
Ghai Essential Pediatrics8th
.pdf
Childhood Malignancies -
Allogenic bone marrow transplantation offers a better chance of cure than conventional chemotherapy for children with ALL who enter a second remission after hematologic relapse.
Late Effects of Treatment
Continued followup of these patients for prolonged periods is necessary. Patients who have received cranial irradiation at a younger age are at risk for cognitive and intellectual impairment and development of CNS neoplasms. There is a risk of development of secondary AML after the intensive use of epipodophyllotoxins (etoposide or teniposide) therapy. Endocrine dysfunction leading to short stature, obesity, precocious puberty, osteoporosis, thyroiddysfunction andgrowthretardation are reported. Patients having received treatment with an anthracycline are at risk of cardiac toxicity.
Down Syndrome and Acute Leukemia
Children with trisomy 21 have a 15-20 fold higher risk of acute leukemia ascomparedtogeneral population with a cumulative risk of developing leukemia of approximately 2.1%. The ratio of ALL to AML in Down syndrome is as for childhood acute leukemia. Approximately one half two-thirds of cases of acute leukemia in children with Downsyndromeare ALL.The exception isduringthefirst 3 yr of life when AML predominates and exhibits a distinctive biology. In addition, approximately 10% of children with Down syndrome develop a preleukemic clone,transientmyeloproliferative disorder, with somatic mutations in hematopoeitic transcription factor GATAl. These children have a high leukocyte count, circulating blasts inperipheralblood, hepatosplenomegaly,effusions, anemia and thrombocytopenia in the neonatal period, which resolves by 3 months. About 20% of children with transient myeloproliferative disorder develop AML. Of the patients with Down syndrome and AML, two-thirds have megakaryocytic leukemia (FAB M7). Children with
Down syndrome and AML have a superior outcome than those not having Down syndrome. The outcome of childrenwithDownsyndrome and ALL is inferiorto those with ALL not associated with Down syndrome.
ACUTE MYELOID LEUKEMIA
Acute myeloid leukemia (AML) also termed as acute non lymphoblastic leukemia, accounts for 15-20% of leukemia in children. AML is a more complex and resistant disease than ALL and results from clonal proliferation of hematopoeitic precursors of myeloid, erythroid and megakaryocytic lineage.
Epidemiology
AMLcan occurat any age but the incidence is more during adolescence; males are affected as frequently as females. Congenital leukemia (occurring during first 4 weeks of life) is mostly AML. While the etiology of AML is not known, there is an association following exposure to ionizing radiation. Down syndrome is the most common genetic predisposing factor associated with risk of developing AMLduringthe first three years of life. Other predisposing factors are Fanconi anemia, Blooms syndrome, Kostmann syndrome and Diamond Blackfan anemia.Medicationsassociated with risk of AML include alkylating agents and epipodophyllotoxins.
Pathogenesis
Genetic aberrations in AML suggest alterations that regulate self renewal and differentiation cooperate in the pathogenesis of AML. Several genetic mutations have been identified in AML (Table 20.8). These include mutationsinFLT3, PTPN11, oncogenic Ras, BCR/ABL and TEL/PDGF R. Mutations and translocation fusion products that impair differentiation and apoptosis (class II mutations) include PML/RARa fusion from t(15;17), AML-1/ETO fusion from t(8;21), CEBPa mutations and
|
Table 20.8: Genetic abnormalities in AML |
|
|
Genetic abnormality |
Frequency (%) |
Clinicalfeatures |
Overall survival (%) |
Gene rearrangement |
|
|
|
t(8;21)(q22;q22) (ETO-AML1) |
12 |
Chloromas common |
75--85 |
inv(1 6)(p13;q22) (MYH 11-CBF ) |
8 |
Eosinophilia |
75-85 |
t(15;17)(q22;q 12) (PML-RARa) |
12 |
FAB M3; Auer rods present; |
90 |
|
|
sensitive retinoic acid t o |
|
Mutations (karyotype normal) |
|
|
|
NPM (nucleophosmin) |
8-10 |
|
75--85 |
CEBPa |
4-6 |
FAB Ml or M2 t ype |
80 |
FLT3-1TD |
10-15 |
|
<35 |
WT1 |
8-10 |
|
35-55 |
Del5 q |
1 |
|
<35 |
M onosomy7 |
2 |
|
<35 |

- Essential Pediatrics
MLL rearrangements. Presence of both types of genetic changesin thehematopoeiticprecursor cells leads to AML. Mutations in GATAl, a gene regulating hematopoietic differentiation of erythroid and megakaryocytic lineage, are primarily found in acute megakaryocytic leukemia in children with Down syndrome.
Classification
AML is divided into several subgroups according to the FABclassification: MO undifferentiated, Ml acute myelo blastic leukemia with minimal maturation, M2 acute myeloblastic leukemiawithmaturation, M3 acute promy elocytic leukemia, M4 acute myelomonocytic leukemia, MS acute monoblastic leukemia, M6 erythroblastic leukemia, M7 acute megakaryoblastic leukemia. About 30-40%patientswithAMLareMl and M2andabout same percentage is M4 and MS. M3 type of AML constitutes about 50-10% and M7 is strongly associated with Down syndrome. Specific chromosomal abnormalities arefound in the various subgroups. Translocation between chromosome 8and21t(8;21) translocation is found almost exclusively in Ml and M2. Almost all patients with M3 carry the translocation t(15;17) and MS is associated with t(9;11). Abnormalities of chromosome 16 are seen in mainly M4 subtype. FABclassification along with the most commonly employed histochemical stains is usually sufficient to distinguish the various subtypes of AML and differentiate it from ALL. Staining for myeloperoxidase activity and positive stain with Sudan black Bis observed in AML. Auer rods, needle shaped accumulation of pri mary granules, are commonly found in M2, M3 and MS subtypes of AML.
