- •Chemical defense against predation
- •Ecology
- •Marine environment
- •Freshwater environment
- •Fossil diatoms
- •Classification
- •Biddulphiales
- •Bacillariales
- •REFERENCES
- •RAPHIDOPHYCEAE
- •REFERENCES
- •XANTHOPHYCEAE
- •Cell structure
- •Cell wall
- •Chloroplasts and food reserves
- •Asexual reproduction
- •Sexual reproduction
- •Mischococcales
- •Tribonematales
- •Botrydiales
- •Vaucheriales
- •REFERENCES
- •PHAEOTHAMNIOPHYCEAE
- •REFERENCES
- •PHAEOPHYCEAE
- •Cell structure
- •Cell walls
- •Flagella and eyespot
- •Chloroplasts and photosynthesis
- •Phlorotannins and physodes
- •Life history
- •Classification
- •Dictyotales
- •Sphacelariales
- •Cutleriales
- •Desmarestiales
- •Ectocarpales
- •Laminariales
- •Fucales
- •REFERENCES
- •PRYMNESIOPHYCEAE
- •Cell structure
- •Flagella
- •Haptonema
- •Chloroplasts
- •Other cytoplasmic structures
- •Scales and coccoliths
- •Toxins
- •Classification
- •Prymnesiales
- •Pavlovales
- •REFERENCES
- •Toxic algae
- •Toxic algae and the end-Permian extinction
- •Cooling of the Earth, cloud condensation nuclei, and DMSP
- •Chemical defense mechanisms of algae
- •The Antarctic and Southern Ocean
- •The grand experiment
- •Antarctic lakes as a model for life on the planet Mars or Jupiter’s moon Europa
- •Ultraviolet radiation, the ozone hole, and sunscreens produced by algae
- •Hydrogen fuel cells and hydrogen gas production by algae
- •REFERENCES
- •Glossary
- •Index
500 CHLOROPLAST E.R.: EVOLUTION OF TWO MEMBRANES
environment. The motile phase of Cricosphaera sp. will tolerate salinities only as low as 0.4% to 0.8% salt and as high as 4.5% to 5.0% salt (see Paasche, 1968, for a review).
Pavlovales
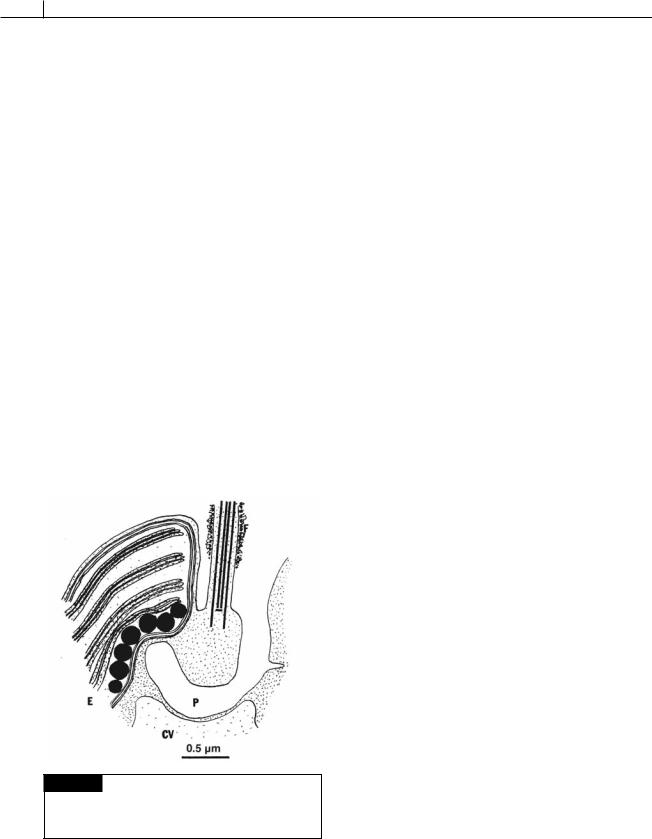
The Pavlovales have cells with two unequal flagella with the haptonema arising between the flagella. In Pavlova, the two unequal flagella are attached some distance below the cell apex (Fig. 22.3(b)) (Green, 1967, 1976; van der Veer, 1969, 1976; Green and Manton, 1970). The longer flagellum is directed forward during swimming and is covered with fine hairs and dense bodies, whereas the short flagellum projects outward and can have fibrillar hairs on it. The flagella and haptonema are attached at the bottom of a depression (Green, 1973). A pit or canal passes from the bottom of the depression, under the base of the long flagellum, and terminates at the inner face of the eyespot (Fig. 22.21). The shape of the cells is variable, and there is a single two-lobed chloroplast with a pyrenoid. An eyespot is present inside the chloroplast. No sexual reproduction is known, and the cells propagate by longitudinal division in the motile state.
