
Gale Encyclopedia of Genetic Disorder / Gale Encyclopedia of Genetic Disorders, Two Volume Set - Volume 2 - M-Z - I
.pdf
von Willebrand disease
KEY TERMS
Amniocentesis—A procedure performed at 16–18 weeks of pregnancy in which a needle is inserted through a woman’s abdomen into her uterus to draw out a small sample of the amniotic fluid from around the baby. Either the fluid itself or cells from the fluid can be used for a variety of tests to obtain information about genetic disorders and other medical conditions in the fetus.
Autosomal dominant—A pattern of genetic inheritance where only one abnormal gene is needed to display the trait or disease.
Autosomal recessive—A pattern of genetic inheritance where two abnormal genes are needed to display the trait or disease.
Biochemical testing—Measuring the amount or activity of a particular enzyme or protein in a sample of blood or urine or other tissue from the body.
Carrier—A person who possesses a gene for an abnormal trait without showing signs of the disorder. The person may pass the abnormal gene on to offspring.
Chorionic villus sampling (CVS)—A procedure used for prenatal diagnosis at 10–12 weeks gestation. Under ultrasound guidance a needle is inserted either through the mother’s vagina or abdominal wall and a sample of cells is collected from around the fetus. These cells are then tested for chromosome abnormalities or other genetic diseases.
Chromosome—A microscopic thread-like structure found within each cell of the body that consists of a complex of proteins and DNA. Humans have 46 chromosomes arranged into 23 pairs. Changes in either the total number of chromosomes or their shape and size (structure) may lead to physical or mental abnormalities.
Deoxyribonucleic acid (DNA)—The genetic material in cells that holds the inherited instructions for growth, development, and cellular functioning.
Desmopressin (DDAVP)—A drug used in the treatment of von Willebrand’s disease.
Diagnostic testing—Testing performed to determine if someone is affected with a particular disease.
DNA testing—Analysis of DNA (the genetic component of cells) in order to determine changes in genes that may indicate a specific disorder.
Endothelial cells—The cells lining the inner walls of the blood vessels.
Factor VIII—A protein involved in blood clotting that requires vWF for stability and long-term survival in the bloodstream.
Gene—A building block of inheritance, which contains the instructions for the production of a particular protein, and is made up of a molecular sequence found on a section of DNA. Each gene is found on a precise location on a chromosome.
Mutation—A permanent change in the genetic material that may alter a trait or characteristic of an individual, or manifest as disease, and can be transmitted to offspring.
Platelets—Small disc-shaped structures that circulate in the bloodstream and participate in blood clotting.
Prenatal testing—Testing for a disease such as a genetic condition in an unborn baby.
Protein—Important building blocks of the body, composed of amino acids, involved in the formation of body structures and controlling the basic functions of the human body.
Skin hematoma—Blood from a broken blood vessel that has accumulated under the skin.
von Willebrand factor (vWF)—A protein found in the blood that is involved in the process of blood clotting.
duced. Type 1 is the most common and mildest form and results when the body produces slightly decreased amounts of typically normal vWF. Type 2 can be classified into five subtypes (A, B, M, N) and results when the body produces an abnormal type of vWF. Type 3 is the rarest and most severe form and results when the body does not produce any detectable vWF.
Genetic profile
The genetics of VWD are complex and involve a gene that produces vWF and is found on chromosome 12. Since two of each type of chromosome are inherited, children inherit two vWF genes. There are different types of changes in the vWF gene that can affect the production
1178 |
GALE ENCYCLOPEDIA OF GENETIC DISORDERS |
of vWF. Some types of changes can cause the vWF gene to produce decreased amounts of normal vWF, while other changes can cause the gene to produce abnormal vWF. Most of the gene changes are significant enough that a change in only one vWF gene is sufficient to cause VWD. Some gene changes only cause VWD if both genes are changed, which often leads to more severe symptoms. Type 1 VWD is called an autosomal dominant condition since it is caused by a change in only one vWF gene. Since type 1 VWD results in only a slight decrease in the amount of vWF produced, the symptoms are often mild and even nonexistent in some patients. Most cases of Type 2 VWD are autosomal dominant since they are caused by a change in only one vWF gene that results in the production of an abnormal protein. An autosomal dominant form of VWD can be inherited from either parent or can occur spontaneously in the embryo that is formed when the egg and sperm cells come together during fertilization.