Clinical Features
Clinical presentation is similar to ALL but more likely to have higher white cell count at presentation and have
higher incidence of infections attimeof presentation. Most patients with AML present with pallor, fatigue, bleeding or fever as manifestations of underlying anemia, thrombocytopenia and neutropenia (Fig. 20.2A). Unlike ALL, lymphadenopathy and massive hepatospleno megaly is not common. However, infants and toddlers with AML have more organomegaly, high leukocyte counts and CNS disease at diagnosis. Theyare mostly M4 and MS subtypes. Disseminated intravascular coagulation may occur with any subgroup, but is common in acute promyelocytic leukemia (M3). Chloromas are localized collections of leukemic cells seen exclusively in patients with AML. They may occur at any site including CNS, neck, bones (typically orbit) and skin. Gingival hypertrophy may be present. Patients with high WBC count may present with signs of leukostasis such as pul monary infiltrates causing respiratory distress or stroke. Central nervous system involvement may occur in up to 15% patients.
Diagnosisisascertainedas in ALL by aperipheralsmear and bone marrow examination; the morphologic, cyto chemical, immunophenotypic and genetic characteristics of blast cells should be determined (Fig. 20.28).
Sometimes the diagnosis of AML is preceded by a prolonged preleukemic phase lasting several weeks or months. Thisis characterized by a lackofone of the normal blood cell lineages, resulting in refractory anemia, a moderate neutropenia orthrombocytopenia. The condition is referred to as a myelodysplastic syndrome. Some patients may show hypoplastic bone marrow that may develop later into an acute leukemia.
Treatment
ThelongtermsurvivalforchildrenwithAMLhasincreased from less than 10% to almost 50% during the past two decades. Thisisduetointensificationoftherapyalongwith
Figs 20.2A and B: A 5-yr-old boy presented with fever, epistaxis and petechiae; (A) Note bilateral subconjunctival bleeding; (B) peripheral smear showed myeloblasts. The arrow points to an Auer rod within a myeloblast, representing pink colored aggregated lysosomes. A diagnosis of acute myeloid leukemia of M2 type was made

improvedsupportivecare.However,comparedto ALL,the cure rate is hampered by low remission rate, an increased relapserate due to the development of resistance to multi ple chemotherapeutic drugs and a greater risk of death in remission due to infections and hemorrhage. The main drugs used for induction therapy are combination of cytosinearabinoside andananthracycline(daunorubicin). The induction regimen most commonly used is cytosine arabinoside(100mg/m2/daygivenascontinuousinfusion for 7 days) plus daunorubicin (45 mg/m2/day for 3 days) with orwithoutadditionaldrugs(etoposide,thioguanine). With the current regimen, remission is induced in about 70-80%patients.Consolidationtherapyincludeshighdose chemotherapy including cytosine arabinoside and etopo side. Risk based approach is also used for treatment of AML. Various prognostic factors in childhood AML have been identified: older age, obesity, MO and M7 subtype of AML FAB classification, presence of CNS disease at diag nosis,absenceofminimalresidualdiseaseandcytogenetics characteristicslikeInv16, t(8;21)and t(15;17) are associated with a favorable outcome. M3 subtype and AML with Downsyndromeare associatedwitha favorable outcome. Patients with favorable genetic features or with normal cytogeneticsaretreatedwithchemotherapyalone.Patients with unfavorable genetic alterations undergo stem cell transplantation in first remission.
Acute Promyelocytic Leukemia
Acutepromyelocyticleukemia (M3), accounting for about 10-15% of patients of AML, is treated differently with all trans retinoic acid or arsenic and systemic chemotherapy (anthracyclines and high dose cytarabine).
Childhood Malignancies -
of 4 yr. The other presents earlier in infancy and early childhood usually below the age of 4 yr, called juvenile CML.
Adult Variety of CML
Though the adult variety of CML is one of the commonest leukemias in adults, it is rare in children accounting for 3-5% cases. The natural history is divided into chronic, accelerated and blast phases.In the chronic phase, patient has nonspecific symptoms such as fever, malaise and weight loss. Occasionally, patients present with acute symptoms such as bone orjoint pain orpriapism.Spleno megaly is the most common physical finding and is usually massive (Fig. 20.3). Mild hepatomegaly and lymphadenopathy may be present. Symptoms of leuko stasis such as headache,dizzinessand visual disturbances may occur rarely. Leukocytosis is present in all cases and 80% patients have leukocyte counts above l,OO,OOO/mm3• The differential count shows all forms of myeloid cells from promyelocytes to polymorphonuclear leukocytes; basophilia is common. Mild anemia and thrombocytosis are common, but thrombocytopenia is rare.Bone marrow examination shows extreme hypercellularity. Myeloid blasts and promyelocytes constitute <20% of cells in the bone marrow in chronic phase. Chronic phase typically progresses to blast crisis which shows sudden rise in leukocytes and blast count and is indistinguishable from acute leukemia. Leukocyte alkaline phosphatase activity is low. Philadelphia chromosome, which involves a reciprocaltranslocation betweenlongarmsofchromosomes 22 and 9; t(9;22) is present in 90% cases. This may be detected by cytogenetics, FISH or RT-PCR.
