
Gale Encyclopedia of Genetic Disorder / Gale Encyclopedia of Genetic Disorders, Two Volume Set - Volume 2 - M-Z - I
.pdf
Wilson disease
Because vitamin D can increase calcium levels, individuals with Williams syndrome and high calcium should not take multivitamins containing vitamin D. If calcium levels remain high after limiting vitamin D and decreasing dietary intake of calcium, an individual with hypercalcemia should see a nephrologist for further management and to monitor kidney function.
Strabismus (crossed eyes) can be treated by patching or by surgery. Ear infections can be treated with antibiotics and surgical placement of ear tubes.
The developmental differences of individuals with Williams syndrome should be treated with early intervention and special education classes. Specific learning strategies that capitalize on the strengths of individuals with Williams syndrome should be used. Physical, occupational, and speech therapy should be provided. Behavioral counseling and medication may help with behavioral problems such as hyperactivity and anxiety.
Prognosis
The prognosis for individuals with Williams syndrome is highly dependent on the medical complications of a particular individual. Individuals with Williams syndrome who have no heart complications, or very minor ones, have a good prognosis. Good medical care and treatment of potential problems allows most individuals with Williams syndrome to lead a long life. The prognosis for individuals with more serious medical complications such as severe heart disease or hypertension is more guarded. Since the medical conditions associated with Williams syndrome are progressive rather than static, it is very important that individuals with Williams syndrome have yearly medical examinations with a health care provider familiar with Williams syndrome.
The range of abilities among individuals with Williams syndrome is very wide and the ultimate functioning of an individual is dependent on his or her abilities. While individuals with Williams syndrome do well in structured environments such as school, their unique abilities and disabilities do not permit them to do as well in unstructured surroundings. Some individuals with Williams syndrome live independently but most live with their parents or in a supervised setting. Many individuals with Williams syndrome can gain employment in supervised settings and do well at tasks that do not require mathematics or visuospatial abilities. It is important to encourage individuals with Williams syndrome towards independence but to recognize that their friendly and outgoing personalities may lead them into abusive situations.
Resources
BOOKS
Bellugi, Ursula, and Marie St. George. Journey from Cognition to Brain to Gene: Perspectives from Williams Syndrome.
Cambridge, MA: MIT Press, 2001.
PERIODICALS
Finn, Robert. “Different Minds.” Discover (June 1991): 55–58.
ORGANIZATIONS
Williams Syndrome Association. PO Box 297, Clawson, MI 48017-0297. (248) 541-3630. Fax: (248) 541-3631. TMonkaba@aol.com. http://www.williams-syndrome
.org/ .
Williams Syndrome Foundation. University of California, Irvine, CA 92679-2310. (949)824-7259. http://www.wsf
.org/ .
Kathleen Fergus, MS, CGC
Williams-Beuren syndrome see Willams syndrome
I Wilson disease
Definition
Wilson disease is a rare, inherited disorder that causes excess copper to accumulate in the body. Steadily increasing amounts of copper circulating in the blood are deposited primarily in the brain, liver, kidneys, and the cornea of the eyes.
Description
Under normal conditions, copper that finds its way into the body through the diet is processed within the liver. This processed form of copper is then passed into the gallbladder, along with the other components of bile (a fluid produced by the liver, which enters the small intestine in order to help in digestive processes). When the gallbladder empties its contents into the first part of the small intestine (duodenum), the copper in the bile enters and passes through the intestine with the waste products of digestion. In healthy individuals, copper is then passed out of the body in stool.
In Wilson disease, copper does not pass from the liver into the bile, but rather begins to accumulate within the liver. As copper levels rise in the liver, the damaged organ begins to allow copper to flow into the bloodstream, where it circulates. Copper is then deposited throughout the body, building up primarily in the kid-
1198 |
GALE ENCYCLOPEDIA OF GENETIC DISORDERS |

KEY TERMS
Anemia—A blood condition in which the level of hemoglobin or the number of red blood cells falls below normal values. Common symptoms include paleness, fatigue, and shortness of breath.
Bile—A substance produced by the liver, and concentrated and stored in the gallbladder. Bile contains a number of different substances, including bile salts, cholesterol, and bilirubin.
Biopsy—The surgical removal and microscopic examination of living tissue for diagnostic purposes.
Cell—The smallest living units of the body which group together to form tissues and help the body perform specific functions.
Ceruloplasmin—A protein circulating in the bloodstream that binds with copper and transports it.
Chromosome—A microscopic thread-like structure found within each cell of the body and consists of a complex of proteins and DNA. Humans have 46 chromosomes arranged into 23 pairs. Changes in either the total number of chromosomes or their shape and size (structure) may lead to physical or mental abnormalities.