Some cases of type 2 VWD and all cases of type 3 VWD are autosomal recessive since they are caused by changes in both vWF genes. A person with an autosomal recessive form of VWD has inherited a changed gene from his or her mother and a changed gene from his or her father. Parents who have a child with an autosomal recessive form of VWD are called carriers, since they each possess one changed vWF gene and one unchanged vWF gene. Many carriers for the autosomal recessive forms of type 2 VWD and type 3 VWD do not have any symptoms, although some people with type 3 VWD are born to parents who have type 1 VWD and may have symptoms. Each child born to parents who are both carriers for VWD has a 25% chance of having VWD, a 50% chance of being a carrier, and a 25% chance of being neither a carrier nor affected with VWD disease. A person with an autosomal dominant form of VWD has a 50% chance of passing the changed gene on to his or her children who may or may not have symptoms.
Demographics
Approximately 1 out of 100 people are affected with VWD, making it the most common inherited bleeding disorder (hemophilia). VWD affects people of all ethnic backgrounds. Approximately 70–80% of people with VWD have type 1 and close to 20–30% have type 2. Type 3 is very rare and occurs in less than one percent of people with VWD.
Signs and symptoms
VWD is usually a relatively mild disorder characterized by easy bruising, recurrent nosebleeds, heavy menstrual periods, and extended bleeding after surgeries and
invasive dental work. There is a great deal of variability in the severity of symptoms, which can range from clinically insignificant to life threatening. Even people within the same family who are affected with the same type of VWD may exhibit different symptoms. An individual with VWD may exhibit a range of symptoms over the course of his or her lifetime and may experience an improvement in symptoms with age. The severity of the disease is partially related to the amount and type of vWF that the body produces, but is also influenced by other genetic and nongenetic factors.
Type 1
Type 1, the mildest form of VWD, is usually associated with easy bruising, recurrent nosebleeds, heavy menstrual periods, and prolonged bleeding after surgeries and invasive work. Many people with type 1 VWD do not have any noticeable symptoms or only have prolonged bleeding after surgery or significant trauma. The amount of vWF produced by the body increases during pregnancy, so prolonged bleeding during delivery is uncommon in people with type 1 VWD.
Type 2
People with type 2 VWD usually have symptoms from early childhood and symptoms may even be present at birth. They usually experience prolonged bleeding from cuts, easy bruising, nose bleeds, skin hematomas, and prolonged bleeding from the gums following teeth extraction and minor trauma. More than 50% of women with type 2 VWD experience heavy periods that may require a blood transfusion. Gastrointestinal bleeding is rare but can be life-threatening. Some women with type 2 VWD exhibit prolonged bleeding during delivery.
Type 3
Type 3 VWD can be quite severe and is associated with bruising and bleeding from the mouth, nose, intestinal, genital and urinary tracts. Type 3 is also associated with spontaneous bleeding into the muscles and joints, which can result in joint deformities. Some women with type 3 VWD experience prolonged bleeding during delivery.
Diagnosis
Diagnostic testing
Many people with VWD have mild symptoms or symptoms that can be confused with other bleeding disorders making it difficult to diagnose VWD on the basis of clinical symptoms. VWD should be suspected in any person with a normal number of platelets in their blood
disease Willebrand von
GALE ENCYCLOPEDIA OF GENETIC DISORDERS |
1179 |
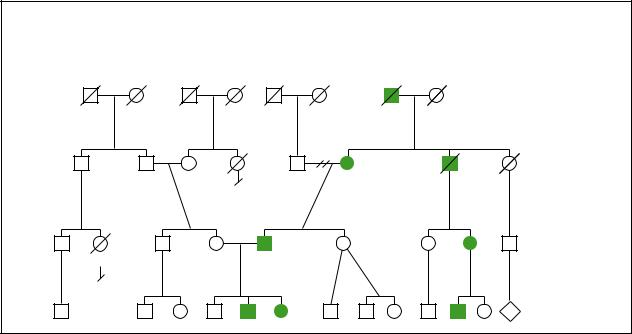
von Willebrand disease
von Willebrand Disease
Autosomal Dominant
|
Died young |
|
d.62y |
Diabetes |
Lung cancer |
HIV |
Mitral valve |
|
prolapse |
2 |
5 |
(Gale Group)
and bleeding from the mucous membranes such as the nose, gums, and gastrointestinal tract. Testing for an individual with suspected VWD often includes the measurement of:
•how long it takes for the bleeding to stop after a tiny cut is made in the skin (the bleeding time)
•the amount of vWF (vWF antigen measurement)
•the activity of vWF (ristocetin co-factor activity)
•the amount of factor VIII (factor VIII antigen measurement)
•activity of factor VIII
People with type 1 VWD usually have an increased bleeding time but they may have an intermittently normal bleeding time. They also have a decreased amount of vWF, decreased vWF activity, and usually have slightly decreased factor VIII levels and activity. People with type 2 VWD have a prolonged bleeding time, decreased activity of vWF, and may have decreased amounts of vWF and factor VIII, and decreased factor VIII activity. Type 3 individuals have undetectable amounts of vWF, negligible vWF activity, factor VIII levels of less than 5–10%, and significantly reduced factor VIII activity. The activity of vWF is reduced for all types of VWD, making it the most sensitive means of identifying all three types of VWD. Patients with borderline results should be tested two to three times over a three month period.