Suggested Reading
Arya LS. Childhood cancer - challenges and opportunities. Indian J Pediatr 2003;70:159-62
Margolin JF, Rabin KR, Steuber CP, Poplack DG Acute lymphoblas tic leukemia. In: Principlesand Practice ofPediatricOncology. Eds. Pizzo PA, Poplack DG. Lippincott Williams and Wilkins, Philadelphia 2011;518-65
Pui CH, Carroll WL, Meshinchi S, Arceci R. Biology, risk stratifica tion and therapy of pediatric acute leukemias: an update. J Clin Oncol 2011;29:551-65
Pui Ch, Robison LL, Look TA. Acute lymphoblastic leukemia. Lan cet 2008;371:1030-43
Zwaan MC, Reinhart D, Hitzler J, Vyas P. Acute leukemias in chil dren with Down syndrome. Hematol Oncol Clin N Am 2010;24:19-34
CHRONIC MYELOID LEUKEMIA
Chronic myeloid or myelogenous leukemia (CML) is a myeloproliferative disorder. CML is primarily a disease of middle age; the peak incidence is in the fourth and fifth decade. However, it may occur at any age including infants andyoungchildren.Twomaintypes ofwelldiffer entiated myelogenous leukemia have been recognized. Oneisclinicallyandhematologicallycomparablewith the adult form of CML and occurs in children above the age
Fig. 20.3: A 6-yr-old boy presentedwith fever, pallor and progressive abdominal distension for 6 months. Significant splenomegaly and hepatomegaly was noted. A diagnosis of chronic myeloid leukemia was made

- Essential Pediatrics
The aim of treatment during chronic phase is to control the increasing white cell counts. This can usually be achieved by single agent chemotherapy with either busulfanorhydroxyurea. However, theseagentsarebeing replaced with P-interferon and tyrosine kinase inhibitor, imatinib mesylate. Majority of patients achieve complete hematologic and cytogenetic response with this therapy and the rate of progression to accelerated or blast crisis is decreased. The blood counts return to normal or near normal in almost all patients within 6-8 weeks. Spleen size also decreases. With conventional treatment, the average survival is 3-4 yr. Survival after development of accelerated phase is usually less than a year and after blast transformation only a few months. Allogeneic stem cell transplantation is now used for patients who do not respond to tyrosine kinase inhibitors.
Juvenile Chronic Myeloid Leukemia
JCML, also termed as juvenilemyelomonocyticleukemia, is an uncommon malignancy accounting for less than 2% leukemias inchildren. Patients with neurofibromatosis are at high risk for this condition. JCML is a disease of infancy and early childhood below the age of 5 yr, has a more acute and severe course with frequent lymphadenopathy, anemia, hepatosplenomegaly, skin involvement (eczema, xanthoma and cafe au lait spots), infection and thrombo cytopenia (Fig. 20.4).
Peripheral smear shows leukocytosis (usually less than 1,00,0000/mm3) with the full spectrum of granulocytic precursors and increased normoblasts; monocytosis is often striking. Thrombocytopenia and anemia are common. Leukocyte alkaline phosphatase scoreis normal or low and fetal hemoglobin levels are elevated. Bone marrow aspirates show an increased cellularity with predominance of granulocytic cells in all stages of maturation; megakaryocytes are normal or decreased.
Fig. 20.4: A 1-yr-old boy presented with fever, rashes, anemia and massive splenomegaly and hepatomegaly. He was diagnosed with juvenile myelomonocytic leukemia
Most patients have normal karyotypes or nonspecific chromosomal abnormalities. Philadelphia chromosome is negative; monosomy 7 is found in 30% patients.
JCML has a fulrninant andrapidlyfatal course. Manage ment involves supportive care including packed red cell and platelet transfusions, treatment of infections and allogeneic stem cell transplant if a matched sibling donor is present. Even with transplant, there is 30-50% event free survival rate at 3 yr. Cis-retinoic acid has been tried with some benefit.
Suggested Reading
Altman AJ, CeciliaFU. Chronic leukemias ofchildhood. In: Principles and Practice of Pediatric Oncology Eds. Pizzo PA, Poplack DG. Lippincot Williams & Wilkins, Philadelphia 2011;611-37
Goldman JM. How I treat chronic myeloid leukemia in the imatinib era? Blood 2007;110:2828-37
LYMPHOMA
Lymphomas are the third most common malignancy in childrenand adolescents, after leukemiaandbrain tumors. About 60% are non-Hodgkin lymphoma and 40% are Hodgkin lymphoma. Lymphomas are uncommon below the age of 5 yr and the incidence increases with age.
Hodgkin Lymphoma
Hodgkin lymphoma, a lymphoreticular neoplasm primarily of Bcell lineage involving lymph nodes andthe lymphatic system has unique molecular, histologic, immune phenotypic and clinical features. Hodgkin lymphoma occurs in 5-7/1,00,000 population. Hodgkin lymphoma isuncommonbelow theageof5 yr andexhibits three distinct forms in developing countries: The childhood form (younger than 14 yr), a young adult form (15-44 yr) and an older adult form (55-74 yr). There is a significant malepreponderance (10:1) in children affected below 7 yr of age with an almost equal sex distribution (1:1) beyond 12 yr of age.
The majority ofpatients achieve disease remission with multiagent chemotherapy with or without radiotherapy. Therapy is based on risk stratified approach based on disease stage and the presence of adverse prognostic factors.
Epidemiology
The etiology of Hodgkin lymphoma is believed to be multifactorial. Siblings have a seven fold increase in the risk and multiple studies have confirmed a gender concordanceof sibling pairs. A strongevidenceforgenetic susceptibility comes from a 100-fold increased risk in monozygotic twins compared with dizygotic twins. Epidemiologic studies suggest link between Hodgkin lymphoma and viral illnesses like Epstein-Barr virus (EBV). EBV viral DNA can be found in Reed-Sternberg cells suggesting that monoclonal proliferation of the neoplastic clone takes place after EBV infection. EBV-

positive classic Hodgkin lymphoma tumors differ geographically and are more common in developing countries. EBY infection is commonly seen in young children with mixed-cellularity Hodgkin lymphoma. Immune deficiency and autoimmune conditions (rheu matoid arthritis, SLE, sarcoidosis) are known to be associated with increased risk of Hodgkin lymphoma.
