
- •Section b gasoline (petrol) engines
- •Section c diesel engines
- •Section b water and its boiling point
- •C) molecules in motion
- •Section d engine-cooling system
- •Section b lubrication
- •Section c engine lubricating system
- •Section d filters
- •Unit four electricity Section a electrolysis
- •Uses of Electrolysis
- •Section b batteries
- •Section c automobile electric system
- •Section d battery-powered automobiles
- •Section b ferrous metals
- •Tensile strength and hardness section c
- •Section d aluminium bronze
- •Grammar exercises
- •Unit 1 advantages and disadvantages of diesel engines
- •Unit 2 comparative characteristics diesel and gasoline engines
- •Unit 3 basic parts of the automobile
- •Unit 4 where diesel engines are used
- •Unit 5 Temperature Regulators of Cooling System
- •In machihes and machinery.
- •Unit 8 the instruments in a car
- •Unit 9 Battery chargers
- •Vocabulary Список сокращений
- •Literature used
- •Contents
Section b batteries
Electrolytic cells have several uses. Some cells are used for producing an electric current. They store an electrical charge in the electrolyte. Batteries contain this type of cell. Some batteries contain primary cells and others contain secondary cells.
Primary cells do not have a long life. It is impossible to recharge them. The primary cells in fig. (f) are called dry cells. These do not contain a liquid. The electrolyte is semi-solid. New cells produce approx. 1.5 V each. They generally consist of an ammonium chloride electrolyte (NH4CI), a carbon rod and a zinc casing. The rod is the anode and the casing is the cathode. The zinc casing corrodes with use, and the cells do not usually produce a current for more than twenty four hours.
It is possible to recharge a secondary cell many times. With careful use, secondary cells have a long life. Fig. (g) shows a car battery. These generally contain six lead-acid cells. These normally consist of a sulphuric acid electrolyte, lead electrodes and a plastic casing. The plastic casing does not normally corrode. Each cell produces 2V. Therefore a car battery normally produces 12 V. However, lead-acid batteries do not always consist of six cells. Some only have three. It is also possible to manufacture batteries with more than six cells for large vehicles.
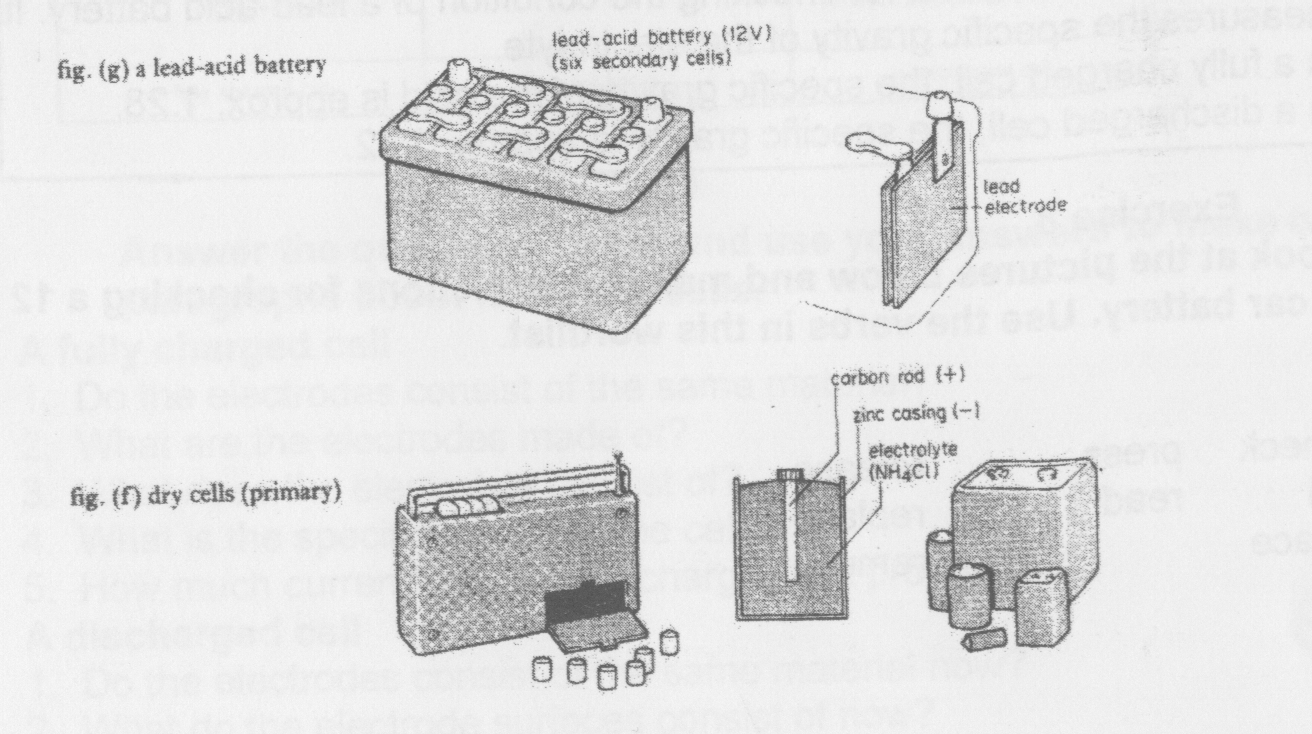
Exercise 5 Are these statements true or false? 1. Batteries store an electrical charge. 2. It is possible to recharge primary cells. 3. The dry cells in fig. (f) are secondary cells.