Once a patient is diagnosed with VWD, further testing such as vWF multimer analysis and ristocetininduced platelet aggregation (RIPA) may need to be performed to determine the subtype. Multimer analysis evaluates the structure of the vWF, and RIPA measures how much ristocetin is required to cause the clumping of platelets in a blood sample. The vWF multimer analysis is able to differentiate people with a structurally normal vWF (type 1) from people with a structurally abnormal vWF (type 2) and is often able to identify the subtype of patients with type 2 VWD. People with type 1 VWD usually have normal to decreased RIPA concentrations. Depending on the subtype, patients with type 2 VWD either have increased or decreased RIPA. RIPA is usually absent and the multimer analysis shows undetectable vWF in people with type 3 VWD.
In some cases DNA testing can be a valuable adjunct to biochemical testing. The detection of gene alteration(s) can confirm a diagnosis and can determine the type and subtype of VWD. It can also help to facilitate prenatal testing and testing of other family members. Unfortunately, as of 2001, many people with VWD possess DNA changes that are not detectable through DNA testing. A person who has a mother, father, or sibling diagnosed with VWD should undergo biochemical testing for VWD. If the relative with VWD possesses a detectable gene change, then DNA testing should also be considered.
1180 |
GALE ENCYCLOPEDIA OF GENETIC DISORDERS |
Prenatal testing
If one parent has been diagnosed with an autosomal dominant form of VWD or both parents are carriers for an autosomal recessive form of VWD, then prenatal testing can be considered. If the parent with an autosomal dominant form of VWD possesses a detectable gene change or both parents who are carriers for an autosomal recessive form of VWD possess detectable mutations, then DNA testing of their fetus would be available. DNA testing can be performed through amniocentesis or chorionic villus sampling. If the DNA change in the parent(s) is unknown then prenatal testing can sometimes be performed through biochemical testing of blood obtained from the fetal umbilical cord, which is less accurate and is associated with a higher risk of pregnancy loss.
Treatment and management
VWD is most commonly treated by replacement of vWF through the administration of blood products that contain vWF or through treatment with desmopressin (DDAVP, 1-deamino-8-D-arginine vasopressin). DDAVP functions by increasing the amount of factor VIII and vWF in the bloodstream. Treatment with blood products or DDAVP may be started in response to uncontrollable bleeding or may be administered prior to procedures such as surgeries or dental work. The type of treatment chosen depends on the type of VWD and a patient’s response to a preliminary treatment trial.
Treatment with desmopressin
DDAVP is the most common treatment for people with type 1 VWD. About 80% of people with type 1 VWD respond to DDAVP therapy. Treatment with DDAVP can also be used to treat some people with type 2 VWD. Patients with Type 2B VWD should not be treated with this medication since DDAVP can induce dangerous platelet clumping. Type 3 VWD should not be treated with DDAVP since this medication does not increase the level of vWF in type 3 patients. DDAVP should only be used in people who have been shown to be responsive through a pre-treatment trial transfusion with this medication.
DDAVP can be administered intravenously or through a nasal inhaler. DDAVP has relatively few side effects although some people may experience facial flushing, tingling sensations, and headaches after treatment with this medication. Often treatment with this medication is only required prior to invasive surgeries or dental procedures.
Treatment with blood products
Patients who are unable to tolerate or are unresponsive to drug-based treatments are treated with concentrated factor VIII obtained from blood products. Not all factor VIII concentrates can be used since some do not contain enough vWF. The concentrate is treated to kill most viruses, although caution should be used since not all types of viruses are destroyed. If the factor VIII concentrates are unable to manage a severe bleeding episode, then blood products called cryoprecipitates, which contain concentrated amounts of vWF, or platelet concentrates should be considered. Caution should be used when treating with these blood products since they are not treated to kill viruses.
