
- •Foreword
- •Preface
- •Contents
- •1.1 Introduction
- •1.2 Prologue
- •1.9 Expansion of the Greater Omentum
- •3: Distal Gastrectomy
- •4: Total Gastrectomy
- •5.2 Part II: Thoracic Manipulation
- •6: Right Hemicolectomy
- •7: Appendectomy
- •8.6 Internal Pudendal Artery and Its Branches
- •8.13 Lateral Ligament
- •8.16 Fascia Propria of the Rectum: Part II
- •9: Sigmoidectomy
- •13: Hemorrhoidectomy
- •14: Right Hemihepatectomy
- •15: Left Lateral Sectionectomy
- •16: Laparoscopic Cholecystectomy
- •17: Open Cholecystectomy
- •Bibliography

Contents
\ 1\ Anatomy of the Stomach and Surrounding Structures, Part I: For Those Who Seek Theoretical Basis Through
Understanding of Developmental Process \ 1 1.1 Introduction\ 1 1.2 Prologue\ 2 1.3 Rotation of the Stomach\ 4 \1.4\ The Beginning of Intestinal Rotation\ 6 \1.5\ Collision of the Pancreas and Duodenum\ 8 1.6 Completion of Intestinal Rotation\ 10 1.7 Collision of the Transverse Mesocolon\ 12 \1.8\ Collision of the Ascending and Descending Colons\ 15 \1.9\ Expansion of the Greater Omentum \ 15
\ 2\ Anatomy of the Stomach and Surrounding Structures,
Part II: For Those Who Value Practical Knowledge\ 21
3 Distal Gastrectomy\ 33
4 Total Gastrectomy\ 91
\ 5\ Ivor Lewis Esophagectomy: A Curative Operation
for Esophageal Cancer\ 133 5.1 Part I: Abdominal Manipulation \ 133 5.2 Part II: Thoracic Manipulation\ 153
6 Right Hemicolectomy\ 187
7 Appendectomy\ 209
\ 8\ Anatomy of the Rectum and Surrounding Structures \ 223 8.1 Pelvis and Ligaments\ 223 \8.2\ Iliopsoas, Internal Obturator, and Piriformis Muscles\ 224 \8.3\ Structure of the Pelvic Floor: Part I\ 225 \8.4\ Structure of the Pelvic Floor: Part II\ 227 \8.5\ Internal Iliac Artery and Its Branches \ 229 \8.6\ Internal Pudendal Artery and Its Branches \ 229 \8.7\ Anatomy of the Nervous System
(Somatic Nervous System) \ 231 \8.8\ Anatomy of the Nervous System
(Autonomic Nervous System) \ 231
xi
xii |
Contents |
|
|
\8.9\ Structure of the Retrorectal Space: Part I\ 234 \8.10\ Structure of the Retrorectal Space: Part II\ 236 \8.11\ Structure of the Prerectal Space\ 239 \8.12\ End Point of Preand Retro-Rectal Dissection\ 239 8.13 Lateral Ligament\ 239 8.14 Three-Dimensional Construction
of the Rectum and Nerves\ 242 \8.15\ Fascia Propria of the Rectum: Part I \ 244 \8.16\ Fascia Propria of the Rectum: Part II\ 246
9 Sigmoidectomy \ 247
10\ \ Low Anterior Resection of the Rectum\ 263
11\ \ Abdominoperineal Resection of the Rectum \ 291
12\ \ Surgical Repair of Rectal Prolapse:
The Altemeier Procedure\ 305
13 Hemorrhoidectomy\ 319
14 Right Hemihepatectomy\ 327
15 Left Lateral Sectionectomy \ 369
16 Laparoscopic Cholecystectomy\ 399
17 Open Cholecystectomy\ 419
18 Pancreaticoduodenectomy: Whipple Procedure\ 435 \19\ Anatomy of the Inguinal Canal and Surrounding Structures\ 481
20\ \ Repair of Inguinal Hernia: Mesh Plug\ 495
Bibliography \ 515
Anatomy of the Stomach |
1 |
and Surrounding Structures, Part I: |
For Those Who Seek Theoretical
Basis Through Understanding
of Developmental Process
Abstract
This chapter describes the anatomy of the stomach, the pivotal organ in the upper abdomen, and surrounding structures based on the author’s embryological understanding. The intestinal primordium consists of the foregut, midgut, and hindgut. The stomach and proximal duodenum arise from the foregut. The gastrointestinal tract is anchored to the body wall by the mesentery, as are the stomach and duodenum. The stomach has the dorsal mesogastrium, as its main mesentery, and the ventral mesogastrium, which are both supplied by the celiac artery. Following rotation of the stomach and rotation of the midgut, the two major events during fetal life, these two mesenteries are significantly deformed, distended, anchored, and fused with adjacent mesenteries. The pancreas arises from the duodenal wall and extends through the mesoduodenum into the dorsal mesogastrium. The pancreas is therefore embedded in the mesentery. The principal concept behind gastric cancer surgery is to resect the mesogastrium while sparing the pancreas. A full understanding of the contour of the mesogastrium is therefore important to performing a reasonable operation.