Secondary cells normally have a long life.
Car batteries generally contain six primary cells.
Lead-acid cells produce approx. 2 V each.
Lead-acid batteries are used in cars and other vehicles.
It is impossible to manufacture lead-acid batteries with more than six cells.
Exercise 6 Make sentences from this table.
A dry cell Lead-acid batteries
|
(does not) (do not) |
have has...
produce
corrode
|
a long life sulphuric acid ammonium chloride
a lead electrodes1.5 V. approx. 12 V.
a plastic casing. |
Exercise 7 Translate into Russian.
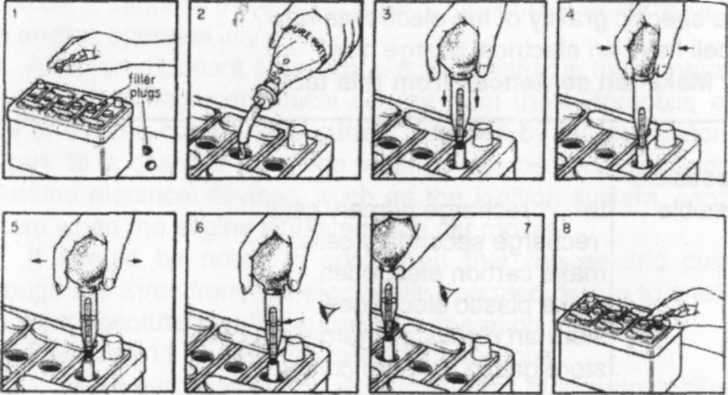
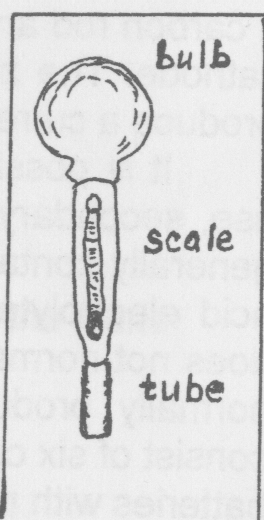
A hydrometer is used for checking the condition of a lead-acid battery. It measures the specific gravity of the electrolyte. In a fully charged cell, the specific gravity of the acid is approx. 1.28 In a discharged cell, the specific gravity is approx. 1.12.
Exercise 8
Look at the pictures below and make 8 instructions for checking a 12 V car battery. Use the verbs in this wordlist.
Check Fill place
|
Press read |
Release Replace remove
|
Exercise 9
With use, the composition of a lead-acid cell changes slowly. The diagram below shows the different compositions of a fully charged and discharged cell.
Answer the questions below and use your answers to make two paragraphs about lead-acid cells. A fully charged cell
Do the electrodes consist of the same material?
What are the electrodes made of?
What does the electrolyte consist of?
What is the specific gravity of the cell?
How much current does a fully charged cell produce?
A discharged cell
Do the electrodes consist of the same material now?
What do the electrode surfaces consist of now?
What is the specific gravity of the electrolyte now?
Does the cell have an electrical charge now?
Exercise 10 Make ten sentences from this table.
It is |
impossible possible |
to recharge primary cells. recharge secondary cells. make carbon electrodes. make plastic electrodes. store an electrical charge in a cell. store gas in an open container. use diesel fuel in a petrol engine. |

 zinc casing
zinc casing