Cirrhosis—A chronic degenerative disease of the liver, in which normal cells are replaced by fibrous tissue. Cirrhosis is a major risk factor for the later development of liver cancer.
Deoxyribonucleic acid (DNA)—The genetic material in cells that holds the inherited instructions for growth, development, and cellular functioning.
Gallbladder—A small, pear-shaped organ in the upper right hand corner of the abdomen. It is connected by a series of ducts (tube-like channels) to the liver, pancreas, and duodenum (first part of the small intestine). The gallbladder receives bile from the liver, and concentrates and stores it. After a meal, bile is squeezed out of the gallbladder into the intestine, where it aids in digestion of food.
Gene—A building block of inheritance, which contains the instructions for the production of a particular protein, and is made up of a molecular sequence found on a section of DNA. Each gene is found on a precise location on a chromosome.
Glucose—One of the two simple sugars, together with galactose, that makes up the protein lactose, found in milk. Glucose is the form of sugar that is usable by the body to generate energy.
Hepatitis—A viral disease characterized by inflammation of the liver cells (hepatocytes). People infected with hepatitis B or hepatitis C virus are at an increased risk for developing liver cancer.
Jaundice—Yellowing of the skin or eyes due to excess of bilirubin in the blood.
Toxic—Poisonous.
neys, the brain and nervous system, and the eyes. Wilson disease, then, is a disorder of copper poisoning occurring from birth.
Genetic profile
Wilson disease is inherited in an autosomal recessive manner. Autosomal recessive refers to the pattern of inheritance in which each parent carries a gene for the disease on one of his or her chromosome pairs. When each parent passes on the chromosome with the gene for Wilson disease, the child will be affected with the disease. Both males and females can be affected with Wilson disease. If an individual is a carrier of the Wilson disease gene, they do not have any symptoms of this disease. In order to be affected, an individual must inherit two copies of the gene, one from each parent. Many cases of Wilson disease may not be inherited but occur as a spontaneous mutation in the gene.
The gene for Wilson disease is located on chromosome 13. The name of the gene is called ATP7B and is thought to be involved in transporting copper. As of 2001, over 70 different mutations of this gene have been identified, making diagnosis by genetic testing difficult.
Demographics
Wilson disease affects approximately 1 in 30,000 to 1 in 100,000 individuals and can affect people from many different populations. Approximately 1 in 90 individuals are carriers of the gene for Wilson disease.
Signs and symptoms
Symptoms typically present between the ages of three and 60, with age 17 considered to be the average age a diagnosis is made. About half of all patients
disease Wilson
GALE ENCYCLOPEDIA OF GENETIC DISORDERS |
1199 |

Wilson disease
Copper deposits are visible as a ring around the iris in patients with Wilson disease. Copper deposits in other organs as well and must be removed to avoid severe mental and physical development disorders. (Photo Researchers, Inc.)
experience their first symptoms in the liver. The illness causes swelling and tenderness of the liver, sometimes with fever, mimicking more common disorders, such as viral hepatitis and infectious mononucleosis. Abnormal levels of circulating liver enzymes reveal that the liver is being seriously damaged. This form of damage is referred to as “fatty degeneration.” Without medical intervention, the liver damage will progress to actual cirrhosis. An oftenfatal manifestation of liver disease is called fulminant hepatitis. This extremely severe inflammation of the liver (hepatitis) results in jaundice, fluid leaking into the abdomen, low protein circulating in the blood, abnormalities of the blood clotting system, swelling of the brain, and anemia due to the abnormal destruction of red blood cells.
Neurological symptoms are the first to occur in half of all patients due to copper accumulation in the brain and nervous system. The average age of onset for neurological symptoms is 21 years. These symptoms include tremors of the hands, uncontrollable movements of the limbs, stiffness, drooling, difficulty swallowing, difficulty talking, and headache. There is no change in a patient’s intelligence.
About one third of all patients with Wilson disease have a variety of psychiatric symptoms as the first signs of the disease. These symptoms include inability to cope, depression, irritability, increased anger, and inappropriate behavior. Patients often have trouble completing tasks at work or in school.
Other symptoms that can affect patients with Wilson disease, and may occur before or after a diagnosis has been made, include joint disorders, symptoms of arthritis, and skeletal problems such as osteoporosis. Patients have occasionally been affected with kidney stones and
abnormal handling of glucose in their body, and women may have menstrual cycle irregularities including temporary stopping of their regular cycle.