Pathology
Lymph nodes are the most common tissue on which the diagnosis of Hodgkin lymphoma is made. However, liver, spleen, bone marrow or lung may provide material for histological examination. It is necessary to obtain the entire node by excision biopsy for proper histologic examination. Fine needle aspiration biopsy and frozen section material are not optimal. The WHO classification of Hodgkin lymphoma recognizes two major subtypes: (i) nodular
lymphocytic-predominant Hodgkin lymphoma (NLPHL), (ii) classical Hodgkin lymphoma.
The NLPHL subtype of Hodgkin lymphoma is charac terized by large cells with multilobed nuclei referred as popcorn cells. These patients are generally asymptomatic and present with localizednonbulky disease. The hallmark of classic Hodgkin lymphoma is the Reed-Sternberg cell. This is a binucleated or multinucleated giant cell that is often characterized by a bilobed nucleus with two large nucleoli, giving an owl eye appearance to the cells. There are four varieties of this subgroup each characterized by the number of Reed-Sternberg cells, characteristics of inflammatory milieu and the presence or absence of fibrosis (nodular sclerosis, mixed cellularity, lymphocyte rich and lymphocyte depleted). Nodular sclerosis is the most common type in developed countries, whereas in developing countries including India, the mixed cellularity type is common, accounting for 60% cases (Table 20.9).
On immunophenotyping, classic Hodgkin lymphoma are positive for CD15 and CD30 and may be positive for CD20, whereas NLPHL is negative for CD15 and CD30 but positive for CD20 and CD45.
Clinical Features
Children with Hodgkin lymphoma present with painless cervical or supraclavicular lymphadenopathy; the nodes
Table 20.9: Histological subtypes of Hodgkin lymphoma
Histology |
Frequency |
Prognosis |
Nodular lymphocyte |
10% |
Excellent |
predominance |
|
|
Classical Hodgkin lymphoma |
|
|
Nodular sclerosis |
20-50% |
Very good |
Mixed cellularity |
20--40% |
Good |
Lymphocyte rich |
<10% |
Excellent |
Lymphocyte |
5-15% |
Poor |
depletion |
|
|
Childhood Malignancies -
are firm and rubbery in consistency (Fig. 20.5). Cervical lymph nodes are the most frequent (80%) site of primary involvement; 50% patients may also have mediastinal adenopathy and superior mediastinal syndrome. Less commonly, axillary or inguinal lymphadenopathy is the presenting feature. About 20-30% of children present with systemic 'B' symptoms, as defined by the Ann Arbor staging criteria, with fever over 38° C, night sweats and unexplained weight loss of >10% body weight at presen tation. The frequency of these symptoms increases with advanced disease and indicate an unfavorable prognosis. The presence of unexplained pruritus should prompt complete physical examination and chest radiography.
Fig. 20.5: A 12-yr-old boy presented with intermittent fever and significant bilateral cervical lymphadenopathy. Lymph node biopsy showed features of Hodgkin lymphoma
Besides presence of 'B' symptoms, other prognostic factors include stage of disease, histopathological subtype (risk increases from lymphocyte predominant to nodular sclerosis to mixed cellularity to lymphocyte depletion), bulky mediastinal disease, extensive splenic involvement and more than 5 nodal sites in stage III. Bone marrow involvement rarely results in cytopenias and has been associated with a variety of paraneoplastic syndromes that may be the presenting feature of the disease. Other uncommon sites of involvement include the gastro intestinal tract and skin. Splenic involvement occurs in 30--40% cases.
Diagnostic Workup and Staging
Evaluation of a patient includes careful physical exami nation with assessment of all lymph node bearing areas. Chest radiograph provides information about enlarge ment of the mediastinum. CT scan of the chest provides information about pulmonary parenchyma, chest wall, pleura and pericardium that may not be apparent on chest X-ray. CT scan of the abdomen and pelvis is done to

- Esse n taI e a t s _______________________________
:;;:,;;;; ;i;;;,.;;,.P.;;di;,;;;,;,r;.ic;,,;;;;;_
examine for involvement of the viscera and lymph nodes. Although lymphangiography is a reliable method for retroperitoneal lymph nodes, it is rarely performed in children. Bone marrow biopsy should be performed in all children with systemic symptoms and in advanced stage (III andIV)disease. A staging laparotomy is not performed because of concerns related to operative morbidity and splenectomy. Diagnostic staging and workup are outlined in Tables 20.10 and 20.11.
Table 20.10: Modified Ann Arbor staging for Hodgkin lymphoma
Stage Involvement
Management
Treatment modalities have varied from total nodal radiation therapy to chemotherapy to combination of chemotherapyandradiotherapywith significantimprove mentin survivalratethroughoutthelastthreedecades.All children generally receive combination chemotherapy as initial treatment.