Keywords
Stomach · Mesentery · Mesogastrium
Anatomy · Embryology
1.1\ Introduction
In surgery for gastrointestinal malignancy, it is generally recommended to remove the tumor- bearing organ together with its mesentery [1]. This would not be very difficult if the stomach and intestine were a straight tube. But in reality, two events occurring in the embryonic stage—rotation of the stomach and rotation of the intestine—result in the formation of a complex three-dimensional (3D) structure of the mesentery. This major movement of the stomach and intestine causes torsion of the mesentery, which was originally a simple plane, and subsequent linked events of collision and fusion. This elaborately programmed, significant, in utero event is a true spectacle show that opens with bulging of the dorsal mesentery of the stomach and closes with formation of the extensive greater omentum as the grand finale.
To properly dissect this complex mesentery at its base, surgeons seek to release fusions and restore the pre-collision structure of the mesentery. Therefore, understanding the anatomy of the stomach, the core organ of the major movement, and surrounding structures significantly influences the quality of not only curative surgery for gastric cancer but also other surgeries such as pancreaticoduodenectomy and curative surgery for colon cancer. In this chapter, we try to clearly reproduce this spectacle show on paper, while also detailing the blood vessels and membranes commonly handled by surgeons during surgery.
© Springer Nature Singapore Pte Ltd. 2020 |
1 |
H. Shinohara, Illustrated Abdominal Surgery, https://doi.org/10.1007/978-981-15-1796-9_1 |
|

2 |
1 Anatomy of the Stomach and Surrounding Structures, Part I: For Those Who Seek Theoretical Basis… |
|
|
Why does the omental bursa end in the middle of the transverse mesocolon? Why is the greater omentum made of multiple layers of membranes? Why is the main pancreatic duct curved sharply at the head of the pancreas? Why does the inferior pancreaticoduodenal artery, the first branch of the superior mesenteric artery, often form a common duct with the first jejunal artery at its root? Readers who have such questions are strongly encouraged to read this chapter. After viewing the spectacle show, it should be easier to understand and accept certain degrees of variation, such as the right hepatic artery originating from the superior mesenteric artery.
1.2\ Prologue
The show begins around the fifth fetal week when one intestinal primordium is connected to the abdominal wall via the mesentery (Fig. 1.1). The superficial peritoneum is a monolayered squamous epithelium composed of mesothelial cells, and so is a genuine “membrane.” Mesothelial cells show no clear cell boundaries when stained with standard procedures but show clear boundaries between polygonal cells when treated with silver nitrate. Outside the peritoneum is the extra- peritoneal fat, spaced by a small gap containing loose connective tissue. When viewed from the body surface side, the fat tissue is located anterior to the peritoneum and is therefore referred to as preperitoneal fat during surgery for inguinal hernia. The inner and outer surfaces of extraperitoneal fat are covered by a thin connective tissue membrane referred to as the subperitoneal fascia. The inner (peritoneal side) fascia is specifically referred to as the subretroperitoneal fascia, again a name based on the observation from the peritoneal side. Now we are using the term “fascia.” The fascia is essentially a dense connective tissue that has no epithelial cell arrangement as observed in the peritoneum, meaning that it is not a true “membrane.” Therefore, the question of how many fascias are present between A and B often makes no sense. The fascia present out-
side the extraperitoneal fat makes contact with the fascia transversalis, the main player in the inguinal anatomy, again spaced by a small gap containing loose connective tissue. Outside the fascia transversalis are abdominal muscles. The layers between the peritoneum and the fascia transversalis are collectively referred to as the retroperitoneal space, which is again referred to as the preperitoneal space during surgery for inguinal hernia. In any case, although we might find it a little uncomfortable to refer to the layer of fat or loose connective tissue as a space, the term “space” is used instead of “cavity” to indicate an artificially expanded space. A number of such spaces are present in the abdomen, including the retrorectal space as detailed in Chap. 8. The kidney is embedded in the retroperitoneal space, as shown in the lower left corner of Fig. 1.1. The kidney is initially located in the pelvis and gradually ascends within the layer of extraperitoneal fat.
The mesentery is a fat fold that anchors the intestine to the abdominal wall and has a double- layered structure in which the peritoneum and the subperitoneal fascia surround its surface in an omega shape. The peritoneum and the subperitoneal fascia are spaced by a small gap containing loose connective tissue and can be separated into two layers. Fat corresponds to the “intermediate layer” of the mesentery and serves as a conduit for blood vessels, lymphatics, and nerves to reach the intestine. In this book, this fat layer is often referred to as “flesh.” In the schematic diagrams (cross-sectional view) that frequently appear in this book, aside from a few exceptions, the peritoneum is shown as a thick line, the fascia as a thin line, and the gap between the two layers, consisting of loose connective tissue, as an equally spaced, ladder-like short thin lines connecting the two layers. The intermediate layer is lightly shaded.