Diagnosis
The diagnosis of Wilson disease can be performed relatively easily through several different tests, however, because Wilson disease is so rare, diagnosis is often unfortunately delayed. The tests used to diagnose Wilson disease can be performed on patients who have or have not already shown symptoms of the disease. It is extremely important to make a diagnosis as soon as possible since liver damage can occur before there are any signs of the disease.
An easy way to diagnose Wilson disease is to measure the amount of a glycoprotein found in the blood called ceruloplasmin. Low levels of ceruloplasmin can diagnose the disease in about 80% of affected patients. This procedure is not as effective for women taking birth control pills, pregnant women, or infants less than six months of age.
A second test involving an eye examination to detect a characteristic ring of copper deposited in a membrane of the cornea (referred to as Kayser-Fleischer rings) is very easy to perform and is very useful in diagnosing patients who have already exhibited symptoms. This test is not as effective in persons without symptoms. This diagnostic test cannot be used by itself to make a diagnosis because some patients with liver disease but not Wilson disease will test positive.
A third test for diagnosing Wilson disease involves measuring the amount of copper in the liver. This can be accomplished by sampling a portion of the liver in a procedure called a biopsy. This is one of the most effective ways to diagnose Wilson disease, however, the procedure itself is more difficult to perform than the others.
Other tests are also useful, for example, measuring the amount of copper passed into the urine daily (high in Wilson disease). Another lab test measures the ability of a patient’s ceruloplasmin to bind with a form of copper (decreased in Wilson disease). And finally, as discussed under genetic profile, some patients can be diagnosed through a DNA test to determine whether or not they carry two genes for Wilson disease. This test does not always prove to be useful in certain patients and is most useful when testing the brothers and sisters of affected patients.
Treatment and management
Treatment involves life-long administration of either D-penicillamine or trientine hydrochloride. Both of these
1200 |
GALE ENCYCLOPEDIA OF GENETIC DISORDERS |
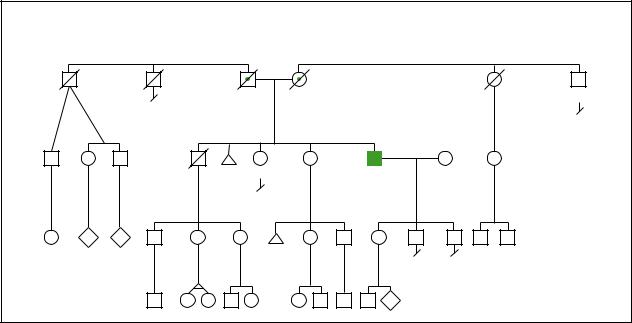
|
|
|
Wilson Disease |
|
|
Wilson |
|
|
|
|
Autosomal Recessive |
|
|
||
|
d.79y |
d.84y |
|
|
|
|
disease |
|
|
|
d.72y |
d.69y |
|
d.66y |
75y |
|
|
|
|
Diabetes |
|
Heart attack |
|
|
|
d.66y |
69y |
60y |
53y |
51y |
|
|
|
Possible |
|
Liver cirrhosis |
|
|
|
|
|
dementia |
|
|
speech |
|
|
|
|
|
|
|
problems |
|
|
3 |
4 |
3 |
|
|
|
|
|
|
|
3 |
2 |
|
P |
|
|
(Gale Group)
drugs remove copper deposits throughout the body by binding to the copper which is removed from the body in urine. Zinc acetate and a low copper diet are other ways to treat Wilson disease.
Penicillamine has a number of serious side effects:
•joint pain
•neurological problems
•systemic lupus erythematosus
•decreased production of all blood elements
•interference with clotting
•allergic reactions
Careful monitoring is necessary. When patients have side effects from penicillamine, the dose can sometimes be lowered to an effective level that causes fewer difficulties. Alternatively, steroid medications may be required to reduce certain sensitivity reactions. Trientine has fewer potential side effects, but must still be carefully monitored.
Treatment with zinc is also an effective way to remove excess copper from the body. Zinc is a metal that works to block copper absorption and bind copper in the intestinal cells until it is all released into the stool approximately one week later. The benefit of treatment with zinc is that there are no toxic side effects, however, the zinc is a slower acting agent than the other drugs. It takes four to eight months for the zinc to be effective in reducing the overall amount of copper in the body.
Finally, patients with Wilson disease are encouraged to follow a diet low in copper, with an average copper intake of 1.0 mg/day. Foods to avoid for their high levels of copper include liver and shellfish. Patients are also instructed to monitor their drinking water for excess levels of copper and drink distilled water instead.
