
- •Nucleotides as metabolic regulators
- •ATP is not quite what it seems
- •GTP-binding proteins, G proteins, or GTPases
- •G proteins
- •The GTPase cycle: a monostable switch
- •Switching off activity: switching on GTPase
- •The G protein receptor kinase family
- •Receptor mechanisms obviating G proteins
- •Monomeric GTP-binding proteins
- •Ras proteins discovered as oncogene products
- •Subfamilies of Ras
- •Structure
- •Post-translational modifications
- •GTPases everywhere!
- •Mutations of Ras that promote cancer
- •Functions of Ras
- •RasGAPs
- •RasGAP
- •Mechanism of GTPase activation
- •Guanine nucleotide exchange factors (GEFs)
- •Essay: Activation of G proteins without subunit unit dissociation
- •Pheromone-induced mating response in yeast
- •Monitoring subunit interactions in living cells by FRET
- •References

GTP-Binding Proteins and Signal Transduction
Other molecules having GAP activity are more likely to be pure negative regulators of Ras. One of these is neurofibromin, the product of the NF1 gene, which is associated with neurofibromatosis type 1 (or von Recklinghausen’s neurofibromatosis). In neurofibroma-derived cell lines deficient in this protein, 30–50% of the Ras is present in the GTP-bound form (as opposed to less than 10% in normal cells).
Neurofibromin contains a segment clearly related to the catalytic domain of RasGAP, but otherwise the NF1 gene product possesses no identifiable domains or motifs and shares no sequence similarity with any other mammalian signalling proteins (see Figure 4.20). These findings are all consistent with the idea that neurofibromin is purely and simply a GTPase activator.
Guanine nucleotide exchange factors (GEFs)
The identification of proteins that catalyse GTP/GDP exchange on Ras, (guanine nucleotide exchange factors, or GEFs) was achieved by studying mutations in yeast and other simple eukaryotes, in which an exchange factor is deficient.
In yeast (S. cerevisiae), the RAS gene products regulate adenylyl cyclase,134 mediating the response to starvation and leading to spore formation. By mutating non-RAS genes that destroy the ability to activate cyclase, it is possible to identify other proteins that contribute to this signalling pathway. Some of these mutations can be overridden (bypassed) by the presence of constitutively activated RAS and are therefore likely to lie upstream of the GTPase. The CDC25 gene product is such a protein.135 Deletion is lethal, but can be compensated by expression of constitutively activated RAS, strongly suggesting that the CDC25 gene product acts as an upstream activator, probably a RAS-GEF.136 In addition, the effect of deletion can be overcome by expression of the C-terminal portion of CDC25, which is understood to house its catalytic activity. The catalytic domain of a human GEF Sos1 (see page 332) can restore cyclase activity in yeast lacking CDC25.137
Essay: Activation of G proteins without subunit unit dissociation
The standard textbook description of the G-protein cycle assumes that the-subunits dissociate entirely from their partners and become independent entities. However, the evidence for this idea, based mainly on biochemical experiments carried out in cell-free systems, and never strong, is becoming ever slimmer.138 This is, at best, an oversimplification. We wish to ask whether
von Recklinghausen’s neurofibromatosis is one of the most common of human hereditary disorders, having an estimated incidence of
1 in 3500 individuals worldwide. Almost half of the patients have no previous family history of the disease, so with 1 in 10 000 individuals harbouring a new mutation, it follows that
NF1, of all human genes, must be one of the most prone to mutation. It predisposes to benign and malignant tumour formation, especially in cells derived from the neural crest.
111

Signal Transduction
Most work in this field has been confined to the investigation of the activation (and inhibition) of adenylyl cyclase, initially in turkey red blood cell membranes and later in other cell-
derived and reconstituted systems. The turkey
red cell membrane cyclase is particularly amenable since it is totally dependent on the presence of a
stimulating hormone and is rather slow, allowing the collection of many data points. There have been no comparable attempts to elucidate the mechanism of activation of phospholipase C.
and do truly dissociate in every case. The main arguments favouring dissociation are:
•Over-expression, or provision of purified -subunits to isolated membrane preparations, causes activation of downstream effector enzymes such as adenylyl cyclase or phospholipase C.
•Provision or over-expression of -subunits tends to oppose the activation due to -subunits in some experimental systems such as platelet membranes.