Fig. 22.21 A drawing of a section through the apical area of Pavlova granifera showing the contactile vacuole (CV), eyespot (E), long flagellum (F), and pit (P). (After Green, 1973.)
The algae in the Pavlovales have unusual sterols with two hydroxyl groups called pavlovals (Ghosh et al., 1998).
REFERENCES
Anderson, O. R., Swanberg, N. R., and Bennett, P. (1983). Fine structure of yellow-brown symbionts (Prymnesiida) in solitary radiolaria and their comparison with similar Acantharian symbionts. J. Protozool. 30:718–22.
Balch, W. M., Kilpatrick, K., Holligan, P. M., and Cucci, T. (1993). Coccolith production and detachment in
Emiliania huxleyi (Prymnesiophyceae). J. Phycol. 29:566–75.
Bhattacharya, D., and Ehlting, J. (1995). Actin coding regions: gene family evolution and use as a phylogenetic marker. Arch. Protistenk. 145:155–64.
Brennan, S. L., Lowenstein, T. K., and Horita, J. (2004). Seawater chemistry and the advent of biocalcification. Geology 32:473–6.
Brown, R. M. (1969). Observations on the relationship of the Golgi apparatus to wall formation in the marine chrysophycean alga, Pleurochrysis scherfferlii
Pringsheim. J. Cell Biol. 41:109–23. Chrétiennot-Dinet, M-J., Giraud-Guille, M. M., Vaulot,
D., Putaux, J-L., Saito, Y., and Chanzy, H. (1997). The chitinous nature of filaments ejected by Phaeocystis (Prymnesiophyceae). J. Phycol. 33:666–72.
Christensen, T. (1962). Alger Botanik, Vol. 2, No. 2. Copenhagen: Munksgaard.
Corstjens, P. L. A. M., and Gonzales, E. L. (2004). Effects of nitrogen and phosphorus availability on the expression of the coccolith V-ATPase (subunit C ) of Pleurochrysis (Haptophyta). J. Phycol. 40:82–7.
Edvardsen, B., Eikrem, W., Green, J. C., Andersen, R. A., Moon-van der Stay, S. Y., and Medlin, L. K. (2000). Phylogenetic reconstructions of the Haptophyta inferred from 18S ribosomal DNA sequences and available morphological data. Phycologia 39:19–35.
Faber, W. W., and Preisig, H. R. (1994). Calcified structures and calcification in protists. Protoplasma 181:78–105.
Fistarol, G. O., Legrand, C., and Granéli, E. (2003). Allelopathic effect of Prymnesium parvum on a natural plankton community. Mar. Ecol. Progr. Ser. 255:115–25.
Fournier, R. O. (1969). Observations on the flagellate Diacronema vlkianum Prauser (Haptophyceae). Br. Phycol. J. 4:185–90.
Fujiwara, S., Tsuzuki, M., Kawachi, M., Minaka, N., and Inouye, I. (2001). Molecular phylogeny of the

PRYMNESIOPHYTA 501
Haptophyta based in the rbcL gene and sequence variation in the spacer region of the Rubisco operon. J. Phycol. 37:121–9.