Other treatments and precautions
Medications called fibrinolytic inhibitors can be helpful in the control of intestinal, mouth, and nose bleeding. Estrogens such as are found in oral contraceptives increase the synthesis of vWF and can sometimes be used in the long-term treatment of women with mild to moderate VWD. Estrogens are also sometimes used prior to surgery in women with type 1 VWD. Some topical agents are available to treat nose and mouth bleeds. Patients with VWD should avoid taking aspirin, which can increase their susceptibility to bleeding and people with severe forms of VWD should avoid activities that increase their risk of injury such as contact sports.
Prognosis
The prognosis for VWD disease is generally fairly good and most individuals have a normal lifespan. The prognosis can depend, however, on accurate diagnosis and appropriate medical treatment.
Resources
BOOKS
Handin, Robert I. “Disorders of the Platelet and Vessel Wall.” In
Harrison’s Principles of Internal Medicine. Edited by Anthony S. Fauci, et al. New York: McGraw-Hill, 1998.
Sadler, J.E. “Von Willebrand Disease.” In The Metabolic and Molecular Basis of Inherited Disease. Edited by C.R. Scriver, et al. New York: McGraw Hill, 1995.
PERIODICALS
Ginsburg, David. “Molecular Genetics of von Willebrand Disease.” Thrombosis and Haemostasis 82, no. 2 (1999): 585–591.
Nichols, William C., and David Ginsburg. “Von Willebrand’s Disease.” Medicine 76 (Jan. 1997): 1.
Voelker, Rebecca. “New Focus on von Willebrand’s Disease.”
Journal of the American Medical Association 278 (October 8, 1997): 1137.
disease Willebrand von
GALE ENCYCLOPEDIA OF GENETIC DISORDERS |
1181 |
von Willebrand disease
ORGANIZATIONS
Canadian Hemophilia Society. 625 President Kennedy, Suite 1210, Montreal, QUE H3A 1K2. Canada (514) 848-0503. Fax: (514) 848-9661. chs@hemophilia.ca. http://www
.hemophilia.ca/english/index.html .
Haemophelia Society—Von Willebrand Support Services. Chesterfield House, 385 Euston Road, London, NW1 3AU. UK 0171 380 0600. Fax: 0171 387 8220. melissa @haemophilia-soc.demon.co.uk. http://www.haemophiliasoc.demon.co.uk/vwd%20services1.html .
National Hemophilia Foundation. Soho Building, 110 Greene Street, Suite 406, New York, NY 10012. (212) 219-8180.http://www.hemophilia.org/home.htm .
OTHER
Mannucci, Pier. “Desmopressin (DDAVP) in the Treatment of Bleeding Disorders: The First Twenty Years.” The Treat-
ment of Hemophilia Monograph Series. No. 11 (1998).
http://www.wfh.org/InformationAboutHemophilia/ Publications/Monographs/Treatment_Series/TOH_PDF/ TOH11_DDAVP.pdf .
Paper, Renee. “Gynecological Complications in Women with Bleeding Disorders.” The Treatment of Hemophilia Monograph Series. No. 5 (1996). http://www.wfh.org/ InformationAboutHemophilia/Publications/Monographs/ Treatment_Series/TOH_PDF/TOH5_VWD.pdf .
World Federation of Hemophilia. “Protocols for the Treatment of Hemophilia and von Willebrand Disease.” No. 14 (1998).http://www.wfh.org/InformationAboutHemophilia/ Publications/Monographs/Treatment_Series/TOH_PDF/ TOH14_Protocols_Treatment.pdf .
Vrolik type of osteogenesis imperfecta see
Osteogenesis imperfecta
1182 |
GALE ENCYCLOPEDIA OF GENETIC DISORDERS |

W
I Waardenburg syndrome
Definition
Waardenburg syndrome (WS) encompasses several different hereditary disorders, the main features of which variably include abnormal pigmentation, hearing loss, and a subtle difference in facial features. Certain other physical anomalies occur less frequently in WS.