•Provision of fluoride ions, or stable analogues of GTP (GTP S, etc.) to
G proteins in detergent solution in the presence of high concentrations of Mg2 enhances their tendency to dissociate. These manipulations are an essential component in the strategies applied in subunit purification.
•The - and -subunits of the retinal G protein transducin certainly dissociate from each other but also from the membrane when activated by rhodopsin.139 However, 8% remains attached to the membrane as the intact heterotrimer and it is possible that it is this fraction that activates the effector, cyclic GMP phosphodiesterase.
•Structural studies of GDP-bound, heterotrimeric G proteins and of complexes of activated -units with their effectors have shown (1)
that the switch regions are obscured by the -subunits in the inactive G protein and (2) that contact with the effectors is made through the same regions.
•-Subunits are unable to bind to -subunits and effector molecules such as adenylyl cyclase or phospholipase C simultaneously. The binding regions for both classes of molecule overlap each other.140
Although it is clear that G protein subunits can dissociate under conditions promoting activation and certainly do so when pressed hard enough (fluoride plus detergent plus Mg2 ), it is far from clear that this is what actually happens in cell membranes.
In support of the idea of non-dissociation is that the process of adenylyl cyclase activation is a first-order reaction, dependent only on the amount of activated receptor. From this it follows that the Gs is permanently coupled to adenylyl cyclase and indeed, is maintained through a 3000-fold purification of the enzyme. When this is carried out in the presence of an activating guanine nucleotide (GppNHp), a 1:1:1 stoichiometry of cyclase to s to is retained.141,142 There is no need to invoke the dissociation of the -subunits from the complex in order for sGTP to cause activation, even though this might deliver a stronger stimulus. The important possibility that arises is that the presence of both subunits may be required in order for the G protein to fulfil its authentic physiological role. What would happen if the subunits are prevented from dissociating?
112

GTP-Binding Proteins and Signal Transduction
Pheromone-induced mating response in yeast
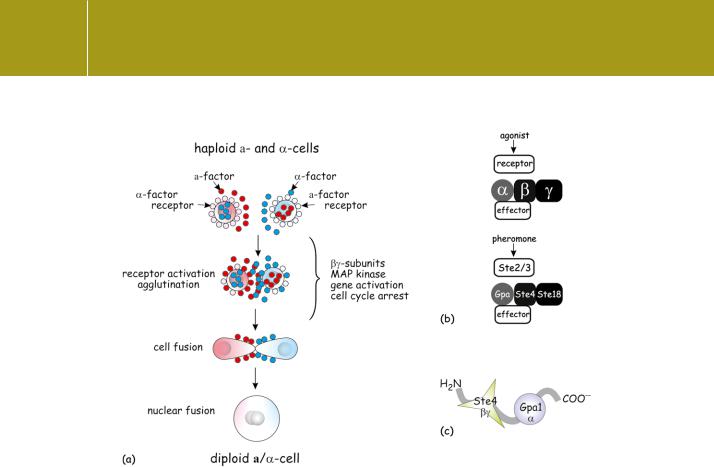
An excellent system in which to challenge the ideas concerning the dissociation of G protein subunits and their relationship to receptors is provided by the mating response of the yeast (S. cerevisiae) in which two haploid cells fuse to form a diploid cell. The haploid cells must be of opposite mating type, a and (analogous to sex). Mating is initiated by the reciprocal binding of pheromones called mating factors, a factor from a cells binding to receptors on cells and vice versa. The a- and -type mating factors are oligopeptides (12 and 13 residues) similar to mammalian gonadotrophin releasing hormone (GnRH).143 In response to binding, the haploid cells undergo cell cycle arrest at the late G1 phase. Cells of opposite mating type attach to each other, fuse, and eventually give rise to diploid /a cells (Figure 4.22a). The receptors for the secreted pheromones are products of the genes STE2 and STE3. The signals are transduced by the products of the genes GPA, STE4, and STE18. The homology between these products and mammalian signalling molecules is shown in Figure 4.22b.
Deletion of GPA is lethal because the free -subunits then activate a pathway leading to growth inhibition. However, mammalian -subunits of any class (also chimeric mammalian/yeast -subunits), that can bind to the -subunits, are able to restore viability. However, they do not restore the signal transduction pathway leading to the generation of diploid cells. Indeed, the presence of genes coding for all of the G protein subunits is
required. Consequently, using the production of diploid cells as a read-out, it is possible to ask searching questions concerning the status and interactions of the various components of the signal transduction pathway. This has been achieved by the use of mutants in which any one of the genes has been substituted by a fusion construct, for example composed of the N- and C- terminal segments of different absent proteins (Figure 4.22b, c).