The intestinal primordium consists of the foregut (giving rise to the stomach, proximal duodenum, hepatobiliary system, and pancreas), the midgut (the distal duodenum to proximal transverse colon), and the hindgut (the distal trans-
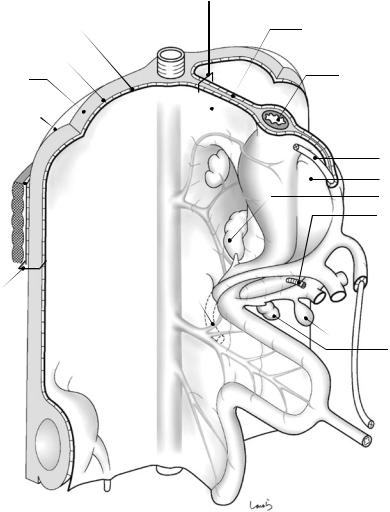
1.2 Prologue |
3 |
|
|
Peritoneum
Subperitoneal fascia (internal side)
Extraperitoneal fat
Subperitoneal fascia (external side)
Fascia transversalis
Abdominal wall  muscles
muscles
Retroperitoneal space
R kidney 
CA
SMA
IMA
Mesentery
Aorta
LGA
SPA
CHA
GDA
IPDA
*
Dorsal mesogastrium
Intermediate layer
Esophagus
Ductus venosus (Arantius’ duct)
Ventral
mesogastrium Dorsal pancreas
Proper hepatic a.
 TP
TP
 UP
UP
 Common bile duct Gallbladder
Common bile duct Gallbladder
Ventral pancreas
 Ligamentum
Ligamentum
teres
Vitelline duct
Fig. 1.1 Primitive gastrointestinal tract and the various derivatives in the fifth week of development
verse colon to rectum) [2, 3]. They are anchored to the posterior wall via dorsal mesentery. The dorsal pancreas arises from the duodenum [4], grows in the dorsal mesoduodenum, and eventually extends into the dorsal mesogastrium. The cell mass within the dorsal mesogastrium gives rise to the spleen. The dorsal mesogastrium is slightly concave on the left side (Fig. 1.1). The foregut is also anchored to the anterior wall via its ventral mesentery (ventral mesogastrium and ventral mesoduodenum), which gives rise to the liver (not shown in the figures before Sect. 1.6).
The common bile duct arises from the liver, gives rise to the gallbladder, and then forms a common orifice (major duodenal papilla, indicated by asterisk symbol in Fig. 1.1) with the ventral pancreas, which also arose within the ventral mesoduodenum, as a communication path to the intestinal tract. These facts support the premise that all abdominal organs, including the hepatobiliary system, pancreas, and spleen, have their own mesentery.
The digestive tract, hepatobiliary system, pancreas, and spleen are vascularized by three
4 |
1 Anatomy of the Stomach and Surrounding Structures, Part I: For Those Who Seek Theoretical Basis… |
|
|
arteries that arise from the aorta and pass through the mesentery: (1) the celiac artery (CA); (2) the superior mesenteric artery (SMA); and (3) the inferior mesenteric artery (IMA). These three arteries supply organs arising from the foregut, midgut, and hindgut, respectively. The CA, the most divergently supplying artery of the three, is forced to immediately divide into three branches: the left gastric artery (LGA), splenic artery (SPA), and common hepatic artery (CHA). The subsequent rotation of the stomach causes these arteries to run a crooked course. Moreover, the gastroduodenal artery (GDA) and the inferior pancreaticoduodenal artery (IPDA) usually form a clear anastomosis referred to as the pancreaticoduodenal arcade between the celiac and superior mesenteric artery systems. The IPDA is the first branch of the SMA.
Blood oxygenated in the placenta returns to the fetus through the umbilical vein. The umbilical vein passes through the liver while distributing blood to the hepatic sinusoids and leads to the main portal vein. Thereafter, it becomes the ductus venosus (Arantius’ duct) draining into the inferior vena cava. The hepatic sinusoids become the transverse portion of the portal vein (TP) after birth. The umbilical vein, after serving its purpose, becomes obliterated and is replaced by a cord; however, the portion connected to the hepatic sinusoids remains as the umbilical por-
tion of the portal vein (UP). This cord is referred to as the ligamentum teres. The ductus venosus is also obliterated and is itself replaced by a cord (ligamentum venosum) that connects the UP and the inferior vena cava. The ligamentum venosum is attached by the ventral mesentery of the stomach (lesser omentum). The direction of blood flow in the TP is reversed after birth (Fig. 1.2).