Prognosis
Without treatment, Wilson disease is always fatal. With treatment, symptoms may continue to worsen for the first six to eight weeks. After this time, definite improvement should start to be seen. However, it may take several years (two to five) of treatment to reach maximal benefit to the brain and liver. Even then, many patients are not returned to their original level of functioning. Patients with Wilson disease need to maintain some sort of anticopper treatment for the rest of their lives in order to prevent copper levels from rising in the body. Interruptions in treatment can result in a relapse of the disease which is not reversible, and can ultimately lead to death.
Resources
BOOKS
Scheinberg, I. Herbert. “Wilson’s Disease.” In Harrison’s Principles of Internal Medicine. Edited by Anthony S. Fauci, et al. 14th ed. New York: McGraw-Hill, 1998.
GALE ENCYCLOPEDIA OF GENETIC DISORDERS |
1201 |

Wiskott-Aldrich syndrome
PERIODICALS
Gow, P.J., et al. “Diagnosis of Wilson’s Disease: An Experience Over Three Decades.” Gut 46 (March 2000): 415–419.
Hariharan, Ramesh, and L. Fred Herbert. “Wilson’s Disease.” Hospital Practice 31 (August 15, 1996): 556+.
Robertson, W.M. “Wilson’s Disease.” Archives of Neurology 57, no. 2 (February 2000): 276–7.
ORGANIZATIONS
American Liver Foundation. 75 Maiden Lane, Suite 603, New York, NY 10038. (800) 465-4837 or (888) 443-7222.http://www.liverfoundation.org .
National Organization for Rare Disorders (NORD). PO Box 8923, New Fairfield, CT 06812-8923. (203) 746-6518 or (800) 999-6673. Fax: (203) 746-6481. http://www
.rarediseases.org .
Wilson’s Disease Association. 4 Navaho Dr., Brookfield, CT 06804. (800) 399-0266.
WEBSITES
Wilson’s Disease Association.
http://www.medhelp.org/wda/wil.htm .
Katherine S. Hunt, MS
I Wiskott-Aldrich syndrome
Definition
Wiskott-Aldrich syndrome (WAS) is a rare inherited disorder marked by a low level of blood platelets, eczema, recurrent infections, and a high risk of leukemia or lymph node tumors.
Description
WAS was named for the two physicians who first reported the disorder. In 1937, Dr. A. Wiskott, a physician working in Munich, described two affected boys of German ancestry who had repeated infections, a skin rash, and poor blood-clotting ability. Nearly twenty years later, Dr. R.A. Aldrich reported similar symptoms in members of an American family of Dutch ancestry.
The syndrome is caused by a defect (mutation) in a specific gene called the WAS gene that normally codes for the protein named Wiskott-Aldrich Syndrome Protein (WASP). This vital protein is a component of cells that are important in the body’s defense against infection (lymphocytes). The same protein also functions in the cells that help prevent bleeding (platelets). A less severe form of the disease, X-linked thrombocytopenia, affects mainly the platelets.
Genetic profile
WAS is inherited as an X-linked genetic disorder and will therefore only affect males. The gene responsible for WAS is located on the short arm of the X chromosome. Since males have only one X chromosome, they only have one copy of the gene. If that copy carries the abnormal gene, they will have WAS. In contrast, females have two X chromosomes. They will have a normal copy of the gene on one chromosome even if an abnormal gene is on the other because the abnormal gene is very rare. The normal copy on one X chromosome is usually sufficient to prevent females from having WAS. However, women who have one abnormal copy of the WAS gene are designated as carriers. While they will not have WAS, they have a 50% risk of passing the gene to each of their sons, who would then have WAS. Carrier females also have a 50% risk of passing the defective copy of the gene to their daughters, who then become carriers.
Researchers identified the gene for WAS in 1994 and pinpointed its location on the short arm of the X chromosome (Xp11.22-p11.23). As of 2000, over 100 different mutations have been found in the gene among WAS patients. The fact that there are many mutations may explain some of the variability of symptoms among boys with WAS. However, even within the same family, affected individuals with the identical WAS gene mutation may have different degrees of severity of the disease. The mild form, X-linked thrombocytopenia, is also caused by mutations in this same gene.
Demographics
The WAS syndrome affects one in every 250,000 male children and occurs worldwide. In the year 2000, scientists estimated that about 500 Americans have WAS.
Signs and symptoms
Increased susceptibility to infections, eczema, and excessive bleeding are the hallmarks of WAS, although the symptoms can vary signficantly from one patient to another. The immune system of patients with WAS produces too few B and T cells. B cells are the cells in the body that make antibodies. There are many types of T cells. Both B and T cells are needed to defend the body against infection. Because both types of cells are affected, WAS patients are subject to repeated infections from bacteria, fungi, and viruses. Ear infections, meningitis, and pneumonia are common in boys with WAS.