Description
In 1951, Dr. Petrus Waardenburg reported a syndrome of dystopia canthorum, heterochromia of the irides, and hearing loss. Dystopia canthorum (also called telecanthus) describes a subtle but unusual facial feature in which the inner corners of the eyes (canthi) are spaced farther apart than normal, yet the eyes (pupils) themselves are normally spaced. The result is that the eyes appear to be widely spaced, even though they are not. Heterochromia means different-colored, and irides is the plural form of iris—the colored portion of the eye. Thus, someone with heterochromia of the irides has differentcolored eyes, often one brown and one blue. Another feature not originally noted by Dr. Waardenburg, but now considered a major sign of WS is a white forelock (white patch of hair extending back from the front of the scalp). In fact, disturbances in pigmentation (coloring) of various parts of the body are consistent features of WS. Uncommon but serious physical anomalies associated with WS include Hirschprung disease (intestinal malformation), spina bifida, cleft lip/palate, and musculoskeletal abnormalities of the arms.
Five types of WS have been defined based on clinical symptoms or genetic linkage. As of 2000, six different genes were associated with WS. Most families show autosomal dominant inheritance, but autosomal recessive inheritance and sporadic (single) cases are also seen. People with WS are not at increased risk for mental retardation, and vision loss is not more common. For the
KEY TERMS
Dystopia canthorum—A wide spacing between the inner corners of the eyes, with the eyes themselves having normal spacing. Also called telecanthus.
Heterochromia irides—A medical term for individuals with different-colored eyes.
Hirschsprung disease—A deformation in which the colon becomes enlarged (megacolon), caused by abnormal nerve control of that portion of the large intestine.
Hypopigmentation—Decreased or absent color (pigment) in a tissue.
Neural crest cells—A group of cells in the early embryo, located on either side of the area that will eventually develop into the spinal cord. The cells migrate (move) away from the area and give rise to various body structures, including melanocytes (pigment producing cells), certain structures of the face and head, and parts of the nervous system.
Neurocristopathy—A disorder that results from abnormal development and/or migration of the neural crest cells in the embryo.
Sensorineural—Type of hearing loss due to a defect in the inner ear (sensing organ) and/or the acoustic nerve.
Synophrys—A feature in which the eyebrows join in the middle. Also called blepharophimosis.
majority of those with WS, hearing loss is the only major medical problem they will have.
WS1 is the “classic” form of WS, and if someone uses just the name Waardenburg syndrome (with no modifying number), they are most likely referring to the
GALE ENCYCLOPEDIA OF GENETIC DISORDERS |
1183 |
Waardenburg syndrome
group of disorders as a whole or just WS1. WS2 may occasionally be referred to as WS without dystopia canthorum. WS3 is also known as Klein-Waardenburg syndrome, as well as WS with upper limb anomalies. Alternate names for WS4 include WaardenburgHirschprung disease, Waardenburg-Shah syndrome, Shah-Waardenburg syndrome, and Hirschprung disease with pigmentary anomaly.
Genetic profile
Since Dr. Waardenburg’s original description of his patients in 1951, many more families with the same or similar symptoms have been reported. By 1971, it became clear that a proportion of families have WS without dystopia canthorum. At that point, Waardenburg syndrome was divided into two distinct types, WS1 and WS2. In addition, a few individuals with typical signs of WS1 were found to also have musculoskeletal symptoms. This form of the disorder was named Klein-Waardenburg syndrome, now also known as WS3. Further, some researchers noted yet a different pattern of anomalies involving pigmentation defects and Hirschprung disease, which eventually became known as WS4. Finally, genetic testing of WS2 families has shown at least two subtypes—those that show genetic linkage are designated as WS2A and WS2B.
The four major types of WS have all been studied through DNA (genetic) analysis. There is some agreement between the clinical subtypes of WS and mutations in different genes, but genetic analysis has also served to confuse the naming scheme somewhat. The different types of WS, their inheritance patterns, and the genes associated with them, are listed below.
WS1
A number of different mutations in a single copy of the PAX3 gene on chromosome 2 are responsible for all cases of WS1, meaning it is always inherited as an autosomal dominant trait. The PAX3 gene plays a role in regulating other genes that have some function in producing melanocytes (pigment-producing cells). PAX3 was formerly known as the HUP2 gene.
WS2A
People who have typical signs of WS2 are designated as having WS2A only if genetic testing shows them to have a mutation in the MITF gene on chromosome 3. As with WS1, all cases of WS2A appear to be autosomal dominant. There is evidence that MITF is one of the genes regulated by PAX3.
WS2B
Some individuals with typical WS2 have had normal MITF gene analysis. A search for a different WS2 gene showed that some cases are linked to a gene on chromosome 1. This gene has been tentatively designated WS2B until its exact chromosomal location and protein product are identified. WS2B displays autosomal dominant inheritance.