Gallois, R. W. (1976). Coccolith blooms in the Kimmeridge Clay and origin of the North Sea Oil. Nature 259:473–5.
Geisen, M., Billard, C., Broerse, A. T. C., Cros, L., Probert, I., and Young, J. R. (2002). Life-cycle associations involving pairs of holococcolothophorid species: intraspecific variation or cryptic speciation?
Eur. J. Phycol. 37:531–50.
Ghosh, P., Patterson, G. W., and Wikfors, G. H. (1998). Sterols of some marine Prymnesiophyceae. J. Phycol. 34:511–4.
Gonzales, E. L. (2004). The proton pump of the calcifying vesicle of the coccolithophore, Pleurochrysis. In Biomineralization, ed. E. Baeuerlein, pp. 217–8. Weinheim: Wiley-VCH.
Green, J. C. (1967). A new species of Pavlova from Madeira. Br. Phycol. Bull. 3:299–303.
Green, J. C. (1973). Studies in the fine structure and taxonomy of flagellates in the genus Pavlova. II. A freshwater representative, Pavlova granifera (Mack) comb. nov. Br. Phycol. J. 8:1–12.
Green, J. C. (1976). Notes on the flagellar apparatus and taxonomy of Pavolva mesolychnon van der Veer, and on the status of Pavlova Butcher and related genera within the Haptophyceae. J. Mar. Biol. Assoc. UK 56:595–602.
Green, J. C., and Hibberd, D. J. (1977). The ultrastructure and taxonomy of Diacronema vlkianum
(Prymnesiophyceae) with special reference to the haptonema and flagellar apparatus. J. Mar. Biol. Assoc. UK 57:1125–36.
Green, J. C., and Leadbeater, B. S. C. (1972).
Chrysochromulina parkae sp. nov. (Haptophyceae) a new species recorded from S.W. England and Norway. J. Mar. Biol. Assoc. UK 52:469–74.
Green, J. C., and Manton, I. (1970). Studies in the fine structure and taxonomy of flagellates in the genus Pavlova. I. A revision of Pavlova gyrans, the type species. J. Mar. Biol. Assoc. UK
50:1113–30.
Green, J. C., and Pienaar, R. N. (1977). The taxonomy of the order Isochrysidales (Prymnesiophyceae) with special reference to the genera Isochrysis Parke,
Dicrateria Parke and Imantonia Reynolds. J. Mar. Biol. Assoc. UK 57:7–17.
Green, J. C., Course, P. A., and Tarran, G. A. (1996). The life-cycle of Emiliania huxleyi: A brief review and a study of relative ploidy levels analyzed by flow cytometry. J. Marine Systems 9:33–44.
Greyson, A. J., Green, J. C., and Leadbeater, B. S. C. (1993). Structure and physiology of the haptonema in Chrysochromulina (Prymnesiophyceae). II. Mechanisms of haptonemal coiling and the regeneration process. J. Phycol. 29:686–700.
Guillard, R. R. L., and Hellebust, J. A. (1971).
Growth and production of extracellular substances by two strains of Phaeocystis poucheti. J. Phycol. 7:330–8.
Haberman, K. L., Ross, R. M., Quetin, L. B., Vernet, M., Nevitt, G. A., and Kozlowski, W. (2002). Grazing by Antarctic krill Euphausia superba on Phaeocystis antarctica: an immunochemical approach. Mar. Ecol. Progr. Ser. 241:139–49.
Hamm, C. E., Simson, D. A., Merkel, R., and Smetacek, V. (1999). Colonies of Phaeocystis globosa are protected by a thin but tough skin. Mar. Ecol. Progr. Ser. 187:101–11.
Hansen, P. J., Nielsen, T. G., and Kaas, H. (1995). Distribution and growth of protists and mesozooplankton during a bloom of Chrysochromulina spp. (Prymnesiophyceae, Prymnesiales). Phycologia 34:409–16.