WAS patients also have thrombocytopenia, a decreased number of platelets. Platelets are the specialized blood cells that help to form blood clots and prevent uncontrolled bleeding. The platelets may also be smaller
1202 |
GALE ENCYCLOPEDIA OF GENETIC DISORDERS |

KEY TERMS
Amniocentesis—A procedure performed at 16–18 weeks of pregnancy in which a needle is inserted through a woman’s abdomen into her uterus to draw out a small sample of the amniotic fluid from around the baby. Either the fluid itself or cells from the fluid can be used for a variety of tests to obtain information about genetic disorders and other medical conditions in the fetus.
Anemia—A blood condition in which the level of hemoglobin or the number of red blood cells falls below normal values. Common symptoms include paleness, fatigue, and shortness of breath.
Chorionic villus sampling (CVS)—A procedure used for prenatal diagnosis at 10–12 weeks gestation. Under ultrasound guidance a needle is inserted either through the mother’s vagina or abdominal wall and a sample of cells is collected from around the fetus. These cells are then tested for chromosome abnormalities or other genetic diseases.
Eczema—Inflammation of the skin with redness and other variable signs such as crusts, watery discharge, and itching.
Gene—A building block of inheritance, which contains the instructions for the production of a particular protein, and is made up of a molecular
than normal. Some of the earliest symptoms of the syndrome are hemorrhage from circumcision, bloody diarrhea, and a tendency to bruise very easily.
Anemia and an enlarged spleen (splenomegaly) are seen in some patients. About 10% of patients develop malignancies, usually leukemia or tumors in the lymph nodes (non-Hodgkin’s lymphoma).
Diagnosis
The diagnosis of WAS is usually suspected in male infants who have excessive bleeding, eczema, and frequent bacterial or viral infections. Special blood tests can then be ordered to confirm WAS. The blood of patients with Wiskott-Aldrich will show a low platelet count and a weak immune (antibody) response. It is also possible to confirm the diagnosis by obtaining a small sample of the patient’s blood and analyzing the DNA for a mutation in the WAS gene. Knowledge of the exact mutation combined with information about how much WAS protein the
sequence found on a section of DNA. Each gene is found on a precise location on a chromosome.
Immune system—A major system of the body that produces specialized cells and substances that interact with and destroy foreign antigens that invade the body.
Mutation—A permanent change in the genetic material that may alter a trait or characteristic of an individual, or manifest as disease, and can be transmitted to offspring.
Platelets—Small disc-shaped structures that circulate in the blood stream and participate in blood clotting.
Prenatal diagnosis—The determination of whether a fetus possesses a disease or disorder while it is still in the womb.
Syndrome—A group of signs and symptoms that collectively characterize a disease or disorder.
Thrombocytopenia—A persistent decrease in the number of blood platelets, usually associated with hemorrhaging.
X-linked—Located on the X chromosome, one of the sex chromosomes. X-linked genes follow a characteristic pattern of inheritance from one generation to the next.
defective gene can produce may help predict how severe a form of the disease an individual will have.
Carrier testing
If the specific WAS gene mutation is identified in an affected child, that child’s mother can then be tested to confirm that she carries the gene. Other members of the mother’s family may also want to consider testing to find out if they carry the same gene mutation. The first step in studying other family members is for a geneticist or genetic counselor to obtain a detailed family history and construct a pedigree (family tree) to determine which family members should be offered testing.
Prenatal diagnosis
In families in which there has been one child born with WAS, prenatal testing should be offered in subsequent pregnancies. There is a 50% chance with each subsequent pregnancy that the mother, who is a carrier, will
syndrome Aldrich-Wiskott
GALE ENCYCLOPEDIA OF GENETIC DISORDERS |
1203 |
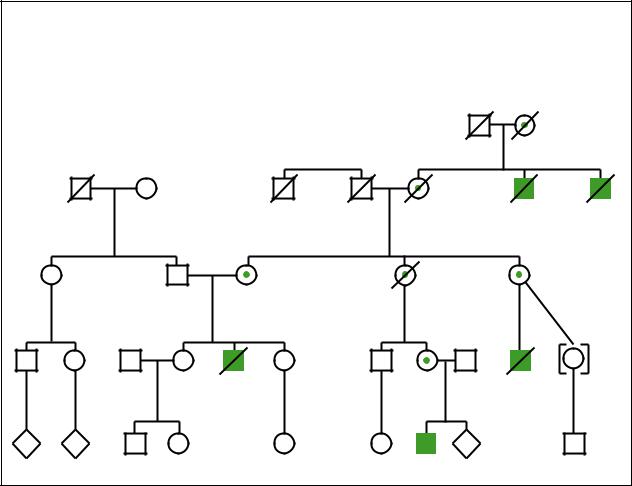
Wiskott-Aldrich syndrome
Wiskott-Aldrich Syndrome
X-Linked Recessive
Died Died young young
|
|
d.58y |
55y |
64y |
62y |
Car accident |
|
3
36y |
35y d.8y |
32y |
d.11y |
|
Pneumonia |
|
|
2 |
N |
|
|
2 |
P |
|
5y |
2y |
4y |
|
5y |
(Gale Group) |
|
|
|
|
|
transmit the abnormal copy of the gene to her baby. The key is to first identify the particular WAS gene mutation in the child with WAS. Then, early in a pregnancy, cells can be obtained from the developing fetus by chorionic villus sampling or amniocentesis, and checked for the same mutation. Women who carry the abnormal WAS gene and are considering prenatal diagnosis should discuss the risks and benefits of this type of testing with a geneticist or genetic counselor.