With theemerging concept ofriskdirectedtherapymost children are treated with combination chemotherapy alone or in combination with radiotherapy. Superior treatment results and absence of leukemogenesis and permanent gonadal toxicity have made ABVD the prefer red front line regimen for Hodgkin lymphoma; however
ISingle lymph node region (I) or one extralymphatic the concerns of this protocol include cardiomyopathy and
site (IE)*
IITwo or more lymph node regions on same side of diaphragm (II) or one or more lymph node regions on same side of diaphragm plus local extralymphatic extension (IIE)*
IIILymphnode regions on both sides of the diaphragm (III) which may be accompanied by local extralymphatic extension (IIIE)*
IV |
Diffuse involvement of one or more extralymphatic* |
|
organ or sites |
ANo B symptoms
B Presence of at least one of the following B symptoms: Unexplained weight loss >10% baseline during 6 months before staging
Recurrent unexplained fever >38°C Recurrent night sweats
XBulky tumor**
*E lesion: Localized cxtranodal extension of Hodgkin lymphoma from a contiguous or nearby nodal site is noted with the designation E
••Defined as either a single mass of tumor tissue exceeding 10 cm in largest diameter or a mediastinal mass extending one-third of the maximum transverse intrathoracic diameter
Table 20.11: Diagnostic evaluation In Hodgkin lymphoma
Physical examination with measurement of lymph nodes Complete hemogram with ESR, C reactive protein
Liver and renal functions tests, alkaline phosphatase Lactate dehydrogenase
Chest X-ray, mediastinal mass to thoracic cavity ratio CT scan of neck, chest and abdomen
Bone marrow biopsy (all children except stages IA/IIA) Biopsy from lymph node or involved extranodal site
Bone scan (if bone pain or raised serum alkaline phosphatase) CT scan brain (if indicated)
Cerebrospinal fluid examination (if indicated)
PET-CT scan (higher sensitivity for stage and residual mass than conventional imaging)
Surgical staging with lymph node sampling and lymphan giography (selected cases)
CT computed tomography; ESR erythrocyte sedimentation rate; PET positron emission tomography
pulmonary fibrosis. The dose of radiation therapy used ranges between15and25 Gy. Severalstudieshavedemon strated that chemotherapy alone is effective therapy for pediatric Hodgkin lymphoma. The advantage of this approach is elimination of radiation associated adverse effects like myocardial dysfunction, musculoskeletal growth deficits and second malignancy.
With favorable clinical presentation (localized nodal involvement [stageI, II, IIIA], absence of B symptomsand no evidence of bulky disease treatment) consists of 2-4 cycles of chemotherapy (ABVD/others) and low dose involvedfieldradiation. Several studies havereduced the dose of radiation in patients achieving a favorable response to chemotherapy. Unfavorable clinical presen tation is defined as presence of B symptoms, bulky mediastinal/peripheral lymphadenopathy, extranodal extension of diseaseand advanced disease [stageIIIB-IV]. Localized disease (stage I, II, IIIA) with unfavorable features may be treated similarly to advanced stage disease in some protocols or given a therapy of inter mediate intensity. Unfavorable disease or localized disease with B symptoms are treated with 4-6 cycles of ABVD with/without radiotherapy (Table 20.12). Other combi nations include COPP/ABVD, MOPP/ABVD, OPPA, Stanford V regimen and BEACOPP regimes.
The role of additional radiotherapy in stage III and IV disease remains controversial. Adjuvant radiotherapy presents no survival advantage thoughbetterlocal tumor control is obtained. The use of hemopoietic stem cell transplantation (HSCT) as initial therapy remains controversial because of the overall excellent prognosis of children with advanced and unfavorable Hodgkin lymphomato chemotherapy alone or in combination with radiotherapy. At present HSCT should be reserved for patients after relapse or for those who are refractory to conventional therapy.
Suggested Reading
Dinand V, Arya LS. Epidemiology of childhood Hodgkin's disease; is it different in developing countries? Indian Pediatr 2006;43:141-7
Hudson MM, Onciu M, Donaldson 55. Hodgkin lymphoma. In: Prin ciples and Practice of Oncology. Eds. Pizzo PA, Poplack DG, Lippincott Williams and Wilkins, Philadelphia 2011;638-62

Childhood Malignancies -
Table 20.12: Commonly used drug combinations for Hodgkin lymphoma
ABVD |
Inj Doxorubicin or Adriamycin |
|
Inj. Bleomycin |
|
Inj. Vinblastine |
|
Inj. Dacarbazine |
|
Inj. Dexamethasone |
Keep off therapy from day 16-28. Repeat on day 28
COPP Inj. Cyclophosphamide Inj. Oncovin (Vincristine) Tab Prednisolone
Cap Procarbazine
Keep off therapy from day 16-28. Repeat on day 28
Non-Hodgkin Lymphoma
Non-Hodgkin lymphoma (NHL) comprises a hetero geneous group of lymphoid neoplasms derived from cells oftheimmunesystem.NHLmostcommonlyoccursduring the second decade of life and occurs less frequently in childrenlessthanthreeyearsofage.TogetherwithHodgkin lymphomatheycomprisethethirdmostcommonchildhood malignancy. Lowgrade lymphomas, which are common in adults are rare in children. Pediatric NHL are high grade, diffuse and aggressive with propensity for dissemination. With current treatment regimes about 80% of children and adolescents with NHL will survive for at least 5 yr.
Epidemiology
There is male preponderance, with male to female ratio of 3:1. Lymphomas are uncommon before3 yr of age. Also age specific trend of incidence of NHL have been obser ved that correlate with histologic subtype. Burkitt and Burkitt like lymphoma characteristically occurs in child ren between5 and 15 yr whereas the incidence of lympho blastic lymphoma is reasonably constant across all age groups. Diffuse large B cell lymphoma is a disease of older adolescents. In equatorial Africa, 50% of all cancers are lymphomas (Burkitt lymphoma being predominant). In United States and Europe, one-third of childhood NHL are lymphoblastic, one-half small, noncleaved cell lymphomas (Burkitt and non-Burkitt or Burkitt like) and the rest are large cell lymphomas. In India, lymphoblastic lymphoma is more common. NHL is also characterized on basis of their T or B cell nature. NHL may follow previous chemotherapy for Hodgkin disease, or be asso ciated with immunodeficiency and DNA repair defi ciency syndromes (Wiskott-Aldrich syndrome, X-linked 1ymphoproliferative disorders, ataxia-telangiectasia), acquired immunodeficiency syndrome and organ trans plantation (post-transplant lymphoproliferative disease). Infection with malaria and EB virus are considered risk factors for Burkitt lymphoma.