1.3\ Rotation oftheStomach
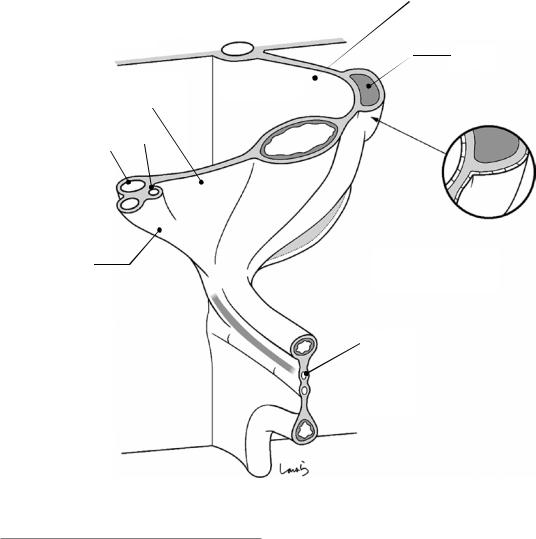
Around the sixth week of gestation, the dorsal mesogastrium begins to inflate toward the left, like a sail swelled out by the wind (small arrow in Fig. 1.3). This also causes a major shift of the dorsal pancreas and spleen, which are within the mesentery, to the left. This causes a clockwise rotation of the stomach along the longitudinal axis, resulting in the dorsal and ventral margins becoming the greater and lesser curvatures and the right and left walls becoming the posterior and anterior walls, respectively. The swollen mesentery forms a sac-like structure called the omental bursa and later becomes the greater omentum [5]. The ventral mesogastrium attached to the liver also moves to the right in a clockwise direction, which facilitates stomach rotation (large arrow in Fig. 1.3). This mesentery becomes the lesser omentum.
IVC |
Ductus venosus |
Ligamentum venosum |
TP |
TP |
UP |
|
Umbilical v. |
Ligamentum |
|
|
|
|
|
teres |
Before birth |
|
After birth |
Fig. 1.2 Changes in the direction of hepatic blood flow before and after the birth
1.3 Rotation oftheStomach |
5 |
|
|
Ductus venosus (Arantius’ duct)
Ventral |
LGA |
mesogastrium |
|
|
UP |
|
TP |
|
PHA |
Ventral pancreas
SMA
Gerota’s fascia 
Zuckerkandl’s 
fascia
Fig. 1.3 Embryo during the sixth week of development
Spleen
|
Greater curvature |
|
Dorsal pancreas |
RGEA |
Dorsal mesogastrium |
(Omental bursa) |
|
|
Pancreaticoduodenal |
|
arcade |
SMV
RGEA: R gastroepiploic a.
Figure 1.4 shows a schematic diagram of the dorsal mesogastrium, which is like a sail widely swelled out to the left by the wind, and the ventral mesogastrium, which is pushed to the right by the expanding dorsal mesogastrium. The right margin of the ventral mesogastrium surrounds the vessel group communicating to the liver, consisting of the portal vein, proper hepatic artery, and common bile duct. This structure is referred to as the hepatoduodenal ligament.
The kidney ascends while excluding extraperitoneal fat within the retroperitoneal space. This movement of the kidney while pushing the subretroperitoneal fascia from behind causes the
subretroperitoneal fascia to migrate behind the kidney, like seaweed wrapping around a sushi roll. This subretroperitoneal fascia surrounds the kidney with fat and is given a local name, the renal fascia, whose anterior and posterior aspects are referred to as the anterior and posterior layers of the renal fascia, respectively. The definition of the well-known Gerota’s fascia is ambiguous, where some definitions include the perirenal fat. For clinical purposes, the anterior layer of the renal fascia can be regarded as Gerota’s fascia. For reference, the posterior layer of the renal fascia is often referred to as Zuckerkandl’s fascia.
6 |
1 Anatomy of the Stomach and Surrounding Structures, Part I: For Those Who Seek Theoretical Basis… |
|
|
Ventral mesogastrium
PV PHA
Common bile duct 
Hepatoduodenal lig.
Dorsal mesogastrium
Aorta
Spleen
Omental bursa
Stomach
 Dorsal pancreas
Dorsal pancreas
SMV
 SMA
SMA
Fig. 1.4 Schematic diagram of the mesogastrium after rotation of the stomach
1.4\ The Beginning of Intestinal
Rotation
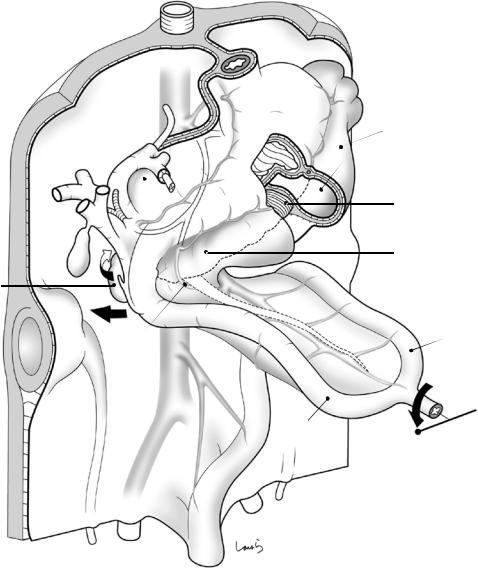
The impact of stomach rotation is also evident in the duodenal loop as it begins to bulge to the right during growth. The head of the dorsal pancreas, which is connected to the duodenum, also extends to the right, protruding out of the dorsal mesogastrium (see Fig. 1.5). Meanwhile, the ventral pancreas hides behind the duodenum
along with the common bile duct. This right shifting of the duodenum triggers the rotation of the intestine, the final major event in the midgut in fetal life. This is a major counterclockwise, three-quarter (270-degree) rotation around the SMA, which extends straight to the vitelline duct, as the rotation axis. The initial 90-degree rotation causes the shift of the proximal limb of the midgut loop to the right and the distal limb to the left (Fig. 1.6).