Treatment and management
Standard treatments for individuals with WAS include antibiotics for infections and platelet transfusions to limit bleeding. Immune globulin is given to strengthen the individual’s immune system. Eczema can be treated with corticosteroid creams applied directly to the skin. The spleen is sometimes removed to reduce the risk of bleeding. In individuals with WAS, however, removal of the spleen also increases the risk of infection unless
antibiotics are given to prevent infections. About 50% of individuals with WAS are helped by treatment with transfer factor, which is a substance derived from the T cells of a healthy person. Transfer factor is given to improve both blood clotting and immune functions. Bone marrow transplantation has been successful in a number of cases. It has been most successful in boys under five years of age when the donor is a sibling whose tissue type closely matches that of the individual with WAS. As of 2000, attempts were also being made to treat individuals with WAS with umbilical cord blood from unrelated newborns in cases when the individual diagnosed with WAS has no matched sibling donor.
Prognosis
The prognosis for males diagnosed with WiskottAdrich syndrome is poor. The average individual lives about four years; those who survive into adolescence often develop cancer. Death usually occurs from severe
1204 |
GALE ENCYCLOPEDIA OF GENETIC DISORDERS |

bleeding or overwhelming infection in the first few years of life.
Resources
BOOKS
Belmont, J. W., and J. M. Puck. “T Cell and Combined Immunodeficiency Disorders.” In The Metabolic & Molecular Bases of Inherited Disease. Edited by C. R. Scriver, et al. New York: McGraw-Hill, 2001.
PERIODICALS
Kuska, B. “Wiskott-Aldrich Syndrome: Molecular Pieces Slide Into Place.” Journal of the National Cancer Institute 92 (January 5, 2000): 9–11.
ORGANIZATIONS
Immune Deficiency Foundation. 40 W. Chesapeake Ave., Suite 308, Towson, MD 21204. (800) 296-4433. Fax: (410) 3219165. http://www.primaryimmune.org/inside.htm .
WEBSITES
“Entry 301000: Wiskott-Aldrich Syndrome.” OMIM—Online Mendelian Inheritance in Man. http://www.ncbi.nlm.nih
.gov/entrez/dispomim.cgi?id=301000 .
NORD—National Organization for Rare Disorders, Inc.
http://www.rarediseases.org .
Sallie Boineau Freeman, PhD
I Wolf-Hirschhorn syndrome
Definition
Wolf-Hirschhorn syndrome (WHS) refers to a condition that is caused by a missing part (deletion) of the short arm of chromosome 4. This missing genetic material results in severe developmental retardation, a characteristic facial appearance, and may include a variety of other birth defects.
Description
This syndrome was reported in 1965 in published reports by Wolf and Hirschhorn, who described that the characteristics of the syndrome were associated with a deletion of part of the short arm of chromosome 4. The short arm of a chromosome is called the “p” arm. Thus, this syndrome is also known as 4p-syndrome or deletion 4p syndrome, and occasionally as Wolf syndrome.
A normal human karyotype consists of 23 pairs of chromosomes. Each pair is numbered 1 through 22 and the 23rd pair are the sex chromosomes. On each chromosome are hundreds of genes that determine how our bod-
ies look and function. WHS is a contiguous gene syndrome. A contiguous gene syndrome occurs when a chromosome is either missing material (deletion) or has extra material (duplication) of several genes in the same region of the chromosome. Each time that the deletion or duplication of those genes occur, they cause specific characteristics that come to be known as a particular syndrome. This is in contrast to having just one particular gene cause a syndrome. Some patients who have WHS may have a small deletion on 4p, while others may be missing up to half of 4p. For this reason, some individuals have a less severe case of WHS than others do. The band 4p16.3 needs to be deleted in order for an individual to have full expression of WHS.