Using such an approach, the mating response of STE4 / ( -subunit knockout) cells was restored by genes coding for chimeras composed of the N-terminus of Ste4 coupled to the C-terminus of Gpa1.12 These were fully functional in the transduction of pheromone signals inducing growth arrest and mating. Since the chimeric construct allows no possibility of subunit dissociation, it is likely that the activated receptor acts to induce conformational changes in the heterotrimer, exposing hidden binding interfaces that allow communication with downstream effectors. The implication is that the fusion construct is fully competent to convey signals ascribed to both the - and the -subunits. It follows that the wild-type proteins, although capable of dissociating, may
not actually do so. If they do tend towards detachment, then it is likely that they remain in very close proximity to each other throughout the cycle. Indeed, it begins to appear that most of the molecules involved in signal
113
Signal Transduction
Fig 4.22 Pheromone-induced mating response of yeast.(a) Haploid yeast cells (a and mating types) generate a and pheromone mating factors that bind to specific receptors on cells of the opposite type. This initiates a series of events that include agglutination, cell cycle arrest and fusion to create diploid cells. In the presence of nutrients, the diploid cells may undergo multiple divisions. In starvation, they undergo meiosis forming four haploid spores. Sinceand a pheromone receptors are G-protein-linked receptors coupled to adenylyl cyclase, the production of diploid cells can be used to test the integrity of the signal transduction pathway. (b) Representation of a construct containing Ste2 (receptor) fused to a chimera of Gpa1 (yeast, N-terminal segment) and S (rat, C-terminal segment). This supports efficient signal transduction. (c) Representation of a construct containing Ste4 ( -subunit) fused to Gpa1 ( -subunit). This transmits signals as efficiently as the native G protein subunits.
transduction – receptors, G proteins, effectors – are close neighbours at all times and under all conditions of activation.
Monitoring subunit interactions in living cells by FRET
A direct way of detecting the association or dissociation of fluorescent molecules is provided by the phenomenon of fluorescence resonance energy transfer (FRET). Excitation energy may be transferred from one fluorophore (a fluorescent molecule or moiety) to another. The requirements are (1) that the two fluorophores should be in close proximity to one another (within 10 nm),
(2) that the emission spectrum of the donor fluorophore should overlap the excitation spectrum of the acceptor, and (3) that the emission dipole moment of the donor should be aligned sufficiently with the excitation dipole moment of the acceptor. When these conditions are met, excitation of the donor
114

GTP-Binding Proteins and Signal Transduction
fluorophore results in emission from the acceptor. The transfer of excitation energy is a radiationless process and no fluorescence is emitted by the donor.
A biological application of FRET is illustrated in Figure 4.23 for cyan and yellow fluorescent proteins (CFP and YFP, mutated forms of green fluorescent protein). These have spectra that overlap, such that the fluorescence emission spectrum of CFP (solid line: 450–600 nm) falls within the excitation spectrum of YFP (dotted line: 450–550 nm). An energy transfer event occurs when a photon is absorbed by the donor (CFP) followed by the emission of a photon from the acceptor (YFP). If the donor and acceptor move apart, the energy transfer efficiency will decrease and vice versa. In this example, emission from YFP, in response to the absorption of violet light by CFP, will decline. At the same time, blue fluorescence emission from CFP will begin to appear.
To investigate the association of G protein - and -subunits, it is necessary to tag them with different fluorophores. This is most effectively achieved
by transfecting cells so that the subunits are expressed as fusion proteins, for example with YFP and CFP. In an experiment illustrated in Figure 4.23c,144 HEK cells expressing G i1 fused with YFP and G 1 fused with CFP, exhibit FRET (yellow emission from G i in response to illumination at 436 nm). If, during stimulation, the - and -subunits separate from one another, the YFP emission would be expected to drop and the CFP fluorescence to rise as the transfer efficiency falls. However, what we see is the converse. FRET increases during stimulation (yellow line) and CFP fluorescence decreases (blue line). This indicates that the fluorophores, and thus the subunits, move closer together (or become better aligned for FRET). The t½ of the onset of the fluorescence changes ( 1 s) and of their reversal on withdrawal of the stimulating hormone ( 38 s) (Figure 4.23c) closely match the rates of opening and closure of GIRK channels introduced as effectors into the same cells, indicating that it is the non-dissociated subunits that are functional.