Pathology
There are 4 major pathological subtypes of NHL in children. These include Burkitt or Burkitt like lymphoma,
25 mg/m2 |
IV day 1 and 15 |
10 mg/m2 |
IV day 1 and 15 |
6 mg/m2 |
IV day 1 and 15 |
375 mg/m2 |
IV day 1 and 15 |
0.15 mg/kg |
IV day 1 and 15 |
600 mg/m2 |
IV day 1 and 8 |
1.5 mg/m2 (max 2 mg) |
IV day 1 and 8 |
40 mg/m2 (max 60 mg) |
PO day 1 to 14 |
100 mg/m2 |
PO day 1 to 14 |
lymphoblastic lymphoma, diffuse large B cell lymphoma. and anaplastic large cell lymphoma. All pediatric NHL are high grade andaggressivewhileNHL in adults is often indolent.
Clinical Presentation
NHL in children has distinct clinical and behavior pro pertieswhencomparedtoadults.Lymphomasinadultsare commonly low or intermediate grade and are dominantly nodal, have variable growth fraction with poor longterm outcome. NHL in children is high grade, extranodal with high growth fraction and good outcome.
Children with NHL typically present with extranodal diseaseinvolvingthemediastinum,abdomen, orhead and neck region (Figs 20.6A and B). Intrathoracic NHL most often T cell lymphoma maypresent with features of supe rior mediastinal or superior vena caval syndrome. There may be associated pleural and/or pericardial effusion. Cervical adenopathy, abdominal pain, ascites, palpable abdominal mass, intestinal obstructionor intussusception (typically B cell disease), cranial nerve palsy, bone involvement, jaw swelling and cytopenias due to bone marrow involvement are other features.
Diagnosis
NHL are rapidly growing tumors; prompt diagnosis is therefore essential. Almost two-thirds of patients have widespread disease at the time of diagnosis, involving bone marrow, central nervous system or both. Selection of the appropriate lymph node or mass for histological diagnosis is.necessary. Histology is the primary means for diagnosis and is supplemented, if possible, with immunophenotypic and cytogenetic studies. Ifthe clinical condition is not suitable for biopsy, due to a large media stinal mass causing superior vena cava syndrome, the diagnosis may be made with less invasive procedures, e.g. percutaneous needle aspiration of accessible lymph node, examination of body fluids (e.g. pleural fluid) or bone marrow. Table 20.13 shows the St Jude staging system, which is applicable to all types of childhood NHL. In newly diagnosed patients, a detailed workup and relevant investigations should be done (Table 20.11).
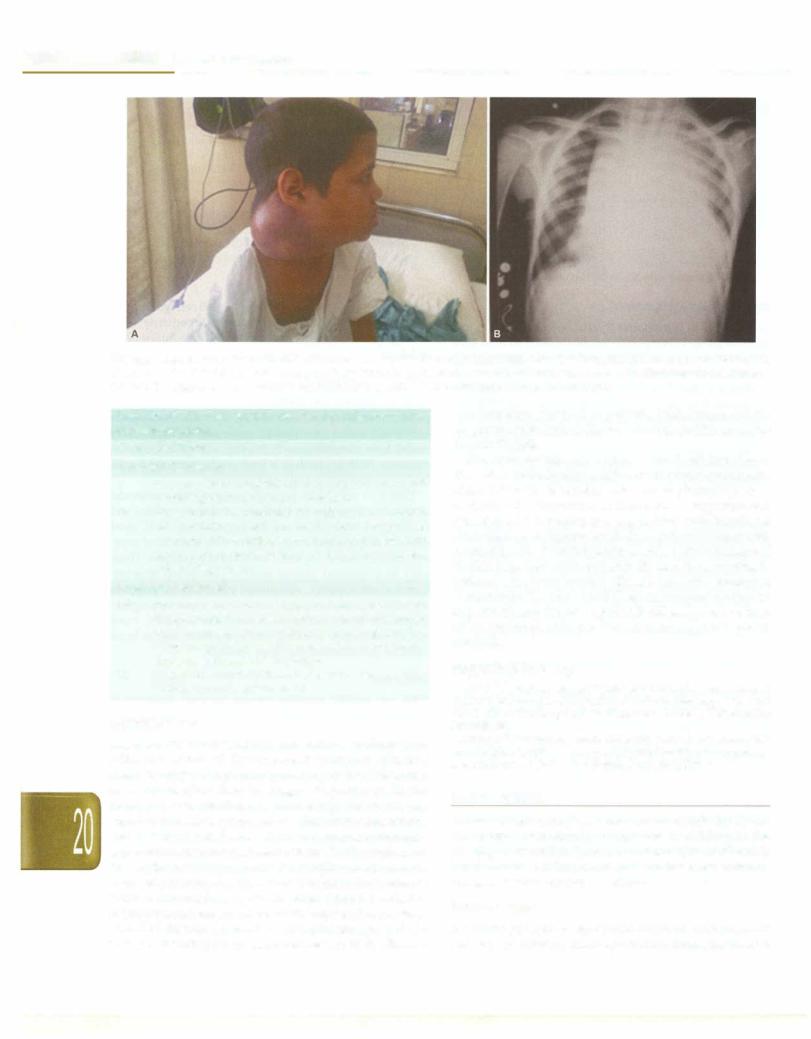
__e_s s_e_n.t1.·a _1_P_e_d_ia_t_r.ics_________________________________
Figs 20.6A and B: (A) A 10-yr-old child with fever was diagnosed as Burkitt lymphoma. Note significant right cervical lymphadenopathy; (B) chest X-ray of a 7-yr-old boy with continuous fever. Note the mediastinal mass with shift of mediastinum to the right and left-sided pleural effusion. The diagnosis of non-Hodgkin T-lymphoblastic lymphoma was confirmed on lymph node biopsy
Table 20.13: St Jude staging system for childhood non Hodgkin lymphoma
Stage Definition
Low-risk (localized)
ISingletumor (extranodal);singleanatomicarea (nodal)
excluding mediastinum and abdomen
IISingletumor(extranodal) withregionalnode involve ment; primary gastrointestinal tumor (completely resected, with or without involvement of mesenteric node); two or more tumors or nodal areas on one side of diaphragm
High-risk (advanced)
IIIPrimary intrathoracic (mediastinal, pleural and thymic) tumor; extensive primary intra-abdominal
disease;paraspinal or epidural tumors regardless of other tumor sites; two or more nodal or extranodal areas on both sides of diaphragm
IV Any of the above with central nervous system and/ or bone marrow involvement
Management
The dramatic improvement in the survival of patients with NHL is because of development of highly effective chemotherapy and supportivecare.Surgeryhaslimited role in treatment other than for diagnostic purposes. Radio therapy is also restricted to emergency situations, e.g. superior vena cava syndrome or spinal cord compression due to paraspinal disease. Different chemotherapeutic regimens areusedfor treatment of B a nd T celllymphomas. The regimens for lymphoblastic lymphoma are usually based on protocols for ALL. These are intensive protocols that use combinations of 8 to 10 drugs. Cranial irradiation or prophylactic intrathecal chemotherapy is given in stage III and IV disease. Chemotherapy is give for a period of 1 to 2 yr depending on the stage and extent of the disease.