Hawkins, E. K., and Lee, J. J. (2001). Architecture of the Golgi apparatus of a scale-forming alga biogenesis and transport of scales. Protoplasma 216:227–38.
Hibberd, D. J. (1976). The ultrastructure and taxonomy of the Chrysophyceae and Prymnesiophyceae (Haptophyceae): A survey with some new observations on the ultrastructure of the Chrysophyceae.
Bot. J. Linn. Soc. 72:55–80.
Holligan, P. M., Viollier, M., Habour, D. S., Camus, P., and Champagne-Phillipe, M. (1983). Satellite and ship studies of coccolithophore production along a continental shelf. Nature 304:339–42.
Igarashi, T., Satake, M., and Yasumoto, T. (1996). Prymnesium-2: a potent ichthyotoxic and hemolytic glycoside isolated from the red tide alga Prymnesium parvum. J. Am. Chem. Soc. 118:479–80.
Inouye, I., and Kawachi, M. (1994). The haptonema. In The Haptophyte Algae, ed. J. C. Green, and B. S. C. Leadbeater, Systematics Assn. Special Vol. 51, pp. 73–89. Oxford: Clarendon Press.
Janse, I., van Rijssel, M., van Hall, P. J., Gerswig, G. J., Gottschel, J. C., and Prins, R. A. (1996). The storage glucan of Phaeocystis globosa (Prymnesiophyceae) cells. J. Phycol. 32:382–7.
Jordan, R. W., and Chamberlain, A. H. L. (1997). Biodiversity among haptophyte algae. Biodiversity and Conservation 6:131–52.
Kawachi, M., and Inouye, I. (1994). Ca2 mediated induction of the coiling of the haptonema in

502 CHLOROPLAST E.R.: EVOLUTION OF TWO MEMBRANES
Chrysochromulina hirata (Prymnesiophyceae
Haptophyta). Phycologia 33:53–7.
Kawachi, W., Inouye, I., Maeda, O., and Chihara, M. (1991). The haptonema as a food-capturing device: observations on Chrysochromulina hirata
(Prymnesiophyceae). Phycologia 30:563–73. Klaveness, D. (1972). Coccolithus huxleyi (Lohm.) Kamptn.
II. The flagellate cell, aberrant cell types, vegetative propagation and life cycles. Br. Phycol. J. 7:309–18.
Kreger, D. R., and van der Veer, J. (1970). Paramylon in a Chrysophyte. Acta Bot. Neerl. 19:401–2.
Leadbeater, B. S. C. (1970). Preliminary observations on differences of scale morphology at various stages in the life cycle of “Apistonema-Syracosphaera” sensu von Stosch. Br. Phycol. J. 5:57–69.
Lechtreck, K.-F. (2004). An immunofluorescence study of the haptonema of Chrysochromulina parva
(Prymnesiophyceae). Phycologia 43:635–40.
Legrand, C., Johansson, N., Johnsen, G., Borsheim, K. Y., and Graneli, E. (2001). Phagotrophy and toxicity variation in the mixotrophic Prymnesium patelliferum (Haptophyceae). Limnol. Oceanogr. 46:1208–14.
Linschooten, C., van Bleijswijk, J. D. L., van Emburg, P., de Vrind, J. P. M., Kempers, E. S., Westbroek, P., and de Vrind-de Jong, E. W. (1991). Role of light-dark cycle and medium composition in the production of coccoliths by Emiliania huxleyi (Haptophyceae). J. Phycol. 27:82–6.
Manton, I. (1964). Further observations on the fine structure of the haptonema in Prymnesium parvum. Arch. Mikrobiol. 49:315–30.
Manton, I. (1966). Further observations on the fine structure of Chrysochromulina chiton, with special reference to the pyrenoid. J. Cell Sci. 1:187–92.