1.4 The Beginning of Intestinal Rotation |
7 |
|
|
LGA
 LGEA
LGEA
Dorsal mesogastrium
Ventral mesogastrium
CHA |
|
Omental bursa |
|||
RGA |
RGEA |
Body |
|
|
Dorsal |
|
|
|
|
||
GDA |
|
|
|
pancreas |
|
|
|
|
|
||
|
Head |
|
|
||
|
|
|
|
||
|
|
|
|
||
Ventral
pancreas SMA SMV
IPDA |
Distal limb |
Proximal limb
RGA: R gastric a.
LGEA: L gastroepiploic a.
Fig. 1.5 The intestinal loops after 90-degree counterclockwise rotation of the midgut
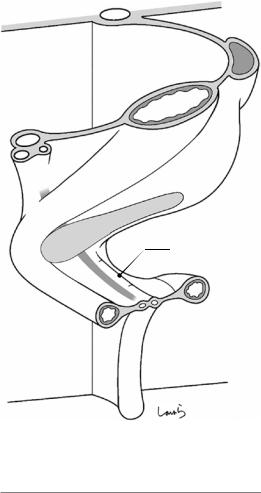
The upcoming major movement of the intestine causes the transformation of the mesentery from a simple plane to a spiral structure. Although many embryology textbooks illustrate this movement of the intestine only, this morphological change in the mesentery is actually more important for surgeons. Of the vessels forming the skel-
eton of the mesentery, three arteries (CA, SMA, and IMA) arise separately from the aorta, while three veins (SPV, SMV, and IMV) converge into the portal vein that drains into the liver. Therefore, an understanding of the torsion of the entire mesentery is helped by thinking that it occurs around the portal vein.
8 |
1 Anatomy of the Stomach and Surrounding Structures, Part I: For Those Who Seek Theoretical Basis… |
|
|
Omental bursa
 Dorsal pancreas
Dorsal pancreas
SMA
SMV
Distal limb
Proximal limb
Fig. 1.6 Schematic diagram of the mesogastrium after the initial 90-degree rotation of the midgut
1.5\ Collision ofthePancreas and Duodenum
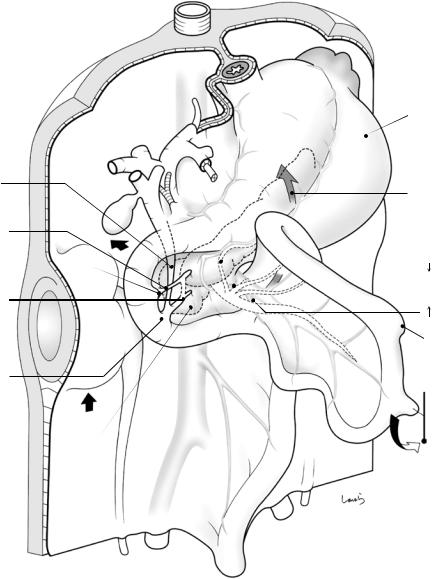
The ventral pancreas migrating behind the duodenum fuses with the dorsal pancreas to form the uncinate process (Fig. 1.7). During this process, the portal vein and the common bile duct are located between the ventral and dorsal pancreas. The ventral pancreatic duct orifice is located slightly inferior to the dorsal pancreatic duct orifice. Once the ventral pancreatic duct becomes the main pancreatic duct after pancreatic fusion, a bridge is formed between the two ducts. This is just like a flood control effort to bring the pancreatic juice produced in the dorsal pancreas into the newly formed main pancreatic duct. This officially turns the ventral pancreatic
duct orifice, which was originally shared with the common bile duct, into the major duodenal papilla (ampulla of Vater). At the same time, the downstream portion of the dorsal pancreatic duct is demoted to the accessory pancreatic duct and its orifice to the minor duodenal papilla. Given this complicated developmental process, it is no wonder that various types of abnormal arrangements of the pancreaticobiliary duct can occur.