The longterm survival in patients with lymphoblastic lymphoma with limited diseaseis80--90%andforadvanced disease 70--80%.
The chemotherapeutic regimens for B cell lymphoma (Burkitt and non-Burkitt) is different.Mostprotocolsconsist of short duration (6 months),intensive alkylating high dose methotrexate, vincristine, anthracyclines, etoposide and cytarabine; CNS prophylaxis is provided with intrathecal chemotherapy.Longtermsurvivalishighlysatisfactorywith survival in more than 90% patientswith limited disease and 75-85% in patients with extensive disease. Survival rates in patients with bone marrow disease have also improved dramatically. The use of anti-CD20 monoclonal antibodies (e.g. rituximab) directed against B cell antigens has been safely combined with standard chemotherapy to improve survival.
Suggested Reading
Gross TG, Perkins SL. Malignant non-Hodgkin lymphomas in children. In: Principles and Practice of Pediatric Oncology. Eds. Pizzo PA, Poplack DG, Lippincott Williams and Wilkins, Philadelphia 2011;663-82
Srinivas V, Soman CS, Naresh KN. Study of the distribution of 289 non-Hodgkin lymphomas using WHO classification among children and adolescents in India. Med Pediatr Oncol 2002;39:40-3
BRAIN TUMORS
Tumors of the central nervous system (Table 20.14) are the second most common neoplasms in children in the developed countries. They account for 25% of all child hood cancers. Medulloblastomas are the most common malignant brain tumors in children.
Epidemiology
Exposure of CNS to significant doses of radiation and presence of certain genetic syndromes increases the risk

Table 20.14: Common pediatric brain tumors Glial tumors
Astrocytoma Low grade High grade Ependymoma
Embryonal tumors
Medulloblastoma
Primitive neuroectodermal tumor Atypical teratoid rhabdoid tumor
Germ cell tumors
Germinoma
Non-germinomatous germ cell tumors
Choroid plexus tumors
Papilloma
Carcinoma
Craniopharyngioma
of developing brain tumors. Meningiomas and malignant gliomas arise within the radiation field several years or decades after radiation therapy. Children who have received cranial or craniospinal radiation for treatment of ALL are at risk for developing these tumors.
Several genetic syndromes predispose to developing CNS tumors (Table 20.15). Of these NFl is the most common disorder. Approximately 15% of patients with NFl develop optic gliomas during their lifetime. Optic gliomas in patients with NFl have a more benign course and may even regress spontaneously. However, the majority of pediatric CNS tumors are sporadic and have no known cause.
Clinical Presentation
Clinical presentation of brain tumors depends on location of the tumor and the rate of growth. Symptoms arise because of raised intracranial pressure or from direct infiltration or compression of parts of the CNS.
Childhood Malignancies -
Symptoms from Raised lntracranial Pressure
Infratentorialtumorsaremorecommonthansupratentorial tumors in children and hence more likely to develop acute or chronic hydrocephalus (Fig. 20.7). Recurrent headaches that are worse at night or early morning and worsen with lying down, early morning vomiting, vision loss, features of VI nerve palsy or sunset sign are indicative of raised intracranial pressure. Acute increase in intracranial pressure maypresent with Cushing triad of hypertension, bradycardia and altered respiration.
Fig. 20.7: A 6-yr-old boy presented with headaches, vomiting and seizures for 6 months. Computed tomography of the brain showed glioma of cerebellum with obstructive hydrocephalus
Symptoms from Compression or Infiltration
Headaches can occur from direct compression of skull and meninges. Vomiting may be due to raised intracranial pressure but can also occur because of direct infiltration of one of the vomitingcentersin the area postrema at thebase of the fourth ventricle. Head tilt may develop in a child as a correction for diplopia arising from a cranial nerve palsy.