WS3
Several people with a severe form of WS1 have been shown by genetic analysis to have a deletion of a small section of chromosome 2. Several genes are located in this section, including the PAX3 gene. Not all patients with WS3 have had the exact same genetic anomaly on chromosome 2, which may explain the variation in symptoms that have been reported. Some families with WS3 have displayed autosomal dominant inheritance, while other individuals with the condition have been sporadic cases.
WS4
Mutations in three different genes—EDNRB, EDN3, and SOX10 on chromosomes 13, 20, and 22 respectively—have been linked to WS4. Those cases of WS4 associated with the EDNRB and EDN3 show autosomal recessive inheritance, while the SOX10-associated cases are dominantly inherited.
Individuals with one of the autosomal dominant types of WS have a 50% risk of passing on the gene each time they have a child. A couple that has a child with WS4 linked to EDNRB or EDN3 faces a 25% risk for recurrence in each subsequent child. WS is quite variable, even within families. For instance, a parent with minimal pigment disturbance, mild facial features, and no hearing loss may have a child with pronounced physical features and deafness, and vice versa. There may be some correlation between specific gene mutations and the incidence of certain symptoms, but precise predictions are not possible.
As of 2000, the six genes listed above were those known to be associated with WS. It is expected, however, that more genes will be identified, especially since only a minority of WS2 cases have shown linkage to the MITF and WS2B genes.
Demographics
The prevalence of WS is estimated at one in 40,000. About 3% of all children with congenital deafness have WS. WS1 and WS2 occur with approximately the same frequency. WS3 and WS4 are much less common than
1184 |
GALE ENCYCLOPEDIA OF GENETIC DISORDERS |
the other types. The majority of people with WS are Caucasian, but members of other ethnic groups may be affected as well.
Signs and symptoms
WS1
Dystopia canthorum is seen in 99% of people with WS1. Other facial features may include decreased length of the nasal bone, a broad/high nasal root (top of the nose), and increased length of the lower face. Seventy percent of people with WS1 have either a medial flare of the eyebrows or synophrys (joining of the eyebrows in the middle, also called blepharophimosis).
Some type of pigmentary disturbance is nearly always present, and involves hypopigmentation (decreased color) of the skin, hair, and/or irides. However, unlike the more common forms of albinism that often involve a generalized lack of pigment in the body, WS is characterized by patches of hypopigmenta- tion—often termed “partial albinism.” A white forelock or premature graying is seen in about 70% of people with WS1. The eyelashes and patches of body hair may also be hypopigmented. Heterochromia of the irides may be complete (25% of patients) or partial (5% of patients). In complete heterochromia, each eye is a different color. In partial heterochromia, an individual iris (in one or both eyes) is composed of two colors. Those people with WS1 who do not have iris heterochromia often have brilliant blue coloring of both eyes.
Although estimates vary, hearing loss of some type is present in about 60% of individuals with WS1. The true prevalence is difficult to determine because of the variable nature of the condition. About 80% of those with hearing loss are affected in both ears (bilateral). Profound hearing loss occurs in some 25% of all people diagnosed with WS1.
Spina bifida (open spine) is seen in a very small percentage of newborns with WS1, as is cleft lip/palate. Hirschprung disease, a deformation in which the colon becomes enlarged (megacolon), is a somewhat more frequent anomaly. Sprengel anomaly (elevated shoulder blade) can also be seen. Overall, about 10% of children with WS1 have one of these anomalies.
WS2
The major clinical distinction between WS1 and WS2 is the absence of dystopia canthorum in WS2. Otherwise, the conditions mostly differ by incidences of the various symptoms. The incidence of hearing loss in WS2 is 80%, with about 30% having a profound loss. Heterochromia of the irides occurs in 50% of patients.
White forelock, premature graying, and hypopigmented skin patches are each found in about 15–20% of people with WS2. Synophrys occurs in only 5% of patients.
WS3
WS3 could be considered a subtype of WS1, since both are associated with the PAX3 gene. The distinction is clinical, with the added feature in WS3 being abnormalities of the muscles and bones of the arms. Some cases of WS3 have been sporadic. Several individuals diagnosed with WS3 have been in families where other members have typical signs of WS1. Thus, in some cases, WS3 can be considered a severe form of PAX3-associ- ated WS, and is a dramatic example of the variability that can occur within families.
WS4
Individuals with WS4 usually do not have dystopia canthorum, and often do not have hearing loss. Hirschprung disease is the major distinguishing feature of WS4. In fact, individuals who carry a single abnormal EDNRB or EDN3 gene (as opposed to two abnormal copies of either gene in WS4) have only Hirschprung disease. A small proportion of people with WS4 have been found to have an abnormal SOX10 gene.