WHS frequently presents prenatally with slow growth (intrauterine growth retardation). Some infants with WHS can be stillborn or die shortly after birth. As many as 1/3 of reported patients have died in the first year of life. Individuals with WHS have been described as having a characteristic facial appearance likened to a “Greek Helmet facies.” This can be described as having a small head size (microcephaly), eyes spaced widely apart (ocular hypertelorism), downturned mouth, short upper lip and short groove between the upper lip and nose (philtrum) or bilateral cleft lip and small chin (micrognathia).
These children have severe developmental retardation. Other significant problems can include heart defects, cleft lip and/or palate, hearing impairment, and eye problems. Most children who have WHS have seizures (approximately 90%). Seizures are one of the major health concerns in children with WHS. These seizures begin between 5 and 23 months of age, however approximately 50% of the individuals stop having seizures between age 3 and 11. Sleeping problems are also common in children who have WHS. Although it seems that most of the literature focuses on children who have WHS, there are adults who have WHS.
Genetic profile
Frequently, with routine chromosome analysis, it is possible to identify that the short arm of chromosome 4 is missing some genetic material. The size of the missing material may vary from patient to patient. At times, the deletion is so small that it cannot be detected by routine chromosome analysis. If a patient is suspected to have WHS and an obvious deletion is not detected by routine chromosome analysis, more detailed studies, including fluorescent in situ hybridization, are warranted and may identify the missing genetic material. WHS may also present as mosaicism. Mosaicism for 4p-syndrome means that the
syndrome Hirschhorn-Wolf
GALE ENCYCLOPEDIA OF GENETIC DISORDERS |
1205 |

Wolf-Hirschhorn syndrome
KEY TERMS
Amniocentesis—A procedure performed at 16–18 weeks of pregnancy in which a needle is inserted through a woman’s abdomen into her uterus to draw out a small sample of the amniotic fluid from around the baby. Either the fluid itself or cells from the fluid can be used for a variety of tests to obtain information about genetic disorders and other medical conditions in the fetus.
Chorionic villus sampling (CVS)—A procedure used for prenatal diagnosis at 10–12 weeks gestation. Under ultrasound guidance a needle is inserted either through the mother’s vagina or abdominal wall and a sample of cells is collected from around the fetus. These cells are then tested for chromosome abnormalities or other genetic diseases.
Corpus callosum—A thick bundle of nerve fibers deep in the center of the forebrain that provides communications between the right and left cerebral hemispheres.
In vitro fertilization—Process by which a woman has her eggs surgically removed and fertilized in the laboratory. The developing embryos can then be transferred to her uterus to hopefully achieve a pregnancy.
individual has some cells that have normal number 4 chromosomes and other cells that are missing some of the genetic material from 4p.
Approximately 85–90% of cases of WHS occur as the result of a new deletion in the affected individual. This is also known as a de novo deletion and simply means that the affected individual’s parents did not have any chromosome arrangement that led to the deletion. In this case, the chance for recurrence in future pregnancies of a couple whom has an affected child is not increased. In the remaining 10–15% of cases, one of the parents of the affected individual carries a balanced translocation. A balanced translocation is a rearrangement in the individual’s chromosomes that causes that individual no problems since they have all the necessary genetic material that they need. However, when they produce eggs or sperm, the eggs or sperm may end up with an unbalanced arrangement and could lead to the conception of a child who has missing or extra genetic material. This could lead to miscarriage or to the birth of a child with conditions, such as WHS.
When a parent is identified as being a carrier of a balanced translocation, with each pregnancy they have an increased chance for having a child with an unbalanced chromosome arrangement. The chance of this is determined by the individual’s specific translocation, how it was identified, and which parent is the carrier of the translocation. Genetic counseling should be offered for any family in which a child is diagnosed to have WHS. Other family members should also be offered counseling and chromosome analysis to determine if they are carriers of a balanced translocation.
Demographics
The incidence of this condition is rare and estimated to be approximately one in 50,000 births. However, as with many genetic conditions, the condition may be misdiagnosed or may not be diagnosed in all individuals who are affected, especially if the condition results in pregnancy loss or loss in the early newborn period. It has been estimated that approximately 35% of individuals who have WHS die within the first two years of life. Also, with the advent of prenatal diagnosis, some fetuses with ultrasound abnormalities may be detected prenatally and the parents may elect to terminate the pregnancy. Approximately two-thirds of reported cases have been females.