The details of the actual molecular rearrangement are not clear. While FRET increases upon stimulation when CFP is located at the N-terminus of the-subunit, it decreases when it is sited at the C-terminus of the -subunit (see Figure 4.2). The simplest explanation involves a rearrangement in which the N- termini of and move closer to the helical domain of , while the C-terminal region of moves away. At all events, the - and -subunits certainly do not dissociate.
Similar FRET investigations indicate that Gi2, Gi3, and Gz also undergo intersubunit rearrangements without dissociating when stimulated. On the other hand, Go and the Gs-like proteins of the slime mould Dictyostelium tell different stories that can be ascribed to subunit dissociation (or very
different forms of rearrangement).145,146 The B/C helical domains of the i andz subunits appear to be essential for stable tethering to -subunits, and substitution of this region with the homologous sequence from Go allows
HEK cells (human embryonic kidney cells): an epithelial cell line that is easy to culture and transfect.
115

Signal Transduction
Fig 4.23 Fluorescence resonance energy transfer (FRET) used to determine subunit dissociation.
(a) Fluorescence excitation and emission spectra of cyan and yellow fluorescent protein (CFP and YFP). (b) FRET results in the emission of yellow light by YFP after the absorption of violet light by CFP. (c) Fluorescence time course of CFP-G 1(blue)and YFP-G i1 (yellow) emission from a single HEK cell during noradrenaline stimulation (NA). Excitation was provided at 436 nm. While the YFP emission registers energy transfer, the emission ratio yellow:cyan (red line) takes account of experimental artefacts (e.g. light scattering) and is a more sensitive measure of FRET.Adapted from Bunemann et al.144
116

GTP-Binding Proteins and Signal Transduction
dissociation (fluorescence ratio decrease in the FRET experiment). On the other hand, the converse situation, insertion of the B/C domain from i1 intoo, fails to induce Gi-like behaviour, so other regions of i must be involved in the stabilization of the intact trimeric formation.146
The stability of the Gi and Gz heterotrimers may provide the key to understanding the specificity of signal transmission between receptors and those effectors, such as the GIRK channels, which are activated by -subunits. As mentioned previously (see page 94), when tested in reconstitution experiments, it has been hard to discern much selectivity, let alone specificity for particular pairs in the activation of effector systems. The stability of the Gi and Gz heterotrimers allows for specificity to be determined by the identity of the -subunit, while the activation signal is carried by the -subunit.
So the best answer to the question of whether heterotrimeric G proteins dissociate on activation is that some do, at least sometimes, and others don’t.
Constructing the mammalian -adrenergic transduction system in insect cells
Earlier we described how the use of S49 cyc lymphoma cells was instrumental in determining the role of the s-subunit of the signal transduction process. More versatile are the Sf9 insect cells.147 With these cells and the baculovirus vector
it is possible to manipulate the expression of all the components – mammalian receptors, -subunits, and -subunits – independently of each other, with regard to identity, specified mutations, and levels of expression. The native adenylyl cyclase can be used to read out the information transfer from the activated receptors.
When expressed in Sf9 cells, the affinity of the mammalian -adrenergic receptor for its ligands is not only very low, but also insensitive to the presence of guanine nucleotides (Figure 4.24a–c). The response of the native (insect) cyclase to the mammalian receptor is also low, and in general the transduction of the signals from the mammalian receptor by the native insect Gs is inefficient. The system displays features of an ‘uncoupled’ (unc) phenotype.148 All this changes when the Sf9 cells express mammalians-subunits in addition to the mammalian receptor (Figure 4.24d). The affinity of ligand binding (isoprenaline) is now high, but importantly, it becomes sensitive to the presence of guanine nucleotides, just as in mammalian cells. In this aspect of receptor function, it appears that the insect -subunits are able to cooperate with the mammalian -subunits. However, the GTPase activity remains insensitive to the presence of stimulating ligands. It requires the additional expression of mammalian -subunits for the system to take on the full characteristics of the mammalian signalling pathway (Figure 4.24).