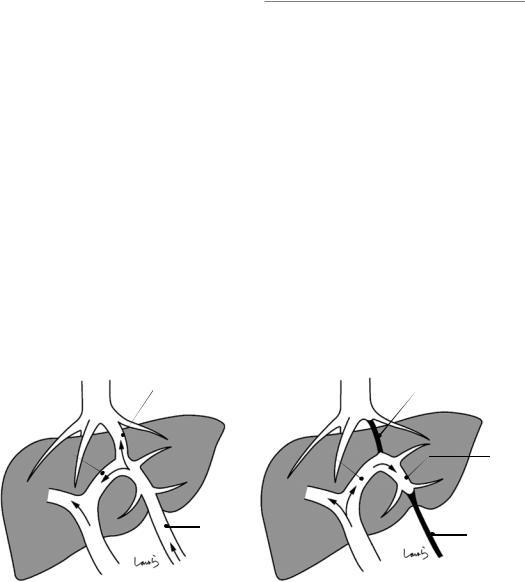
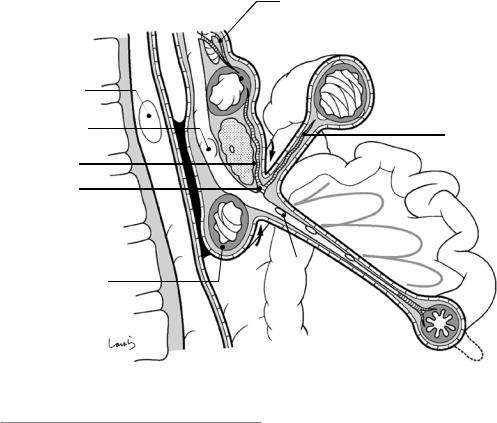
The pancreas and duodenum then collide with the posterior wall and subsequently anchored to the posterior abdominal wall by fusion of the overlying peritoneal membranes (see Fig. 1.8). It should be noted that fusion occurs only at the level of the peritoneum and the subperitoneal fascia; the lower layers remain intact. The middle part of the pancreas collides with the SMA at around its root but does not undergo peritoneal fusion as it is located at the base of the mesentery. The middle part of the pancreas is firmly anchored to the aorta, from an early stage of development, via well-developed connective tissue that surrounds the SMA and contains nerve plexuses. The fused peritoneum formed by collision is the fusion fascia of Treitz on the pancreatic head/duodenal side (to the right of the midline) and the fusion fascia of Toldt on the pancreatic tail side (to the left of the midline). The term “fascia” can now be used because the peritoneum has become connective tissue due to lysis of epithelial cells caused by collision. The fused peritoneum has lost the glossy appearance it used to have before fusion.
Repositioning organs to their original pre- collision positions during surgery is referred to as mobilization or displacement. Mobilization of the duodenum is specifically referred to as the Kocher maneuver, which is an important technique used not only for pancreatoduodenectomy but also for periaortic lymphadenectomy during gastric cancer surgery. With this technique, layer separation should be achieved by entering into the gap between the peritoneum and the visceral fascia (black arrow in Fig. 1.8) while keeping the fusion fascia of Treitz attached to the abdominal wall. Mobilization of the pancreatic tail is
1.5 Collision of the Pancreas and Duodenum |
9 |
|
|
Common |
|
|
bile duct |
|
|
Acc. |
|
|
pancreatic |
|
|
duct |
RGEV |
|
Minor |
|
|
duodenal |
|
MCV |
papilla |
|
|
|
|
|
Main |
SM |
ARCV |
pancreatic |
A |
|
|
|
|
duct |
* |
A |
|
|
|
|
|
SM |
Major |
|
|
duodenal |
|
|
papilla |
|
|
Uncinate |
|
|
process of |
|
|
pancreas |
|
|
Fig. 1.7 The intestinal loops after 180-degree rotation of the midgut
Dorsal mesogastrium
Treitz lig.
IPDA
90-degree difference in branching direction
J1a
Cecal diverticulum
RGEV: R gastric v. MCV: Middle colic v.
ARCV: Accessory r. colic v.
used for lymphadenectomy around the splenic artery during gastric cancer surgery. Taking into account the continuity with the later-described layer separation procedure for the transverse mesocolon, it is again appropriate to enter into the gap between the subperitoneal fascia on the visceral (pancreatic) side and the fusion fascia of Toldt (white arrow in Fig. 1.8). The subperitoneal fascia in the body/tail part of the pancreas is spe-
cifically referred to as the retropancreatic fascia. The location of a single layer of this fascia on the dorsal aspect of the pancreas after displacement indicates the correctness of the surgical procedure.
Another 90 degrees of rotation is applied to the midgut. This is driven by a string that pulls up the duodenojejunal junction (asterisk in Fig. 1.7) toward the diaphragm, a cord
10 |
1 Anatomy of the Stomach and Surrounding Structures, Part I: For Those Who Seek Theoretical Basis… |
|
|
Zuckerkandl’s fascia
Gerota’s fascia |
|
Treitz fusion |
|
fascia |
|
IVC |
Ao |
PV |
|
Mobilization of |
|
|
A |
IPDA |
V |
||
duodenum |
SM |
SM |
|
|
|
||
Toldt fusion fascia
Mobilization of the pancreatic tail
Retropancreatic fascia
Omental bursa
Fig. 1.8 Transverse cross section showing collision of the pancreas and duodenum
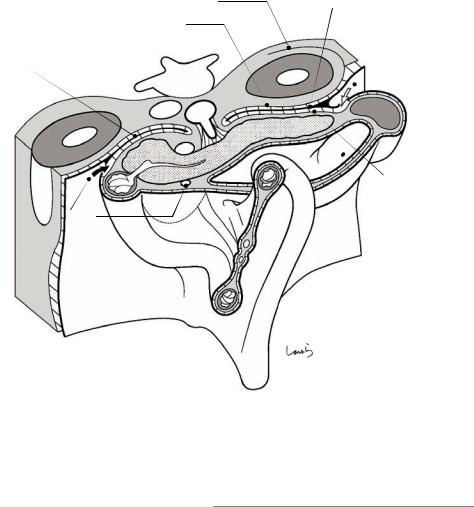
that later becomes the ligament of Treitz. During the rotation of the midgut, the mesentery is twisted at the root of the SMA, more precisely, between the two branches of the SMA, the IPDA, and the first jejunal artery (J1a) (indicated by the thin dotted line). Perhaps for this reason, the two arteries often form a common duct at their root. In this case, the IPDA arises from the back or left side of the SMA (see Fig. 1.9). This course variation should be kept in mind when dissecting the pancreatic uncinate process during pancreatoduodenectomy.