Diagnosis
In the early 1990s, a group of researchers known as the Waardenburg Consortium established criteria for diagnosing someone with WS1. They considered the major criteria of WS1 to be:
•congenital sensorineural hearing loss (not due to some other obvious cause)
•pigmentary disturbance of the iris
•hair hypopigmentation of some type
•dystopia canthorum
•an affected first-degree relative (parent, sibling, or child)
Minor criteria established by the Waardenburg consortium include:
•several areas of hypopigmented skin
•synophrys or medial flare of the eyebrows
•broad and high nasal root
•hypoplastic alae nasi (cartilage and skin around the nostrils)
•premature graying of hair
In order to be diagnosed with WS1, a person must have two major criteria, or one major plus two minor
syndrome Waardenburg
GALE ENCYCLOPEDIA OF GENETIC DISORDERS |
1185 |

Waardenburg syndrome
TABLE 1
Waardenburg Syndrome
Type |
Inheritance |
Gene |
Chromosome |
Demographics |
Symptoms |
WS 1 |
AD |
PAX3 |
2 |
1 in 40,000 for all types; |
Dystopia canthorum (99%) |
|
|
|
|
WS 3 and WS 4 are less |
Medial flare of eyebrow or |
|
|
|
|
common than WS 1 and |
joining of eyebrows in the |
|
|
|
|
WS 2 |
middle (70%) |
|
|
|
|
|
Hypopigmentation |
|
|
|
|
|
of skin, hair, and/or irides |
|
|
|
|
|
Heterochromia of irides (30%) |
|
|
|
|
|
Hearing loss (60%) |
WS 2A |
AD |
MITF |
3 |
|
Same symptoms as WS 1, but |
|
|
|
|
|
without dystopia conthorum |
|
|
|
|
|
Incidence of symptoms varies |
|
|
|
|
|
from WS 1, e.g. hearing loss |
|
|
|
|
|
(80%), heterochromia of irides |
|
|
|
|
|
(50%), joining of eyebrows (5%) |
WS 2B |
AD |
“WS2B” |
1 |
|
See WS 2A |
WS 3 |
AD or sporadic |
Deletions |
2 |
|
Similar symptoms to WS 1 but |
|
|
including PAX3 |
|
|
also features abnormalities of |
|
|
|
|
|
arm muscles and bones |
WS 4 |
AR |
EDNRB |
13 |
|
Usually dystopia canthorum is |
|
AR |
EDN3 |
20 |
|
absent and incidence of hearing |
|
AD |
SOX10 |
22 |
|
loss is reduced |
|
|
|
|
|
Hirschsprung disease |
criteria. A modification of the list for WS2 includes removing dystopia canthorum, and including premature graying as a major criterion. With those modifications, a person with no family history of the condition should have two major criteria to be considered for WS2, and someone with an affected family member need only have one major criterion. Diagnosing WS2 can be more difficult than diagnosing WS1 because of the lack of dystopia canthorum. In addition, some people with a white forelock or premature graying may color their hair, and thus conceal an important sign.
As indicated, the distinction between WS1 and WS3 is clinical, with musculoskeletal anomalies added to the list of criteria for WS1. The criteria for diagnosing WS4 would be similar to those for WS2, with the inclusion of Hirschprung disease as a major criterion, and the probable exclusion of dystopia canthorum, broad nasal root, and severe hearing loss. In addition, by definition WS4 is not linked to PAX3, MITF, or WS2B, and is linked to one of the established WS4 genes (assuming genetic testing is available and informative).
Treatment and management
The primary medical consideration for people with WS is hearing loss. The most effective intervention is hearing aids. It is widely accepted that infants at risk for hearing loss, such as those who may inherit WS from a
parent, can benefit from screening in the newborn period. An undiagnosed hearing deficit can result in delays in speech and learning. Children with profound hearing loss are eligible for special accommodations in their education, and the entire family can benefit by starting to use sign language very early.
Although spina bifida in WS is uncommon, the potential complications are serious. Infants with spina bifida usually have damage to the spinal cord at the level of the open spine, and consequently have either partial or total paralysis below that point. The opening in the spine can be repaired, but the neurological damage to the spinal cord is permanent. Cleft lip/palate is also uncommon in WS, but is a serious birth defect. Children with cleft lip/palate usually require several surgeries, but the outcome of the repair is generally very good. It would be prudent to screen any infant of a parent with WS for Hirschprung disease. Surgical removal or repair of the affected segment of colon is often necessary. Depending on the severity of the musculoskeletal anomalies, a child with WS3 might require some sort of orthopedic intervention, such as casting, bracing, or surgery. A few children with WS3 have had only minor joint contractures of the arms and hands.