Signs and symptoms
It is important to remember that each individual who may have a particular genetic syndrome is a unique individual. Therefore, all individuals with WHS do not have all of the same signs and symptoms. The most important reason for diagnosing an individual with a syndrome is not to put a label on that person. The reason for a diagnosis is so that predictions can be made to determine the needs of that person, based on the history available from other individuals affected with the same condition.
Signs and symptoms that can be associated with WHS include:
•slow growth before birth
•slow growth after birth (postnatal growth deficiency)
•small head size (microcephaly)
•weak cry in infancy
•poor muscle tone (hypotonia)
•seizures
•severe developmental retardation
•severe retardation of motor skills
•crossed eyes (strabismus)
•widely spaced eyes (hypertelorism)
1206 |
GALE ENCYCLOPEDIA OF GENETIC DISORDERS |
•droopy eyelids (ptosis)
•skin folds in the corner of the eyes (epicanthal folds)
•cleft lip and/or palate
•short upper lip and philtrum
•small chin (micrognathia)
•asymmetry of the skull (cranial asymmetry)
•skin tag or pit in front of the ear (preauricular tag or pit)
•downturned mouth
•prominent triangular area of the forehead (glabella)
•scalp defects on the center of the back of the head
•underdeveloped fingerprints (dermal ridges)
•a single crease across the palm of the hands (simian crease)
•misaligned bones in the front part of the foot/clubfoot (talipes equinovarus)
•turned up fingernails
•urinary opening on the underside of the penis (Hypospadias)
•undescended testicles (cryptorchidism)
•dimple at the base of the spine
•heart defects
•curvature of the spine (scoliosis)
•underdeveloped bones of the hands and pelvis
Diagnosis
When WHS is suspected, chromosome analysis should be performed and the laboratory should be informed as to what syndrome is suspected. This ensures that the laboratory carefully looks at chromosome 4 and if the deletion is not visible, then fluorescent in situ hybridization (FISH) can be done specifically for the critical 4p16.3 region of chromosome 4. FISH analysis is a procedure that is used in the laboratory to identify pieces of genetic material that are too small to see by looking at the chromosome under the microscope. Instead, DNA that is specific to a particular area of a chromosome is fluorescently labeled, so that it is visible under the microscope. This labeled DNA is then added to the sample and allowed to attach itself to the particular piece of DNA in question. This enables the laboratory technician to then look under the microscope for the fluorescent spot on the chromosome and identify extra or missing pieces of DNA that are too small to see by just looking at the chromosome alone. With this procedure, those individuals who have deletions so small that they cannot be detected by routine chromosome analysis may be able to have the deletion detected by FISH.
Interestingly, there is a syndrome called Pitt-Rogers- Danks syndrome (PRDS) that has been reported to have similar characteristics to WHS. Several individuals who have initially been diagnosed with PRDS, subsequently had FISH analysis that detected a deletion of 4p, and thus the individuals were reclassified as having WHS. Some feel that PRDS is actually WHS without obvious deletions of 4p.
When a couple has had a child diagnosed with WHS, and a member of that couple carries a balanced translocation, genetic counseling should be offered to discuss reproductive options. One option is choosing sperm or egg donation so that the parent who has the translocation does not pass unbalanced genetic material on to his or her child. Another option is preimplantation genetic diagnosis. Preimplantation genetic diagnosis is a very complex process that involves in vitro fertilization and diagnosing the embryos before they are placed into the mother’s uterus. Thus, only unaffected embryos are transferred to the uterus. Lastly, the options of CVS and amniocentesis for prenatal diagnosis should be discussed. All of these options have allowed couples who have balanced translocations, to realize the dream of having more children when the fear of having another affected child may have otherwise stopped them from choosing to add to their families.
If ultrasound examination reveals findings consistent with the possibility of WHS in a family with no history of WHS, genetic counseling and prenatal diagnosis should be offered. These ultrasound findings may include heart defects, microcephaly, agenesis of the corpus collosum (missing a specific part of the brain), micrognathia, cleft lip and palate, a hole in the diaphragm (diaphragmatic hernia), hypospadius, and clubbed feet. Keep in mind that these findings can also be consistent with other genetic syndromes.
Treatment and management
There is no treatment for the underlying condition of WHS. Treatment and management for patients who have WHS are specific to each individual. For example, some individuals with WHS may have heart defects or a cleft lip and/or palate that may require surgery, while others may not. Therefore, there is no specific treatment for individuals who have WHS, rather, the treatment and management is geared toward that particular individual’s needs and is likely to include several medical specialists. Information about patients who have WHS has been compiled and provides a comprehensive look into the natural history of this condition. It also allows the following management guidelines to be recommended. The collection of this
syndrome Hirschhorn-Wolf
GALE ENCYCLOPEDIA OF GENETIC DISORDERS |
1207 |