The proximal part of the midgut loop in relation to the vitelline duct grows vigorously and gives rise to a major part of the small intestine. In the meantime, the cecal diverticulum is formed in the distal part of the midgut and subsequently gives rise to the distal end of the ileum up to the right transverse colon. Thereafter, the vitelline
duct regresses and disappears or might remain as Meckel’s diverticulum.
1.6\ Completion ofIntestinal Rotation
By the tenth week of pregnancy, the midgut loop rotates another 90 degrees to complete the major rotation of 270 degrees in total (Fig. 1.9). The ligament of Treitz, which has pulled the duodenojejunal junction, is now located to the left of the SMA. The three major branches of the SMA— the IPDA, J1a, and middle colic artery, which were initially on the same plane—are forced to take a 3D branching pattern after mesenteric torsion. This local anatomy should also be kept in mind when performing pancreatoduodenectomy. Tracing along the SMA distally ends up reaching the branch that supplies the terminal ileum (not
1.6 Completion of Intestinal Rotation |
11 |
|
|
MCA |
|
|
MCV |
Treitz lig. |
|
RGEV |
|
|
|
Duodenojejunal |
|
IPDA |
junction |
|
J1a |
||
|
||
ARCV |
|
|
RCA |
|
|
|
ICA |
MCA; Middle colic a.
RCA: R colic a.
ICA: Ileocolic a.
Fig. 1.9 The intestinal loops after completing 270-degree rotation of the midgut
the ileocolic artery, which is considered the terminal branch). The branch ends in the part of the ileum where the vitelline duct used to be.
Figure 1.10 shows a sagittal cross section along the portal vein through to the superior mesenteric vein (SMV). Here, the gastrocolic
trunk of Henle, which is formed by the convergence of the right gastroepiploic vein (RGEV) and the accessory right colic vein (ARCV), drains into the SMV (indicated by two arrows) corresponding to the “center of torsion” of the mesentery.
12 |
1 Anatomy of the Stomach and Surrounding Structures, Part I: For Those Who Seek Theoretical Basis… |
|
|
IVC |
Omental |
PV |
Confluence of |
bursa |
|
L renal v. |
|
|
|
|
|
Confluence of |
|
|
splenic v. |
|
|
RGEV
Gastrocolic trunk of Henle
Treitz fusion fascia
Horizontal part of duodenum
RGA
Duodenal bulb
Transverse colon
ARCV
SMV
 Mesentery
Mesentery
of ileum
J1v
Ileum
Descending colon
Fig. 1.10 Sagittal cross section along the portal vein through to the superior mesenteric vein
1.7\ Collision oftheTransverse Mesocolon
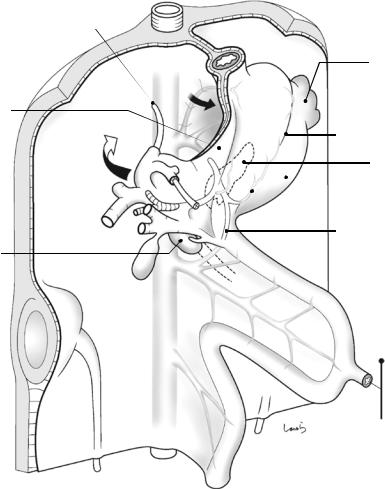
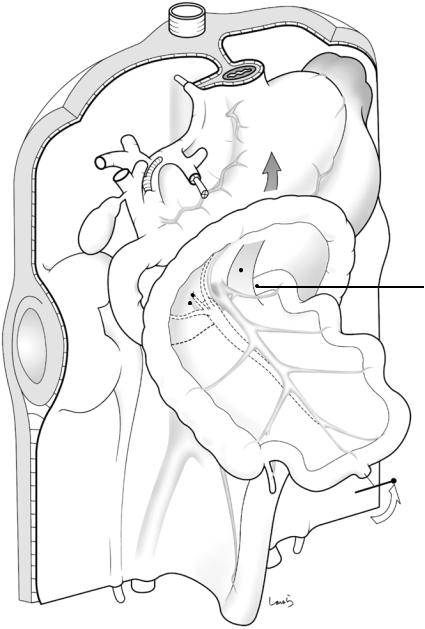
The liver, which we have not mentioned so far, now joins the show. The colon, which has completed the planned rotation and is in almost the final position, is going to be fixed sequentially. First, the transverse mesocolon is inverted cranially, causing a major part of the left half of the mesentery to collide with the dorsal mesogastrium, which now appears inflated like a balloon (Fig. 1.11). After the apposed peritoneal membranes are fused with each other, as always, the wide area of the dorsal mesogastrium in contact with the left transverse mesocolon will be recognized as the anterior layer of the mesentery (while the “former” transverse mesocolon becomes the posterior layer). The right margin of the contact surface corresponds to the right border of the omental bursa. The “new” left transverse mesocolon, generated as described above, has a layer structure consisting of, from top to bottom, the peritoneum, subperitoneal fascia, fat, subperitoneal fascia, fusion fascia, subperi-
toneal fascia, fat, subperitoneal fascia, and peritoneum (italics indicate the mesogastrium-derived anterior layer, also circled in Fig. 1.11). It is really astonishing that a membrane of just a few millimeters in thickness, even in adults, contains these many layers.