Genetic counseling is indicated for any family with WS. Prenatal diagnosis might be an option if genetic testing in the family is informative, but many couples may
1186 |
GALE ENCYCLOPEDIA OF GENETIC DISORDERS |

not choose invasive testing if they would not terminate a pregnancy for WS.
Prognosis
The majority of people with WS lead productive lives. In the absence of severe hearing loss, many people with WS would not be noticed as having a condition by anyone in the general population. If hearing loss is present, it usually does not get worse, and is often amenable to treatment. There is little hope for any preventive measures for WS, since all of the features of the syndrome occur early in embryonic development and are present at birth.
Resources
BOOKS
Gorlin, Robert J., Helga V. Toriello, and M. Michael Cohen.
Hereditary Hearing Loss and Its Syndromes. New York: Oxford University Press, 1995.
PERIODICALS
Mishriki, Yehia Y. “Facial Clues to an Inherited Syndrome.”
Postgraduate Medicine (July 2000): 107–110.
ORGANIZATIONS
Alexander Graham Bell Association for the Deaf, Inc. 3417 Volta Place NW, Washington, DC 20007-2778. (800) 4327543. http://www.agbell.org .
FACES: The National Craniofacial Association. PO Box 11082, Chattanooga, TN 37401. (423) 266-1632 or (800) 3322373. faces@faces-cranio.org. http://www.faces-cranio
.org/ .
National Association of the Deaf. 814 Thayer, Suite 250, Silver Spring, MD 20910-4500. (301) 587-1788. nadinfo@nad
.org. http://www.nad.org .
National Organization for Albinism and Hypopigmentation. 1530 Locust St. #29, Philadelphia, PA 19102-4415. (215) 545-2322 or (800) 473-2310. http://www.albinism
.org .
Research Registry for Hereditary Hearing Loss. 555 N. 30th St., Omaha, NE 68131. (800) 320-1171. http://www
.boystown.org/btnrh/deafgene.reg/waardsx.htm .
Scott J. Polzin, MS
I Walker-Warburg syndrome
Definition
Walker-Warburg syndrome is a congenital disorder of the central nervous system involving fatal neurological lesions. Multiple malformations of the brain, eyes, and muscle tissue distinguish WWS from similar malforma-
tion syndromes. It is also known by the acronym HARD +/- E syndrome (hydroencephalus, agyri, retinal dysplasia, plus or minus “e” for encephalocele).
Description
Affected individuals typically show a combination of severe brain, eye, and muscle defects. Multiple malformations of the brain include type II lissencephaly, a condition in which the brain lacks normal convolutions and is unusually smooth without folds. Eighty-four percent of the babies with WWS have macrocephaly (an enlarged head). In half of these cases, the macrocephaly is apparent at birth, and in a quarter of the cases it develops postnatally. Hydrocephalus, or excessive accumulation of cerebrospinal fluid around the brain, occurs in 95% of infants with WWS. This fluid fills abnormally large ventricles or spaces in the brain. Fifty percent of affected infants have an encephalocele, or gap in the skull that does not seal. The meninges or membranes that cover the brain may protrude through this gap. The formation of an encephalocele may be associated with the failure of the neural tube to close during development of the fetus. A malformed cerebellum characterizes the syndrome as well as distinct muscle abnormalities, including congenital muscular dystrophy.
Ocular defects occur in 100% of infants with WWS. The most common are abnormally small eyes and retinal abnormalities, which arise from the improper development of the light sensitive area at the back of the eye. Cataracts may also be present and more than three quarters of the infants born with WWS have a defect in the anterior chamber of the eye. WWS syndrome leads to severely retarded mental development and is often lethal in infancy.
Genetic profile
WWS is inherited in an autosomal recessive pattern. Offspring of parents who have had one affected infant have a 25% chance of having WWS. The locations of the causitive genes remains unknown.
Demographics
WWS is extremely rare. Cases described in the literature cite siblings with WWS born to consanguineous (closely related) parents as well as cases in families not known to be at risk.
Signs and symptoms
Clinical signs include a malformed head, small eyes, cataracts, retinal abnormalities, and muscle weakness. An
syndrome Warburg-Walker
GALE ENCYCLOPEDIA OF GENETIC DISORDERS |
1187 |