2-Adrenergic receptors expressed together with mammalian Gs heterotrimers
117

Signal Transduction
Fig 4.24 Reconstitution of the -adrenergic response in insect cells. (a) Although they possess G protein (grey disc) coupled to adenylyl cyclase, Sf9 (insect) cells lack -adrenergic receptors and are therefore unresponsive to catecholamines. (b) When transfected with the human -adrenergic receptor (pale green), Sf9 cells generate cAMP at a low rate that is proportional to the extent of receptor expression. No activating ligand is needed for this low level activity. (c) The rate of cAMP generation is greatly enhanced when the transfected cells are stimulated with isoprenaline (yellow ellipse). However, in membrane preparations, the affinity of the receptor is not sensitive to the presence of GTP. The native G protein does not transmit a feedback signal to the mammalian receptor. Nor is the GTPase activity of the insect -subunit sensitive to the presence of the agonist. (d) By additionally transfecting the cells with mammalian s (blue), the affinity of the receptor becomes sensitive to the activation state of the G protein. However, GTPase activity remains insensitive to the presence of the hormone. (e) To establish the complete pathway of forward and backward control, it is necessary to express both the
mammalian - and -subunits (pink). The GTPase activity of the -subunit is now sensitive to the presence of the activating hormone. For details of this experiment, see Lachance et al.148
allow high signal throughput, high ligand binding affinity, and now, ligandenhanced GTPase activity. The conclusion from all this is that the - and the -subunits communicate with the receptor throughout the period of activation. They never really lose sight of each other, nor of the receptors.
That this might really be the case, not just in transfected insect cells, finds support in the frequent reports of the coimmunoprecipitation of receptors together with G proteins and of G proteins with their effector enzymes. Similarly, the purification of 7TM receptors on affinity supports can result in copurification of G protein subunits, frequently of several different classes. As examples, the receptors for somatostatin and for opioids ( -opioid) copurify with - and -subunits to an extent that depends on the state of their activation.149–151 This raises the possibility that receptors, rather than communicating in a linear fashion through one G protein to one catalytic
118

GTP-Binding Proteins and Signal Transduction
H |
R |
G |
E |
(1) |
|
H1 |
R1 |
G |
E |
(2) |
|
|
|
||||
H2 |
R2 |
|
|
|
|
H |
R |
G |
E1 |
(3) |
|
E2 |
|||||
|
|
|
|
||
|
|
G1 |
E1 |
|
|
H |
R |
G2 |
E2 |
(4) |
|
|
|
|
H1  R1
R1  G1
G1
E (5)
H2  R2
R2 G2
G2
Fig 4.25 Pathways of information flow through receptors and G proteins.The communication of signals from receptor to effector is not necessarily a simple linear sequence of steps (1). There are other more complex modes by which receptors, transducers and effectors are linked. Some of these may seem self evident: different receptors accessing one class of G proteins (2) and a single class of G protein regulating more than one type
of effector enzyme (3). The regulated switching of attention of adrenergic receptors between Gs and Gi (4) is considered in Chapters 9 and 12. The synergistic interaction of two receptors and two G proteins in the activation of some isoforms of adenylyl cyclase (5) is discussed in Chapter 5.
effector, may communicate, depending on their state of activation, with different G proteins and thence with different effector enzymes (Figure 4.25).
For yeast cells devoid of the endogenous STE2 (pheromone receptor) and GPA1 ( -subunit) genes, transduction of the mating response can be
achieved by a fusion protein generated from the N-terminus of Ste2 linked to a chimera composed of the N-terminus of Gpa1 and the C-terminus of rats152 (Figure 4.22b). The presence of the Gpa1/ s construct (i.e. not linked to the receptor), while capable of restoring viability to haploid cells lacking Gpa1, fails to restore mating competence. This is due to its inability to recognize the receptor, but there is no problem when the two are fused together as Ste2Gpa1/ s. It follows that the C-terminus of the -subunit operates
mainly to bring the G protein into the proximity of the receptor, allowing guanine nucleotide exchange and ensuring efficient coupling. Questions related to the communication of signals to downstream effectors are not raised in this experiment since for yeast this is a function of the-subunits.
Contrary to standard descriptions of G protein activation, couched in terms of fleeting interactions between receptors and dissociated subunits, the evidence now shows that receptors, G proteins and their effectors remain together as operational ensembles. Some of them may dissociate when activated, but the extent of this under physiological conditions remains uncertain.
119