Figure 1.12a shows a sagittal cross section of the omental bursa slightly to the left of the midline. For the separation of the anterior layer of the left transverse mesocolon during gastric cancer surgery (although now rarely performed), it is appropriate to enter into the gap between the peritoneum and the subperitoneal fascia on the omental bursa side while keeping the fused peritoneum on the mesentery side intact (black arrow in Fig. 1.12a). While advancing within this layer, we pass behind the retropancreatic fascia and eventually reach the layer of the displaced pancreatic tail (white arrow in Fig. 1.12a). If wishing to dissect only the peritoneum over the anterior aspect of the pancreas without displacing the pancreatic tail, we will need to penetrate the subperitoneal tissue anteriorly at the lower edge
1.7 Collision of the Transverse Mesocolon |
13 |
|
|
RGA
SPDA
RGEV
Henle
ARCV
RGEA
MC |
A |
|
MC |
V |
|
A
SM
Dorsal mesogastrium Omental bursa
 L transverse mesocolon
L transverse mesocolon
Post. layer of transverse mesocolon
SPDA: Superior pancreatoduodenal a.
Fig. 1.11 Collision of the transverse mesocolon with the mesogastrium
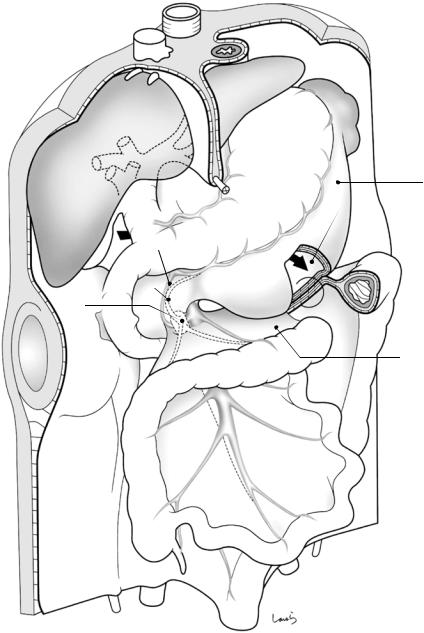
of the pancreas to deliberately transfer to the next upper layer (black thin arrow in Fig. 1.12a). Otherwise, we will continue to migrate under the pancreas, which we do not want to do. It should be noted that the gap between the retroperitoneum and the descending colon (asterisk in Fig. 1.12a) disappears after collision of the ascending and descending colons, as described later.
The right transverse mesocolon, which has not come into contact with the omental bursa, directly collides with the horizontal part of the duodenum and the anterior aspect of the uncinate process of the pancreas, again resulting in fusion of the apposed peritoneal membranes (Fig. 1.12b). At this point, the head/duodenal part of the pancreas has lost its peritoneum
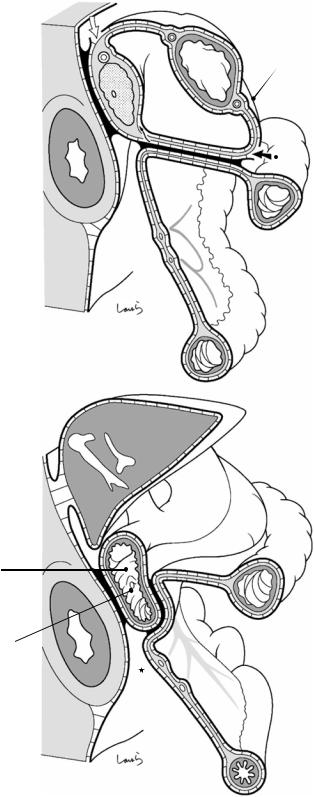
14 |
1 Anatomy of the Stomach and Surrounding Structures, Part I: For Those Who Seek Theoretical Basis… |
|
|
Fig. 1.12 Sagittal cross section of the omental bursa (a) and the duodenum (b) after collision of the transverse mesocolon
a
LGA
SPA 
Omental
bursa
*
b
Descending part of duodenum
Major duodenal papilla
Dorsal mesogastrium
Bursectomy
RGEA