Manton, I. (1967a). Further observations on the fine structure of Chrysochromulina chiton with special reference to the haptonema, “peculiar” Golgi and aspects of scale production. J. Cell Sci. 2:265–72.
Manton, I. (1967b). Further observations on scale formation in Chrysochromulina chiton. J. Cell Sci. 2:411–18.
Manton, I. (1968). Further observations on the microanatomy of the haptonema in Chrysochromulina chiton and Prymnesium parvum. Protoplasma 66:35–53.
Manton, I. (1972). Preliminary observations on
Chrysochromulina mactra sp. nov. Br. Phycol. J. 7:21–35. Manton, I., and Parke, M. (1962). Preliminary observa-
tions on scales and their mode of origin in
Chrysochromulina polylepis sp. nov. J. Mar. Biol. Assoc. UK
42:565–78.
Manton, I., and Peterfi, L. S. (1969). Observations on the fine structure of coccoliths, scales and the
protoplast of a freshwater coccolithophorid. Hymenomonas roseola Stein, with supplementary observations on the protoplast of Cricosphaera carterae. Proc. R. Soc. Lond. [B] 172:1–15.
Manton, I., Sutherland, J., and Oates, K. (1977). Arctic coccolithophorids: Wigwammia arctica gen. et sp. nov. from Greenland and arctic Canada. W. annulifera sp. nov. from South Africa and S. Alaska and Calciarcus alaskensis gen. et sp. nov. from S. Alaska. Proc. R. Soc. Lond. [B] 197:145–68.
Marsh, M. E. (1994). Polyanion-mediated mineralization – assembly and reorganization of acidic polysachharides in the Golgi system of a coccolithophorid alga during mineral deposition.
Protoplasma 177:108–22.
Marsh, M. E. (1996). Polyanion-mediated mineralization – a kinetic analysis of the calciumcarrier hypothesis in the phytoflagellate Pleurochrysis carterae. Protoplasma 190:181–8.
Marsh, M. E. (2004). Biomineralization in coccolithophores. In Biomineralization, ed. E. Baeuerlein, pp. 198–215. Weinheim: Wiley-VCH.
Medlin, L. K., Barker, G. L. A., Baumann, M., Hayes, P. K., and Lange, M. (1994). Molecular biology and systematics. In The Haptophyte Algae, ed.
J. C. Green, and B. S. C. Leadbeater, Systematics Assn. Special Vol. 51, pp. 393–411. Oxford: Clarendon Press.
Moestrup, Ø. (1994). Economic aspects: “blooms”, nuisance species, and toxins. In The Haptophyte Algae, ed. J. C. Green, and B. S. C. Leadbeater, Systematics Assn. Special Vol. 51, pp. 265–85. Oxford: Clarendon Press.
Nielsen, M. V. (1995). Photosynthetic characteristics of the coccolithophorid Emiliania huxleyi (Prymnesiophyceae) exposed to elevated concentrations of dissolved inorganic carbon. J. Phycol. 31:715–19.
Outka, D. E., and Williams, D. C. (1971). Sequential coccolith morphogenesis in Hymenomonas carterae. J. Protozool. 18:285–97.
Paasche, E. (1968). Biology and physiology of coccolithophorids. Annu. Rev. Microbiol. 22:71–86.
Paasche, E. (2002). A review of the coccolithophorid Emiliania huxleyi (Prymnesiophyceae), with particular reference to growth, coccolith formation and calcification-photosynthesis interactions. Phycologia 40:503–29.
Parke, M. (1971). The production of calcareous elements by benthic algae belonging to the class Haptophyceae (Chrysophyta). Proc. II Plank. Conf., pp. 929–38.

PRYMNESIOPHYTA 503
Parke, M., and Manton, I. (1962). Studies on marine flagellates. VI. Chrysochromulina pringsheimii sp. nov. J. Mar. Biol. Assoc. UK 42:391–404.