Table 20.15: Genetic disorders associated with brain tumors
Genetic disorder |
Gene; chromosome |
Neurofibromatosis type 1 |
Neurofibromin; chromosome 17 |
Neurofibromatosis type 2 |
Merlin; chromosome 22 |
Li-Fraumeni syndrome |
P53; chromosome 17 |
Bilateral retinoblastoma |
Rbl; chromosome 13 |
Tuberous sclerosis |
TSC1; chromosome 9; TSC2; |
|
chromosome 13 |
von Hipplel-Lindau |
VHL; chromosome 3 |
Gorlin syndrome |
PATCHl; chromosome 9 |
Turcot syndrome |
APC; chromosome 5 |
Brain tumor |
Otherfeatures |
Optic glioma |
Autosomal dominant |
Acoustic neuromas; |
Peripheral nerve sheath tumors; |
schwannoma |
cardiac sarcoma |
Choroid plexus |
Sarcoma; adrenocortical cancer; |
carcinoma |
breast cancer |
Pineal tumor |
Sarcoma |
Subependymal cell |
Autosomal dominant |
astrocytoma, |
|
malignant glioma |
|
Hemangioblastoma |
Renal, adrenal and pancreatic |
tumors |
tumors |
Medulloblastoma |
Sensitive to radiation; basal cell |
|
carcinoma |
Medulloblastoma; |
Adenomatous polyps in colon |
malignant glioma |
|

__E_s_s_e_n_t1•P•e•d.a1-ia.trs.ic ___________________________
Diencephalic syndrome (emaciation, euphoria and emesis) is associated with tumors in the diencephalon. Parinaud's syndrome of supranuclear upgaze palsy withpupilsreactive to accommodation but not to direct light is associated with tumorsofpinealregionor upperbrainstem. Seizures arevery rarely associatedwith tumors. Low grade tumorsofcerebral cortexmay present with seizures as the main manifestation.
Frontal lobe tumors present with personality changes, seizuresandheadaches. Tumorsin thetemporallobecause seizures and speech changes. Suprasellar tumors are associated with endocrinopathies and visual changes. Tumors compressing or involving the hypothalamic pituitary axis present with endocrinopathies. Tumor should always be ruled out in a new onset diabetes insi pidus. Tumors involving the thalamus lead to motor and sensory deficits. Tectal plate (on top of brainstem) and pineal tumors cause obstructive hydrocephalus. Multiple cranial nerve deficits are classic presentation of brainstem gliomas. Nystagmus, ataxia and vomiting because of increased intracranial pressure are typically present with cerebellar tumors. Spinal tumors may cause back pain, scoliosis, numbness, weakness and impairment of bladder/bowel function.
Diagnosis
Imaging
MRI,withandwithoutcontrast,istheimagingstudyofchoice. CT scan is necessary in the setting of acute presentation suggestive of raised intracranial pressure.
Histology
While the distinction between benign and malignant tumors is critical, location of thetumor in the CNS is often as important a determinant of prognosis as the histology itself. A benign tumor in an unresectable location in the brain has as poor a prognosis as a malignant tumor in a surgically accessible area of the brain. Also, the age of the childdetermines the typeoftreatmentto be usedandhence impacts the prognosis. The grade of the tumor refers to microscopic appearance, 1 being the lowest and 4 being the highest grade. However, the grade does not always reflect theprognosis. Histologicaldiagnosisof braintumors is challenging. Special stains, immunohistochemistry and molecular testing is required to make the diagnosis.
Treatment
Treatment of pediatric brain tumors requires a multi disciplinary approach. Surgery, chemotherapy and radiation therapy are important for therapy. Complete surgical resectionwithout damaging the critical structures of the brain is usually the desired goal. However, this may be difficult to achieve depending on the location of the tumor. Somepatientsmayneedurgentsurgicalintervention to relieve raised intracranial pressure. Radiation therapy
utilizingphotons is most commonly used for treating brain tumors. Chemotherapy may also be used concurrently with radiation therapy as a radiation sensitizer for some tumors. Newer agents such as antiangiogenic drugs and inhibitors of tyrosine kinases, histone deacetylases and the sonic hedgehog pathway are under investigation for brain tumors.
Suggested Reading
Fleming AJ, Chi SN. Brain tumors in children. Curr Prob! Pediatr Adolesc Health Care 2012;42:80-103
Ulrich LJ, Pomory SL. Pediatric brain tumors. Neurol Clin 2003; 21:897-13
RETINOBLASTOMA
Retinoblastomais the most commonprimary ocular tumor of childhood, a tumor of the embryonic neural retina. It has an incidence of 11 new cases per million population (age less than 5 yr). About 90% cases are diagnosed by age 3-4 yr and 98% by 5 yr. Bilateral disease is diagnosed earlier then unilateral disease. There is increased frequencyofretinoblastoma in some developing countries especially Latin America, Africaand Asia including India. Also there is marked disparity in the mortality associated with retinoblastoma between developed and developing countries. 40-70% of children with retinoblastoma in the developing countries die as opposed to only 3-5% in the developed counties, predominantly due to delayed diagnosis and extraocular disease.
Genetics and Inheritance
The retinoblastoma (RBl) gene, encoded on chromosome 13ql4, was the first described tumor suppressor gene. Constitutional loss of one RBl allele causes cancer predisposition and loss of the second allele in developing retinal cells leads to retinoblastoma.
Retinoblastoma can be sporadic or inherited. Sporadic tumors are unilateral, unifocal and occur at an older age while inheritedtumors occur at an earlier age and are often bilateral and multifocal. One-third of all cases have bilateral tumors. All cases with bilateral disease have germline mutaions of RBI and are heritable. Only a small proportion of unilateral tumors are heritable.
Mostcasesofhereditaryretinoblastomahavespontaneous new germline mutation while their parents have both wild type RBI alleles. The risk of anoffspring inheriting an RBI mutation from a parent with germline mutation of RBI is 50% and 97% of these offsprings with the inherited mutation will go onto develop retinoblastoma. A consti tutional (germline) mutation of RBI also causes an increased risk of a second cancer of lung, soft tissue, bladder, skin, bone andbrain lifelong andthis risk is even higher when these patients are treated with radiation therapy for their retinoblastoma. A small proportion (5-10%) of unilateral tumors are hereditary.