Parke, M., Manton, I., and Clarke, B. (1955). Studies on marine flagellates. II. Three new species of
Chrysochromulina. J. Mar. Biol. Assoc. UK 34:579–609. Parke, M., Manton, I., and Clarke, B. (1956). Studies on
marine flagellates. III. Three further species of
Chrysochromulina. J. Mar. Biol. Assoc. UK 35:387–414. Parke, M., Lund, J. W. G., and Manton, I. (1962).
Observations on the biology and fine structure of the type species of Chrysochromulina (C. parva Lackey) in the English Lake District. Arch. Mikrobiol. 42:333–52.
Pringsheim, E. G. (1955). Kleine Mitteilungen über Flagellaten und Algen. I. Algenartige Chrysophyceen in Reinkultur. Arch. Mikrobiol. 21:401–10.
Reynolds, N. (1974). Imantonia rotunda gen. et sp. nov., a new member of the Haptophyceae. Br. Phycol. J. 9:429–34.
Rowson, J. D., Leadbeater, B. S. C., and Green, J. C. (1986). Calcium carbonate deposition in the motile (Crystallolithus) phase of Coccolithus pelagicus. Br. J. Phycol. 21:359–70.
Savage, R. E. (1930). The influence of Phaeocystis on the migration of the herring. Fish. Invest., Lond., Ser. II
12:5–14.
Shilo, M. (1967). Formation and mode of action of algal toxins. Bacteriol. Rev. 31:180–93.
Simonsen, S., and Moestrup, Ø. (1997). Toxicity tests in eight species of Chrysochromulina (Haptophyta). Can. J. Bot. 75:129–36.
Skovgaard, A., and Hansen, P. J. (2003). Food uptake in the harmful alga Prymnesium parvum mediated by excreted toxins. Limnol. Oceanogr. 48:1161–6.
Solomon, C. M., Lessard, E. J., Keil, R. G., and Foy, M. S.
(2003). Characterization of extracellular polymers of
Phaeocystis globosa and P. antarctica. Mar. Ecol. Progr. Ser. 250:81–9.
Tang, K. W. (2003). Grazing and colony size development in Phaeocystis globosa (Prymnesiophyceae): the role of the chemical signal. J. Plankton Res. 25:831–42.
Tasch, P. (1973). Paleobiology of the Invertebrates. New York: John Wiley.
van der Veer, J. (1969). Pavlova mesolychnon (Chrysophyta), a new species from the Tamar Estuary, Cornwall, Acta Bot. Neerl. 18:496–510.
van der Veer, J. (1976). Pavlova calceolata (Haptophyceae), a new species from the Tamar Estuary, Cornwall, England. J. Mar. Biol. Assoc. UK 56:21–30.
van der Wal, P., deVrind, J. P. M., deVrind-deJong, E. W., and Borman, A. H. (1987). Incompleteness of the coccosphere as a possible stimulus for coccolith formation in Pleurochrysis carterae (Prymnesiophyceae). J.
Phycol. 23:218–21.
van Rijssel, M., Hamm, C. E., and Gieskes, W. W. C. (1997). Phaeocystis globosa (Prymnesiophyceae) colonies: hollow structures built with small amounts of polysaccharides. Eur. J. Phycol. 32:185–92.
von Stosch, H. A. (1967). Haptophyceae. In Vegetative Forplanzung, Parthenogenese und Apogamie bie Algen, ed. W. Ruhland, Encyclopedia of Plant Physiology
18:646–56.
Young, J. R., Didymus, J. M., Bown, P. R., Prins, B., and Mann, S. (1992). Crystal assembly and phylogenetic evolution in heterococcoliths. Nature 356:516–18.
Zapata, M., Jeffrey, S. W., Wright, S. W., Rodriguez, F., Garrido, J. L., and Clementson, L. (2004).
Photosynthetic pigments in 37 species (65 strains) of Haptophyta: implications for oceanography and chemotaxonomy. Mar. Ecol. Progr. Ser. 270:83–102.
